Abstract
Although fibrous collagens are major structural components of extracellular matrix in mammals, collagen overproduction is associated with many human diseases including cancers and fibrosis. Collagen is typically identified in biomedical research by western blot and immunohistochemistry; however anti-collagen antibodies employed in these analyses are difficult to prepare and their affinities to collagen can diminish if collagen becomes denatured during analyses. Previously, we discovered that single-stranded collagen mimetic peptides [CMPs, sequence: (GlyProHyp)9] can bind to denatured collagen chains by triple helix hybridization. Here we present collagen-specific staining methods using simple CMPs conjugated to common fluorophores (e.g. carboxyfluorescein), which allow direct detection of collagens and collagen-like proteins in SDS-PAGE and in various mammalian tissue sections. By directly staining SDS-PAGE gels with fluorescently labeled CMPs, both intact (type I, II, and IV) and MMP-1 cleaved collagen (type I) chains as well as complement factor C1q were detected. Collagen bands containing as little as 5 ng were optically visualized while no staining was observed for fibronectin, laminin, and a collection of proteins from mammalian cell lysate. The CMP was unable to stain collagen-like bacterial protein which contains numerous charged amino acids that are believed to stabilize triple helix in place of Hyp. We also show that fluorescently labeled CMPs can specifically visualize collagens in fixed tissue sections (e.g., skin, cornea, and bone) more effectively than anti-collagen I antibody, and allow facile identification of pathologic conditions in fibrotic liver tissues.
Fibrous collagens, a major structural component of the extracellular matrix (ECM), are largely found in connective tissues. Biosynthesis and degradation of collagens which are mediated by growth factors and proteases secreted by cells are widely studied in developmental biology,1-3 wound healing, and aging.4 Numerous human diseases including osteogenesis imperfecta,5 atherosclerosis,6 fibrosis7-10, arthritis,11-14 and tumors15 are associated with abnormalities in either the structure or metabolism of collagens.
Western blot and immunohistochemistry are the two most common techniques for detecting collagens,5,7-15 where a particular type of collagen is identified by antibody binding. However, because the triple helical domains which constitute the major part of the fibrous collagen (type I and II) have a highly repetitive triplet amino acid sequence (Gly-X-Y) and a tight rod-like structure, it is difficult to generate antibodies with high specificities against fibrous collagens.16,17 Therefore, extensive purification and selection steps, which involve multiple immunoaffinity purification against serum proteins and other non-collagenous ECM proteins, are needed to create collagen antibodies with low levels of cross-affinity. For antibodies that recognize the intact triple helical collagen epitopes, their affinity decreases dramatically when they are used in western blot and in formalin-fixed and/or paraffin embedded tissue samples because collagens in those samples are partially denatured. Moreover, antibody detection usually requires overnight reactions and additional detection steps involving secondary antibodies labeled with either a reporter enzyme or a fluorescent dye, which are often tedious and time-consuming. Considering these limitations, we sought to develop a broad-spectrum collagen staining agent that is easy to use and can bind not only to native collagens but also to denatured fibrous collagens.
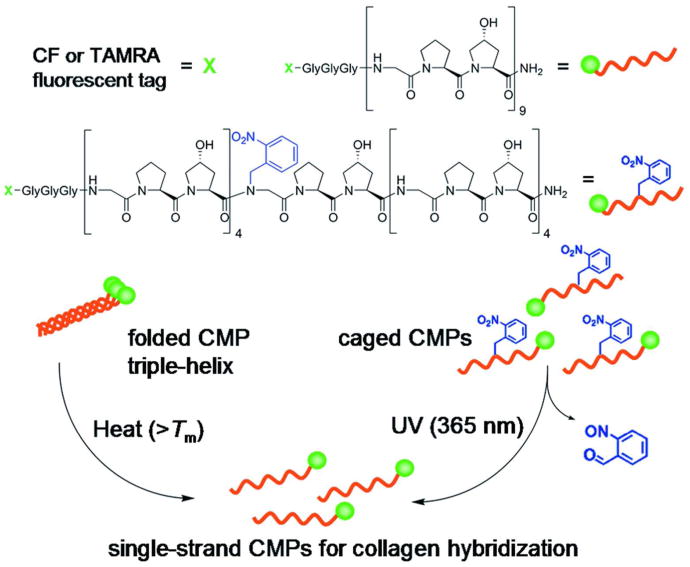
Here we report a convenient collagen-specific staining method that is based on triple helix forming peptide probe which can directly detect collagenous proteins in SDS-PAGE as well as in immunohistochemical staining (Figure 1). Previously, we discovered that single strand collagen mimetic peptides [CMP, sequence: (GPO)x, x = 6-10, O: hydroxyproline] can bind to unfolded collagen chains presumably through the formation of collagen-CMP heterotrimeric complexes.18-25 The binding interaction originates from the unique triple helical structure of the collagens,26 and the inherently strong triple helical folding propensity of the CMPs.27 Because CMPs self-assemble into homotrimers at room temperature which have little driving force for collagen binding, monomeric CMPs were generated by heating the peptide solution above CMP's melting temperature just prior to applying to collagen substrates (Figure 1).20-22,28 Although such thermally activated CMPs were successfully used for collagen tissue scaffold modification, they could not be used for in vivo experiments for concerns associated with heat-induced tissue damage. Recently we've developed a new type of CMP, namely caged CMP [(GPO)4NBGPO(GPO)4, Figure 1], whose triple helical folding can be controlled by UV light.18 The caged CMP contains a photo-cleavable nitrobenzyl group attached to the peptide's central glycine, which sterically prevents the CMP from folding into triple helix; yet removal of the protective cage group by UV irradiation immediately recovers CMP's folding and collagen binding abilities. Taking advantage of this efficient non-thermal trigger, we were able to employ CMP with strong triple helical propensity (high Tm) to perform in vivo collagen targeting studies in mice. This led to a surprising discovery that systemically delivered CMPs can target denatured collagens of tissues undergoing normal or pathologic remodeling.18 Realizing CMPs' effectiveness in targeting denatured collagen from these studies,18,20,21,23 we set out to explore their full capacity for in vitro collagen/gelatin targeting using both the heat and light activation approaches (Figure 1). We were particularly interested in devising a new and easy collagen staining method that can help biomedical researchers and clinicians.
Figure 1.
Structures of fluorescently labeled CMP and nitrobenzyl (NB) caged CMP, designated respectively as CF(GPO)9 and CFNB(GPO)9 for CF labeled peptides, and schematic illustration of the two approaches (heat or UV activation) of generating single-strand CMPs that can hybridize with collagen strands.
The rate of single stranded CMPs self-assembling into homotrimers depends strongly on the concentration of CMP solution, because trimer formation follows a third order folding kinetics.29,30 Previously, for diagnostic imaging18 and tissue scaffold engineering21,22 applications, it was necessary to use high concentrations (50 μM - 0.2 mM) of CMPs in order to achieve binding levels high enough for collagen detection and for activating cellular response in collagen scaffolds. Since homotrimer formation is fast in concentrated CMP solutions, we employed thermal quenching and caging-decaging strategies to minimize the homotrimer formation in those studies. We realized that such strategies are not necessary for in vitro collagen staining application, since the concentration of CMP staining solutions can be very dilute (5-10 μM) with the trimerization half time in the order of hours at room temperature.29,30 Therefore we decided to test staining of SDS-PAGE and tissue sections using dilute solutions of CMPs.
We synthesized a peptide with (GPO)9 sequence conjugated to a fluorophore through a flexible GGG linker to minimize fluorophore's influence on the peptide's triple helical folding process (Figure 1). We chose 5(6)-carboxyfluorescein (CF) and 5(6)-carboxytetramethylrhodamine (TAMRA) as the fluorophores since they are compatible with standard fluorescence microscopes and imaging systems. All peptides were prepared by conventional solid-phase peptide synthesis (SPPS) using Fmoc/HBTU chemistry. The caged CMP was synthesized by inserting Fmoc(N-o-nitrobenzyl)Gly-OH in the middle of standard SPPS; the coupling reaction following the NBGly was run using excess amount of Hyp and PyBroP as previously reported.18 The fluorophores were conjugated to the amino termini of the peptide on the solid phase in the presence of PyAOP and DIPEA (see Supporting Information, Materials and Methods, and Figure S1).31 The CD spectra and thermal melting curves of the fluorescent peptides (Supporting Information Figure S2) confirmed that the triple helical folding propensity of the peptides remains largely unchanged even when they are conjugated to the fluorophores. A sequence-scrambled peptide, CFSG9P9O9 (CF-GGG-PGOGPGPOPOGOGOPPGOOPGGOOPPG) that cannot fold into triple helix was also prepared for comparison.18
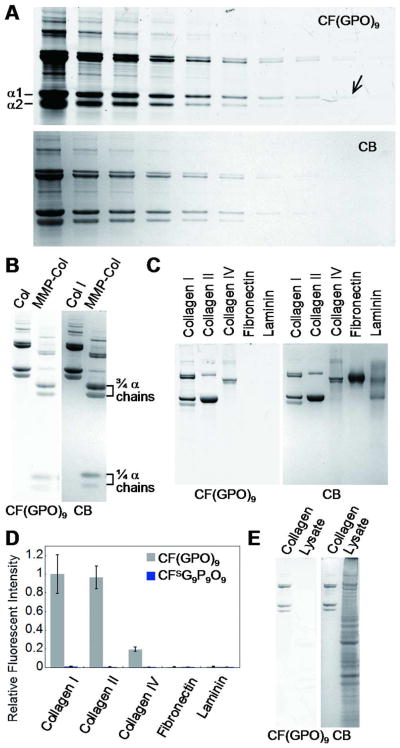
To test the CMP's collagen probing capacity in SDS-PAGE under dilute CMP concentration, denatured type I collagen was resolved by SDS-PAGE. After the electrophoresis, the gel was washed with deionized water three times to remove the remaining SDS, and immersed in a solution of CF(GPO)9 (6 μM) that had been pre-heated to 85 °C which is above the peptide's melting temperature (see Supporting Information, Materials and Methods). After 3 hr of incubation at room temperature followed by washing with deionized water, the gel was photographed using a fluorescent imaging system. The image of the gels showed distinct fluorescent emission from the bands of type I collagen chains (Figure 2A): not only the α1 and α2 chains were visible but also higher molecular weight bands corresponding to the naturally crosslinked collagen chains were prominently visualized with high fluorescence intensity. Under dilute conditions, the thermally melted CMPs remain mostly in single strands immediately after the cooling due to their slow triple helix folding rate as described above.29,30 These monomeric CMPs seem to be able to hybridize with the unfolded collagen chains in the SDS-PAGE which are denatured and densely aggregated in the bands. To estimate the sensitivity of the CMP probe, a dilution series of type I collagen was run. The most dilute band that could be visualized by the CF(GPO)9 probe contained as little as 5 ng of collagen chains (Figure 2A, arrow), which is similar to the sensitivity level of the conventional coomassie brilliant blue (CB) staining (Figure 2A bottom panel). We also found out that enzymatically digested collagen fragments can be readily visualized on SDS-PAGE by the heat activated CF(GPO)9 just as well as the UV activated caged peptide previously reported from our group (Figure 2B).18
Figure 2.
Detection of collagen in SDS-PAGE by heat activated fluorescent CMPs. (A) SDS-PAGE loaded with a dilution series of type I collagen, stained and imaged first with CF(GPO)9 (top panel) followed by coomassie blue (CB) staining (bottom panel). From left to right, each lane was loaded with 4 μg, 2 μg, 1 μg, 500 ng, 250 ng, 125 ng, 62.5 ng, 31.2 ng, 15.6 ng and 7.8 ng of denatured collagen, respectively. The arrow points to the least recognizable band in the image which contains approximately 5 ng of collagen α1 chains. (B) SDS-PAGE of intact and MMP-1 cleaved type I collagens (3 μg in each lane) similarly stained with CF(GPO)9 and CB. (C) SDS-PAGE loaded with collagen type I, II, IV, fibronectin and laminin (2 μg of each protein), and stained with CF(GPO)9 and CB. (D) Comparative fluorescence levels of the ECM protein bands in SDS-PAGE stained by CF(GPO)9 (C) or CFSG9P9O9 (Supporting Information Figure S3). The measured fluorescence intensities were normalized by collagen I, and the experiment was performed in triplicate (±s.d.). (E) SDS-PAGE of collagen I (0.7 μg) and a lysate of HUVECs stained by CF(GPO)9 and CB showing remarkable specificity of CMP for collagen detection. Images of the CF(GPO)9 stained gels were recorded using a Typhoon fluorescent imager (λex = 488 nm), and CB stained gels were photographed using a Gel Doc EQ system.
We further determined the binding specificity of CF(GPO)9 for different collagen types and other major ECM proteins. Fluorescent images of the gel after SDS-PAGE showed that CF(GPO)9 binds to collagen types I, II and IV but has no affinity to fibronectin and laminin (Figure 2C). Same gel stained with the sequence-scrambled peptide CFSG9G9O9 revealed no collagen bands (Supporting Information Figure S3). These results clearly show that the CMPs bind by triple helical hybridization and only to proteins with triple helical domains. Intensity of the fluorescence emission in each collagen lane (Figure 2D) indicated that type IV collagen was stained to a lesser degree than the type I and II collagens. This is most likely because the type IV collagen has lower triple-helical content due to the presence of large globular non-collagenous domains as well as over 20 interruptions in the collagenous domain that break up the triple helix.32 Finally, when CF(GPO)9 was used to stain the SDS-PAGE of whole cell lysate of human umbilical vein endothelial cells (HUVEC), no protein band was visualized (Figure 2E). This result demonstrates the remarkable specificity of the CMP probe for detecting collagen strands.
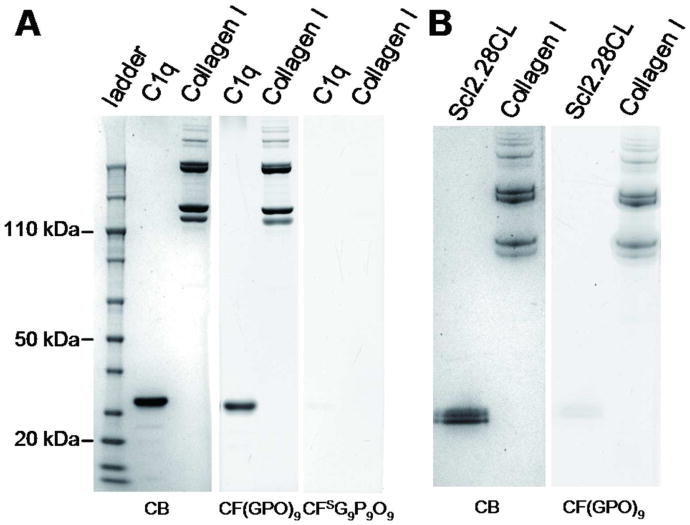
Although the Gly-X-Y triplet repeat is the signature protein sequence of collagen family, similar sequence is also found in several non-collagenous proteins, typically as a triple helix-triple helix association domain of a larger protein (e.g. mannose binding protein and complement factor C1q).26 The Gly-X-Y repeats capable of forming triple helical structure are also found on the surface of bacteria and viruses.19,33,34 We were curious to see if these collagen-like domains can also be detected in SDS-PAGE by CMP binding. We tested two proteins: the complement protein C1q which is constructed of eighteen globular heads connected to six collagen-like triple helical assemblies,35,36 and a recombinant protein, Scl2.28CL, derived from the cell-surface protein (Scl2) of Streptococcus pyogenes previously reported to form collagen-like triple helices.37 The Scl2 folds into lollipop-like structure similar to C1q monomers and is speculated to interact with mammalian collagens and proteins with collagenous domains (e.g. macrophage scavenger receptor38), facilitating their adhesion to host cells and tissues.19,37,38 The collagen-like domain of complement factor C1q is rich in Hyp and Pro, which are strong triple helix stabilizers, respectively in the positions X and Y of the Gly-X-Y repeats;39 however in the Scl 2 protein, these positions are populated by charged amino acids37 which are believed to stabilize collagen triple helix by electrostatic interactions.40
We found out that CF(GPO)9 can stain the SDS-PAGE bands of C1q chains almost as effectively as collagens (Figure 3A). This indicates that the CMPs are capable of hybridizing with the collagen-like domain of the C1q chains. In drastic contrast, CF(GPO)9 failed to stain the Scl2.28CL bands (Figure 3B). In mammalian collagens, hydroxyprolines play a critical role in folding and stabilization of the triple helical structure,27 while collagen-like domains of bacterial proteins that lack Hyp rely, in part, on charge-charge interactions for making stable triple helix.40 Heat denatured mammalian collagens partially recover their triple helical structure and turn into gelatin when cooled.41 The Scl2.28CL chains, however, are unable to refold into triple helix after denaturation.42,43 The results suggest that CMP can hybridize with the denatured strands of the Hyp rich collagen and C1q, but it does not make stable heterotrimeric helices with collagen-like sequences of Scl2, presumably because the CMP is a neutral peptide that cannot participate in electrostatic interactions and because of inherently low triple helix folding propensity of the Pro-poor Scl2 sequence.
Figure 3.
SDS-PAGE of collagen-like proteins stained with CMP. (A) SDS-PAGE loaded with 2 μg of complement factor C1q and type I collagen, stained by CB, CF(GPO)9, or CFSG9P9O9, showing specific visualization of the C1q chains by CF(GPO)9 hybridization. (B) SDS-PAGE loaded with 2.5 μg of type I collagen and streptococcal collagen-like protein Scl2.28CL stained by CF(GPO)9, showing almost no staining of the Scl2.28CL band.
Compared to conventional antibody-mediated detection, CMP probes are more convenient to use. Antibody binding in western blot requires transferring the proteins from PAGE gel onto a polyvinylidene difluoride (PVDF) or nitrocellulose membrane, followed by blocking, and long hours of immunoreactions. In contrast, because of CMP's small size and high affinity, detection of collagen by CMP probes can be performed directly in gels, without transferring and blocking, and in relatively short period of time. In addition, the CMP hybridization relies on protein's overall secondary structure instead of a few well-defined epitopes. Therefore, even fragments of collagen chains can be recognized, a feature useful for studying collagen degradation which is common to many degenerative diseases (e.g. arthritis). The main limitation of the CMP probes is that the probes cannot distinguish different collagen types, since the Pro- and Hyp-rich Gly-X-Y motif recognized by the CMP is universal in all types of collagens. Therefore CMP probes should be considered a broad-spectrum collagen targeting molecule.
While western blot is useful for molecular level detection and quantification of collagens, direct visualization of collagens can help identify the location of collagens in tissue samples and a pathological state of diseased tissues with abnormal collagen remodeling activity. In immunohistochemistry, harvested tissues are often preserved by fixation, followed by cryosectioning and probing by different antibodies to determine the location of biomolecules. The fixation step is needed to keep the cellular components and overall tissue morphology from deterioration during histological study and long term storage; however the fixing procedures, which often include heat (microwave), and treatment with organic solvents (e.g. acetone and alcohols) and crosslinking reagents (e.g. paraformaldehyde), can denature the collagen molecules.44 Although such denaturation can reduce the number of epitopes for antibody binding, it could have an opposite effect on CMP probes. The denaturation may increase the number of binding sites for the CMP probes because the CMP preferentially hybridizes with denatured collagen strands over intact collagen fibers. For this reason, we anticipated that the fluorescent CMP probes could be an ideal collagen staining agent for histology. Since addition of heat activated peptide probes to tissue sections could result in further tissue damage and destruction of other heat sensitive antibodies (for co-staining), the caged CMP that can be activated by UV light was used for staining tissue sections.
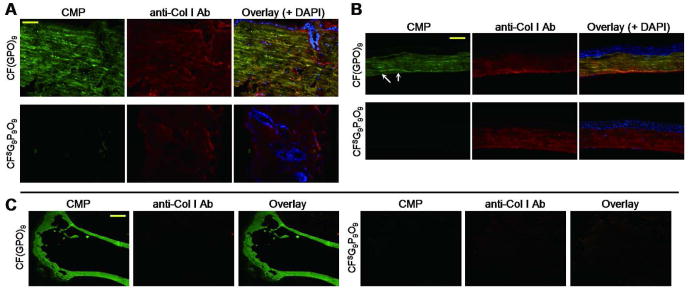
Caged CMP, CFNB(GPO)9 was applied to tissue sections (fixed tissue sections from mouse skin, cornea, and bone), followed by exposure to UV light to activate collagen binding. Anti-collagen I antibody (2nd antibody: anti-rabbit-AlexaFluor594) was also applied to the tissue samples for comparison (see Supporting Information, Materials and Methods). As shown in Figure 4A and B, the decaged CF(GPO)9 effectively stained the collagen-rich dermis layer of the fixed mouse skin and the stroma of the cornea sections. The control groups stained by scrambled peptide CFSG9P9O9 showed no discernible binding under identical experimental conditions (Figure 4A and B, bottom panels). The fluorescent signals from the CF(GPO)9 overlapped largely with those from the antibody, which confirmed the specificity of the probes for the collagen fibrils. Compared to the anti-collagen antibody, the CF(GPO)9 showed more intense signals which also revealed finer details of the collagen fibril organization in the dermis and the corneal stroma (Figure 4A and B). In addition, a bright green line corresponding to the Descemet's membrane of cornea which contains type VIII collagen was clearly visualized by the CMP probe (Figure 4B, arrow). We also noticed that the processing of the tissue seems to enhance the CMP binding. Paraformaldehyde fixed cornea samples were significantly brighter than the fresh unfixed samples when identical CMP staining and imaging protocols were employed (Supporting Information Figure S4). In particular, the mouse tibia bone sections that have undergone acidic demineralization process as well as fixation and paraffin embedding exhibited strong CF(GPO)9 signals but almost no collagen antibody signal was detected (Figure 4C). It is highly likely that the tertiary protein structure of the epitopes targeted by the collagen antibody had been compromised by the heat during the paraffin-embedding, and the highly acidic demineralization process; yet the CMP probe can still target such collagens because it recognizes the unfolded secondary protein structure that is prevalent in collagens.
Figure 4.
Histological tissue staining using photo-activated caged CMPs. Fluorescent micrographs of fixed mouse skin (A) and cornea (B) sections probed by photo-triggered CFNB(GPO)9 (in green, top panels) or scrambled peptide CFSG9P9O9 (in green, bottom panels) and co-stained with anti-collagen I antibody (red), and DAPI (blue). The CFNB(GPO)9 staining revealed fine irregular collagen fibers of the dermis (A) and parallel collagen fibrils in the corneal stroma as well as the collagens in Descemet's membrane (arrows) (B). (C) Fluorescent images of paraffin embedded, demineralized mouse tibia sections stained with photo-triggered CFNB(GPO)9 (green, left panels) or scrambled peptide CFSG9P9O9 (green, right panels), and co-stained with anti-collagen I antibody (red), showing prominent CMP signals and only weak non-specific antibody signals from the collagenous bone. Concentrations of CFNB(GPO)9 and CFSG9P9O9 used in this study are: (A), 25 μM; (B), 2.5 μM; (C), 8 μM. (scale bars: A, 50 μm; B, 100 μm; C, 0.5 mm).
The CMP probe's remarkable ability to target collagens in bones even after acidic demineralization and extensive preservation process demonstrates the robustness and versatility of the CMP mediated collagen staining. Histological preservation and processing often cause alteration or masking of epitopes targeted by immunohistochemical agents. Even for a same target biomolecule, different histological processing (e.g. frozen vs. paraffin-embedded) may require different types of primary antibody. Sometimes, heat- or enzyme-induced antigen retrieval step is necessary to improve the antibody binding.45 In contrast, CMP probes recognize the secondary protein structure, the metastable polyproline-II-like helix that is waiting for triple helix hybridization partners. For this reason, perturbation of collagen's tertiary and quaternary protein structures seem to have little effect on the CMP's binding affinity to collagen strands.
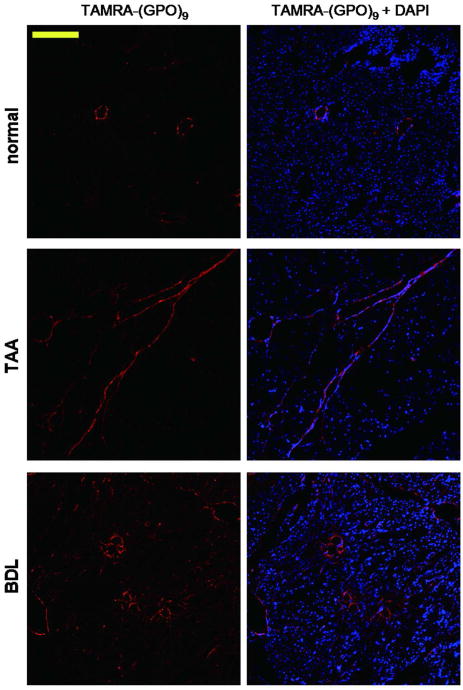
To showcase the CMP's ability to identify pathological conditions, a set of healthy and fibrotic rat liver sections were stained utilizing the photo-triggered CMP probe. Two common fibrosis models were tested: a fibrosis induced by repetition of a toxic insult, thioacetamide (TAA), to the liver, and the secondary biliary fibrosis model induced by bile duct ligation (BDL).46 Because the liver tissues emit strong autofluorescence whose spectrum overlaps with the emission spectrum of CF,47 TAMRA-NB(GPO)9 was used for the liver fibrosis staining. In addition, CuSO4 solution was applied to the tissue sections during optical imaging to selectively reduce the lipofuscin-like background autofluorescence (see Materials and Methods, Supporting Information).48 As shown in Figure 5, TAMRA-(GPO)9 revealed minimal collagen staining in healthy liver: collagens can only be found surrounding the major vessels in the portal area. In the tissue sections of TAA and BDL fibrotic models, the CMP successfully exposed the abnormal presence of collagens.49,50 In the TAA sample, long and thin bridged septa of aggregated collagens were readily seen, and in the BDL model, excess fibrotic collagens were detected around the circular proliferating bile ducts (Figure 5). These results are consistent with the hepatic fibrosis patterns for those classical fibrosis models.46 Compared to Masson Trichrome staining, a common non-immunochemical staining procedure that chemically stains collagen fibers in blue on top of pink colored cellular background (Supporting Information, Figure S5), the fluorescent CMP probe offers more clear and collagen specific imaging as well as simultaneous co-staining with other biomarkers that can be easily distinguished by the multi-color channels of fluorescence microscope. The results demonstrate the potential application of CMP probes not only for histology of clinical biopsies, but also for live imaging and targeting of fibrotic tissues.51
Figure 5.
Identification of fibrotic conditions using CMP staining. Fluorescence micrographs of normal and fibrotic rat liver sections (TAA and BDL) stained with photo-triggered TAMRANB(GPO)9 (10 μM, in red) and DAPI (in blue), showing distinct collagen distributions in fibrotic liver models. (scale bar: 200 μm).
In summary, this study has validated the use of fluorescently labeled collagen mimetic peptides for direct and efficient detection of Hyp rich collagenous proteins in SDS-PAGE and immunohistostaining. Our results indicate that CMPs are highly effective at staining collagens in extensively processed tissue sections which are not easily probed by conventional antibodies. We believe that the fluorescent CMP is an excellent alternative to collagen antibodies for detecting fibrous collagens in various assays and tissue imaging. Although CMP probes cannot distinguish different types of collagens, they offer higher specificity to collagens when compared to conventional staining agents.52 Conventional collagen staining agents such as Sirus Red rely on electrostatic interactions for binding to collagens; negatively charged probes bind to positively charged collagens. Therefore the staining is not specific to collagen and can stain other proteins with high content of basic amino acids.53 Since CMP recognizes the triple helical amino acid sequence of collagen, it is a true broad-spectrum collagen-specific staining agent with almost no binding affinity for non-collagenous proteins as demonstrated in this paper. As a potent collagen targeting molecule, CMP is a structurally simple peptide that is easy to prepare and conjugate to other bioactive moieties. The two orthogonal activation mechanisms (Figure 1: heat activation of CMP and light activation of caged CMP) provide great flexibility for the incorporation of additional functionalities to this peptide: the heat activation system is suitable for conjugation of chemically reactive compounds that may be sensitive to UV light or photo-cleaved by-products, while the light activation system is suitable for conjugation of delicate biomolecules (e.g. proteins) that might be incompatible with heat. The ability to synthesize more complex CMP derivatives that can target collagen strands may lead to new applications in tissue scaffold engineering, collagen-targeted drug delivery and in vivo collagen imaging.
Supplementary Material
Acknowledgments
We thank Drs. Catherine Foss and Collin Torok for the gift of the mouse tibia bone sections, Jie Yan and Yuzhan Kang for the gift of rat liver samples, and Dr. Lijuan He for technical assistance and informative discussion. We also thank Dr. Michael McCaffery of the Integrated Imaging Center at Johns Hopkins University for the access to the Typhoon™ 9410 imager. This work was supported by grants from NIAMS/NIH (R01-AR060484) and DOD (W81XWH-12-1-0555) awarded to S.M.Y., and grants from NIH, K08-EY15523, R01-EY019874 awarded to A.S.J. and P30-EY001765 awarded to Wilmer Microscopy Core Facility, as well as NIH EB011620 awarded to B.B.
Abbreviations
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- Fmoc
fluorenylmethyloxycarbonyl
- HBTU
2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate
- PyBroP
bromotripyrrolidinophosphonium hexafluorophosphate
- PyAOP
(7-azabenzotriazol-1-yloxy)tripyrrolidinophosphonium hexafluorophosphate
- DIPEA
N,N-diisopropylethylamine
- CD
circular dichroism
- MMP
matrix metalloproteinase
- DAPI
4′,6-diamidino-2-phenylindole
Footnotes
Notes: The authors declare no competing financial interest.
Supporting Information: Materials and Methods, MALDI-TOF and CD analysis of the peptides, control gel images, additional fluorescent immuohistostaining images, and Masson Trichrome stained tissue sections. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Nagata K. HSP47 as a collagen-specific molecular chaperone: function and expression in normal mouse development. Semin Cell Dev Biol. 2003;14:275–282. doi: 10.1016/j.semcdb.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Quantock AJ, Young RD. Development of the corneal stroma, and the collagen-proteoglycan associations that help define its structure and function. Dev Dyn. 2008;237:2607–2621. doi: 10.1002/dvdy.21579. [DOI] [PubMed] [Google Scholar]
- 3.von der Mark H, von der Mark K, Gay S. Study of differential collagen synthesis during development of the chick embryo by immunofluorescence: I. Preparation of collagen type I and type II specific antibodies and their application to early stages of the chick embryo. Dev Biol. 1976;48:237–249. doi: 10.1016/0012-1606(76)90088-9. [DOI] [PubMed] [Google Scholar]
- 4.Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees JJ. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138:1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- 5.Willaert A, Malfait F, Symoens S, Gevaert K, Kayserili H, Megarbane A, Mortier G, Leroy JG, Coucke PJ, De Paepe A. Recessive osteogenesis imperfecta caused by LEPRE1 mutations: clinical documentation and identification of the splice form responsible for prolyl 3-hydroxylation. J Med Genet. 2009;46:233–241. doi: 10.1136/jmg.2008.062729. [DOI] [PubMed] [Google Scholar]
- 6.Rekhter MD. Collagen synthesis in atherosclerosis: too much and not enough. Cardiovasc Res. 1999;41:376–384. doi: 10.1016/s0008-6363(98)00321-6. [DOI] [PubMed] [Google Scholar]
- 7.Haist V, Ulrich R, Kalkuhl A, Deschl U, Baumgartner W. Distinct spatio-temporal extracellular matrix accumulation within demyelinated spinal cord lesions in theiler's murine encephalomyelitis. Brain Pathol. 2012;22:188–204. doi: 10.1111/j.1750-3639.2011.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He W, Zhang LN, Ni AG, Zhang ZP, Mirotsou M, Mao L, Pratt RE, Dzau VJ. Exogenously administered secreted frizzled related protein 2 (Sfrp2) reduces fibrosis and improves cardiac function in a rat model of myocardial infarction. Proc Natl Acad Sci U S A. 2010;107:21110–21115. doi: 10.1073/pnas.1004708107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ulsamer A, Wei Y, Kim KK, Tan K, Wheeler S, Xi Y, Thies RS, Chapman HA. Axin pathway activity regulates in vivo pY654-beta-catenin accumulation and pulmonary fibrosis. J Biol Chem. 2012;287:5164–5172. doi: 10.1074/jbc.M111.322123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim KK, Wei Y, Szekeres C, Kugler MC, Wolters PJ, Hill ML, Frank JA, Brumwell AN, Wheeler SE, Kreidberg JA, Chapman HA. Epithelial cell alpha3 beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest. 2009;119:213–224. doi: 10.1172/JCI36940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mo XT, Guo SC, Xie HQ, Deng L, Zhi W, Xiang Z, Li XQ, Yang ZM. Variations in the ratios of co-cultured mesenchymal stem cells and chondrocytes regulate the expression of cartilaginous and osseous phenotype in alginate constructs. Bone. 2009;45:42–51. doi: 10.1016/j.bone.2008.07.240. [DOI] [PubMed] [Google Scholar]
- 12.Shen JB, Yang MZ, Jiang H, Ju DH, Zheng JP, Xu ZH, Liao TD, Li L. Arterial injury promotes medial chondrogenesis in Sm22 knockout mice. Cardiovasc Res. 2011;90:28–37. doi: 10.1093/cvr/cvq378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cairns DM, Uchimura T, Kwon H, Lee PG, Seufert CR, Matzkin E, Zeng L. Muscle cells enhance resistance to pro-inflammatory cytokine-induced cartilage destruction. Biochem Biophys Res Commun. 2010;392:22–28. doi: 10.1016/j.bbrc.2009.12.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rankin KS, Lakey RL, Gerrand CH, Sprowson AP, McCaskie AW, Birch MA. A novel in vitro model to investigate behavior of articular chondrocytes in osteoarthritis. J Rheumatol. 2010;37:426–431. doi: 10.3899/jrheum.080080. [DOI] [PubMed] [Google Scholar]
- 15.Chen PS, Su JL, Cha ST, Tarn WY, Wang MY, Hsu HC, Lin MT, Chu CY, Hua KT, Chen CN, Kuo TC, Chang KJ, Hsiao M, Chang YW, Chen JS, Yang PC, Kuo ML. miR-107 promotes tumor progression by targeting the let-7 microRNA in mice and humans. J Clin Invest. 2011;121:3442–3455. doi: 10.1172/JCI45390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srinivas GR, Chichester CO, Barrach HJ, Pillai V, Matoney AL. Production of Type II Collagen Specific Monoclonal Antibodies. Immunol Invest. 1994;23:85–98. doi: 10.3109/08820139409087791. [DOI] [PubMed] [Google Scholar]
- 17.Chichester C, Barrach HJ, Chichester A, Matoney A, Srinivas GR. Evidence for polyreactivity seen with monoclonal antibodies produced against type II collagen. J Immunol Methods. 1991;140:259–267. doi: 10.1016/0022-1759(91)90379-t. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Foss CA, Summerfield DD, Doyle JJ, Torok CM, Dietz HC, Pomper MG, Yu SM. Targeting collagen strands by photo-triggered triple-helix hybridization. Proc Natl Acad Sci U S A. 2012;109:14767–14772. doi: 10.1073/pnas.1209721109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu SM, Li Y, Kim D. Collagen mimetic peptides: progress towards functional applications. Soft Matter. 2011;7:7927–7938. doi: 10.1039/C1SM05329A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang AY, Mo X, Chen CS, Yu SM. Facile modification of collagen directed by collagen mimetic peptides. J Am Chem Soc. 2005;127:4130–4131. doi: 10.1021/ja0431915. [DOI] [PubMed] [Google Scholar]
- 21.Wang AY, Foss CA, Leong S, Mo X, Pomper MG, Yu SM. Spatio-temporal modification of collagen scaffolds mediated by triple helical propensity. Biomacromolecules. 2008;9:1755–1763. doi: 10.1021/bm701378k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang AY, Leong S, Liang YC, Huang RCC, Chen CS, Yu SM. Immobilization of growth factors on collagen scaffolds mediated by polyanionic collagen mimetic peptides and its effect on endothelial cell morphogenesis. Biomacromolecules. 2008;9:2929–2936. doi: 10.1021/bm800727z. [DOI] [PubMed] [Google Scholar]
- 23.Mo X, An YJ, Yun CS, Yu SM. Nanoparticle-assisted visualization of binding interactions between collagen mimetic peptide and collagen fibers. Angew Chem Int Ed. 2006;45:2267–2270. doi: 10.1002/anie.200504529. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Mo X, Kim D, Yu SM. Template-tethered collagen mimetic peptides for studying heterotrimeric triple-helical interactions. Biopolymers. 2011;95:94–104. doi: 10.1002/bip.21536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HJ, Lee JS, Chansakul T, Yu C, Elisseeff JH, Yu SM. Collagen mimetic peptide-conjugated photopolymerizable PEG hydrogel. Biomaterials. 2006;27:5268–5276. doi: 10.1016/j.biomaterials.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Engel J, Bächinger HP. Structure, stability and folding of the collagen triple helix. In: Brinckmann J, Notbohm H, Müller PK, editors. Collagen. Springer; Verlag Berlin Heidelberg: 2005. pp. 7–33. [Google Scholar]
- 27.Shoulders MD, Raines RT. Collagen Structure and Stability. Annu Rev Biochem. 2009;78:929–958. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan TR, Stahl PJ, Yu SM. Matrix-bound VEGF mimetic peptides: design and endothelial cell activation in collagen scaffolds. Adv Funct Mater. 2011;21:4252–4262. doi: 10.1002/adfm.201101163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boudko S, Frank S, Kammerer RA, Stetefeld J, Schulthess T, Landwehr R, Lustig A, Bächinger HP, Engel J. Nucleation and propagation of the collagen triple helix in single-chain and trimerized peptides: transition from third to first order kinetics. J Mol Biol. 2002;317:459–470. doi: 10.1006/jmbi.2002.5439. [DOI] [PubMed] [Google Scholar]
- 30.Ackerman MS, Bhate M, Shenoy N, Beck K, Ramshaw JAM, Brodsky B. Sequence dependence of the folding of collagen-like peptides. J Biol Chem. 1999;274:7668–7673. doi: 10.1074/jbc.274.12.7668. [DOI] [PubMed] [Google Scholar]
- 31.Stahl PJ, Cruz JC, Li Y, Yu SM, Hristova K. On-the-resin N-terminal modification of long synthetic peptides. Anal Biochem. 2012;424:137–139. doi: 10.1016/j.ab.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc Res Tech. 2008;71:357–370. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bamford DH, Bamford JKH. Collagenous proteins multiply. Nature. 1990;344:497–497. doi: 10.1038/344497b0. [DOI] [PubMed] [Google Scholar]
- 34.Smith MCM, Burns N, Sayers JR, Sorrell JA, Casjens SR, Hendrix RW. Bacteriophage collagen. Science. 1998;279:1834–1834. doi: 10.1126/science.279.5358.1831g. [DOI] [PubMed] [Google Scholar]
- 35.Kishore U, Reid KBM. Modular organization of proteins containing C1q-like globular domain. Immunopharmacology. 1999;42:15–21. doi: 10.1016/s0162-3109(99)00011-9. [DOI] [PubMed] [Google Scholar]
- 36.Tschopp J. Kinetics of activation of the first component of complement (C1) by IgG oligomers. Mol Immunol. 1982;19:651–657. doi: 10.1016/0161-5890(82)90365-0. [DOI] [PubMed] [Google Scholar]
- 37.Xu Y, Keene DR, Bujnicki JM, Höök M, Lukomski S. Streptococcal Scl1 and Scl2 Proteins Form Collagen-like Triple Helices. J Biol Chem. 2002;277:27312–27318. doi: 10.1074/jbc.M201163200. [DOI] [PubMed] [Google Scholar]
- 38.Andersson L, Freeman MW. Functional changes in scavenger receptor binding conformation are induced by charge mutants spanning the entire collagen domain. J Biol Chem. 1998;273:19592–19601. doi: 10.1074/jbc.273.31.19592. [DOI] [PubMed] [Google Scholar]
- 39.Rosano CL, Braun CB, Hurwitz C. A method for serum C1q based on its hydroxyproline content. Clin Chem. 1987;33:398–400. [PubMed] [Google Scholar]
- 40.Mohs A, Silva T, Yoshida T, Amin R, Lukomski S, Inouye M, Brodsky B. Mechanism of stabilization of a bacterial collagen triple helix in the absence of hydroxyproline. J Biol Chem. 2007;282:29757–29765. doi: 10.1074/jbc.M703991200. [DOI] [PubMed] [Google Scholar]
- 41.Leikina E, Mertts MV, Kuznetsova N, Leikin S. Type I collagen is thermally unstable at body temperature. Proc Natl Acad Sci U S A. 2002;99:1314–1318. doi: 10.1073/pnas.032307099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng H, Rashid S, Yu Z, Yoshizumi A, Hwang E, Brodsky B. Location of glycine mutations within a bacterial collagen protein affects degree of disruption of triple-helix folding and conformation. J Biol Chem. 2010;286:2041–2046. doi: 10.1074/jbc.M110.153965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshizumi A, Yu Z, Silva T, Thiagarajan G, Ramshaw JAM, Inouye M, Brodsky B. Self-association of streptococcus pyogenes collagen-like constructs into higher order structures. Protein Sci. 2009;18:1241–1251. doi: 10.1002/pro.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bächinger HP, Morris NP. Analysis of the thermal stability of type II collagen in various solvents used for reversed-phase high performance chromatography. Matrix. 1990;10:331–338. doi: 10.1016/s0934-8832(11)80189-7. [DOI] [PubMed] [Google Scholar]
- 45.Hecke DV. Routine immunohistochemical staining today: choices to make, challenges to take. Journal of Histotechnology. 2002;25:45–54. [Google Scholar]
- 46.Starkel P, Leclercq IA. Animal models for the study of hepatic fibrosis. Best Practice & Research Clinical Gastroenterology. 2011;25:319–333. doi: 10.1016/j.bpg.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 47.Croce AC, De Simone U, Freitas I, Boncompagni E, Neri D, Cillo U, Bottiroli G. Human liver autofluorescence: An intrinsic tissue parameter discriminating normal and diseased conditions. Lasers Surg Med. 2010;42:371–378. doi: 10.1002/lsm.20923. [DOI] [PubMed] [Google Scholar]
- 48.Schnell SA, Staines WA, Wessendorf MW. Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J Histochem Cytochem. 1999;47:719–730. doi: 10.1177/002215549904700601. [DOI] [PubMed] [Google Scholar]
- 49.Tai DCS, Tan N, Xu S, Kang CH, Chia SM, Cheng CL, Wee A, Wei LC, Raja AM, Xiao G, Chang S, Rajapakse JC, So PTC, Tang HH, Chen CS, Yu H. Fibro-C-Index: comprehensive, morphology-based quantification of liver fibrosis using second harmonic generation and two-photon microscopy. J Biomed Opt. 2009;14:044013. doi: 10.1117/1.3183811. [DOI] [PubMed] [Google Scholar]
- 50.Salguero Palacios R, Roderfeld M, Hemmann S, Rath T, Atanasova S, Tschuschner A, Gressner OA, Weiskirchen R, Graf J, Roeb E. Activation of hepatic stellate cells is associated with cytokine expression in thioacetamide-induced hepatic fibrosis in mice. Lab Invest. 2008;88:1192–1203. doi: 10.1038/labinvest.2008.91. [DOI] [PubMed] [Google Scholar]
- 51.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Armendáriz-Borunda J, Rojkind M. A simple quantitative method for collagen typing in tissue samples: its application to human liver with schistosomiasis. Coll Relat Res. 1984;4:35–47. doi: 10.1016/s0174-173x(84)80027-8. [DOI] [PubMed] [Google Scholar]
- 53.Nielsen LF, Moe D, Kirkeby S, Garbarsch C. Sirius red and acid fuchsin staining mechanisms. Biotech Histochem. 1998;73:71–77. doi: 10.3109/10520299809140509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.