Abstract
Purpose
Clinical pathways aimed at reducing hospital length of stay following vascular surgery have been broadly implemented to reduce costs. However, early hospital discharge may adversely affect the risk of readmission or mortality. To address this question, we examined the relationship between early discharge and 30-day outcomes among patients undergoing a high-risk vascular surgery procedure, thoracic aortic aneurysm (TAA) repair.
Methods
Using Medicare claims from 2000 to 2007, we identified all patients who were discharged home following elective thoracic endovascular aneurysm repair (TEVAR) and open repair for nonruptured TAAs. For each procedure, we examined the correlation between early discharge (<3 days for TEVAR, <7 days for open TAA repair) and 30-day readmission, 30-day mortality, and hospital costs. Predictors of readmission were evaluated using logistic regression models controlling for patient comorbidities, perioperative complications, and discharge location.
Results
Our sample included 9764 patients, of which 7850 (80%) underwent open TAA repair, and 1914 (20%) underwent TEVAR. Patients discharged to home early were more likely to be female (66% early vs 56% late), Caucasian (94% early vs 91% late), younger (73 years early vs 74 years late), and have fewer comorbidities (mean Charlson score: 0.7 early vs 1.0 late) than patients discharged home late (all P < .01). As compared with patients who were discharged late, patients discharged home early following uncomplicated open TAA repair and TEVAR had significantly lower 30-day readmission rates ([open: 17% vs 24%; P < .001] [TEVAR: 12% vs 23%; P < .001]) and hospital costs ([open: $73,061 vs $136,480; P < .001] [TEVAR: $58,667 vs $128,478; P < .001]), without an observed increase in 30-day postdischarge mortality. In multivariable analysis, early hospital discharge was associated with a significantly lower likelihood of readmission following both open TAA repair (odds ratio, 0.70; 95% confidence interval, 0.57–0.85; P < .001) and TEVAR (odds ratio, 0.57; 95% confidence interval, 0.38–0.85; P < .01) procedures.
Conclusions
Discharging patients home early following uncomplicated TEVAR or open TAA repair is associated with reduced hospital costs without adversely impacting 30-day readmission or mortality rates. These data support the safety and cost-effectiveness of programs aimed at early hospital discharge in selected vascular surgery patients.
High-risk vascular surgery procedures such as major aortic reconstructions have historically been associated with high complication rates, extended hospital length of stay (LOS), and a significant risk for readmissions. Advances in coordinated clinical care pathways, however, have helped to decrease the risk of postoperative complications, and concordantly, the time needed to recover in the hospital following major vascular surgery prior to discharge.1–3 The practice of discharging patients early is encouraged by financial incentives within current hospital payment systems, as a means to reduce costs, particularly when patients can be sent home versus to a skilled nursing facility.4 Accordingly, many healthcare networks have created incentive programs to reward the early discharge of vascular and other surgical patients.
The benefits of early hospital discharge, however, may be offset by higher rates of readmission, if vascular surgery patients are potentially discharged “too early,” before potentially preventable complications are recognized. Further, the importance of readmission as a quality measure in vascular surgery has gained increasing prominence in recent years, given current changes in healthcare legislation.5 The Center for Medicare and Medicaid Services (CMS) has developed new policies in accordance with the Patient Protection and Affordable Care Act of 2010 to reduce rates of readmission,6,7 and this legislation will soon enact lower rates of remuneration for providers and centers with high rates of readmission.
In this context, we examined the association between early hospital discharge and readmission following thoracic aortic aneurysm (TAA) repair in the national Medicare population. Thoracic endovascular aortic repair (TEVAR) and open TAA repair are both high-risk vascular procedures associated with an increased risk of postoperative complications, which may lead to increased 30-day readmission and mortality. We hypothesized that patients can be discharged home early following complex vascular procedures such as TAA repair, when their hospital course has been uncomplicated, as a means to contain hospital costs without having an increased risk of readmission. As hospital payers and providers increasingly scrutinize hospital costs, LOS, and readmissions following vascular surgery, it is essential to examine the tradeoff between these different quality measures.
METHODS
Data sources and study population
We used the CMS Medicare Provider Analysis and Review database to study patients undergoing TAA repair between January 1, 2000 and December 31, 2007. This nationwide database contains patient and hospital identifiers, demographics, risk factors, hospitalization and procedure dates, complications, hospital charges, treatment costs (inpatient and outpatient), and discharge status for patients ≥65 years of age undergoing surgical repair for TAA. International Classification of Diseases, Ninth Revision (ICD-9) procedure codes were used to identify patients who underwent TEVAR and open repair of nonruptured TAA from the CMS dataset. In addition to these procedural codes for TAA, each patient was required to have a diagnosis code for TAA. As shown in Fig 1, we excluded CMS claims with diagnosis codes for separate thoracic aortic pathologies, including ascending TAAs, thoracoab-dominal aneurysms, thoracic aortic dissections, and “other” aortic pathology. ICD-9 procedure codes associated with debranching procedures such as 39.24 (“aortorenal bypass”) and 39.25 (“aorto-iliac-femoral bypass”) were also excluded from analysis.
Fig 1.

Establishing the cohort of Medicare beneficiaries who were discharged home after undergoing nonruptured TAA repair.
Medicare beneficiaries that underwent TAA repair were excluded from analysis if their median hospital LOS was ≤1 day, if they died during their index admission, or if they were discharged home with hospice care. In addition, we excluded patients that were discharged to skilled nursing facilities or rehabilitation facilities, given that hospital LOS in these patients would be confounded. Our study protocol was approved by the Dartmouth Institutional Review Board.
Definition of LOS and exposure variables
Patients with TAAs were first categorized into two groups based on whether they underwent TEVAR or open TAA repair. Next, we stratified patients by their hospital LOS for each procedure into three evenly sized groups designated as “early discharge,” “normal discharge,” and “late discharge.” Early discharge categories for each procedure type were established at least a day below the geometric mean LOS, defined by CMS for the TAA repair diagnosis-related group (DRG). The geometric mean LOS is what Medicare uses to determine the average LOS for a given procedure when determining hospital payments and any adjustments. For TEVAR, early discharge LOS was defined as 1 to 2 days, normal discharge LOS was 3 to 7 days, and late discharge LOS was >8 days. For open TAA repair, early discharge LOS was defined as a 3 to 6 days, normal discharge LOS was 7 to 10 days, and late discharge LOS was >11 days. The LOS for each procedure did not include any days spent in the hospital preoperatively.
Risk adjustment models were constructed for LOS exposures in each procedure group, which included baseline patient demographic variables for age (continuous and categorical variables), gender, race, urgency of admission (elective, urgent, or emergent), and comorbidity score using the Charlson comorbidity index. In addition, logistic models and stratified analyses were designed to control for whether patients experienced a major postoperative complication based on ICD-9 diagnosis codes for renal failure, pulmonary failure, major gastrointestinal complication, spinal cord ischemia, stroke, or major cerebrovascular complication. These adverse events were identified as the most significant postoperative complications following TAA repair that would be potentially associated with increased LOS and risk for readmission.
Readmission, mortality, and cost analysis
The primary outcomes for the study were 30-day readmission or postdischarge mortality following TEVAR or open TAA repair. Readmission was defined as a readmission to any hospital within 30 days of discharge from index hospitalization for the vascular procedure. Only the first readmission during the first 30 days postoperatively was examined. Postdischarge mortality was defined as death within 30 days that occurred following discharge from the index hospitalization. Analyses of primary outcomes were conducted with and without stratification of patients who experienced any major complication during their index hospitalization.
Health care spending and cost outcomes were calculated using the Medicare Total Charge amount, which represents the total number of all hospital charges (covered and uncovered) for all services provided to the beneficiary during the index hospitalization. Hospital costs included only charges incurred during the day of procedure and postoperative hospitalization period. In addition, we analyzed Medicare-adjusted payments for services as well, which represent the negotiated bundled payment for part A claims. The ratio of hospital costs to adjusted payments was calculated for patients undergoing TEVAR and open TAA repair with different LOS exposures.
Statistical analysis
Analyses of patient variables and outcome variables were performed using χ2 tests for categorical variables and analysis of variance for continuous variables that were normally distributed. The Wilcoxon signed rank-sum test was used to compare non-normally distributed data. We used logistic regression modeling to estimate the effect of early discharge practices on 30-day readmission rates and post-discharge mortality rates while adjusting for patient-level variables. These models accounted for clustering of patient-level outcomes within hospitals. Potential interactions between variables were also explored using multivariate analysis. P values less than .05 (two-sided) were considered to be significant for all statistical tests and models, and the Bonferroni correction was used to control for multiple comparisons. Stata 11.0 statistical software (College Station, TX) was used for all analyses.
RESULTS
We identified a total of 9764 patients that underwent surgical repair for TAAs from 2000 to 2007 and were subsequently discharged home (Fig 1). This cohort included 7850 patients (80%) that underwent open TAA repair and 1914 patients (20%) that underwent TEVAR. Five hundred ninety-five (31%) of the TEVAR procedures occurred prior to 2005, when the first thoracic endograft device became approved by the US Food and Drug Administration (Table I). The median LOS decreased between 2000 and 2007 for patients that underwent TEVAR and open TAA repair (Table I). During this same time period, there was a concurrent decrease in readmission rates from 22% to 16% among patients undergoing TEVAR (P < .05), as well as a decrease in readmission from 21% to 19% among patients who underwent open TAA repair (P < .05). Overall, patients who underwent TEVAR had a lower mean LOS (5.2 days vs 9.0 days; P < .001) and were less likely to be readmitted compared with patients undergoing open TAA repair (17.6% TEVAR, 20.1% open surgical repair; P < .05).
Table I.
Trends in utilization, median length of stay (LOS), and readmission rates among patients undergoing thoracic endovascular aneurysm repair (TEVAR) and open repair who were discharged home
| Years | TEVAR
|
Open TAA repair
|
||||
|---|---|---|---|---|---|---|
| N | Median LOS | Average readmission rate | N | Median LOS | Average readmission rate | |
| 2000–2001 | 109 | 5 | 22.0 | 1553 | 8 | 21.0 |
| 2002–2003 | 198 | 5 | 18.2 | 1852 | 8 | 20.5 |
| 2004–2005 | 559 | 4 | 18.6 | 2262 | 8 | 20.4 |
| 2006–2007 | 1048 | 4 | 16.5 | 2183 | 7 | 19.0 |
TAA, Thoracic aortic aneurysm.
Patient demographics and in-hospital complications that occurred for both TEVAR and open TAA repair cohorts are shown in Table II, stratified into cohorts based on median LOS, defined as “early,” “usual,” and “late” discharge for each procedure. Patients discharged early following both procedures were less likely to be male, have undergone emergent procedures, have experienced a longer intensive care unit stay, and/or experienced a major postoperative complication. This included a lower rate of renal failure, pulmonary failure, spinal cord ischemia, stroke, or gastrointestinal complications (Table II).
Table II.
Characteristics of patients discharged home following thoracic endovascular aneurysm repair (TEVAR) and open thoracic aortic aneurysm (TAA) repair, stratified into early, usual, and late discharge cohorts based on median length of stay (LOS)
| Characteristic | TEVAR
|
Open TAA repair
|
||||
|---|---|---|---|---|---|---|
| Early discharge | Usual discharge | Late discharge | Early discharge | Usual discharge | Late discharge | |
| Number of patients | 538 | 988 | 388 | 2698 | 3218 | 1934 |
| Age (mean years) | 75.3 | 75.8 | 75.4 | 72.2 | 73.0 | 73.6 |
| Male gender (%) | 28.4 | 41.2 | 42.3a | 35.5 | 39.6 | 44.2a |
| Black race (%) | 5.8 | 7.4 | 9.0 | 2.5 | 3.1 | 4.1a |
| Charlson comorbidity score (mean) | 1.32 | 1.37 | 1.32 | 0.54 | 0.72 | 0.91a |
| Emergent (%) | 7.3 | 16.9 | 42.3a | 11.7 | 20.9 | 38.8a |
| Intensive care unit LOS (mean days) | 0.6 | 1.8 | 4.5a | 2.2 | 3.6 | 7.1a |
| Renal complication (%) | 5.0 | 7.1 | 8.3 | 2.8 | 5.0 | 9.2a |
| Pulmonary complication (%) | 4.7 | 8.2 | 24.7a | 11.6 | 17.6 | 33.2a |
| Spinal cord complication (%) | 0.7 | 0.9 | 1.8 | 0.2 | 0.4 | 1.0a |
| Cerebrovascular complication (%) | 2.2 | 5.1 | 7.5a | 1.3 | 3.5 | 4.2a |
| Gastrointestinal complication (%) | 0.0 | 0.1 | 0.3 | 0.0 | 0.0 | 0.2 |
P < .05.
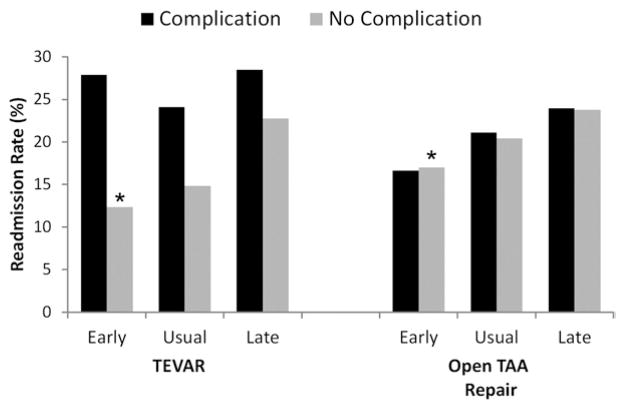
We next compared 30-day readmission rates after stratifying patients into cohorts based on median LOS, defined as “early,” “usual,” and “late” discharge, and whether they had experienced a major postoperative complication following TEVAR and open TAA repair. As shown in Fig 2, there was a significant decrease in readmission rates among patients discharged home early following uncomplicated TEVAR (12.4% readmission if discharged early vs 22.8% readmission if discharged late; P < .001) and open TAA repair (17.0% vs 23.8%; P < .001) as compared with patients discharged late after an uncomplicated postoperative course. The reduction in readmission risk associated with early discharge was also noted among patients who suffered a major postoperative complication following open TAA repair (Fig 2). However, among patients discharged early who suffered a major postoperative complication following TEVAR, there was no difference in readmission rates compared with patients discharged late following complications (27.8% vs 28.5%; P = .6). The association between early discharge and reduced readmission rates was confirmed in risk-adjusted logistic regression models that included in-hospital complications and patient comorbidities for both procedures (Table III). Other independent risk factors for readmission in this model included older age, a Charlson comorbidity index >2, and the presence of a major in-hospital complication. Finally, no significant interactions were found that confounded the association between early discharge and readmission, including year of procedure.
Fig 2.
Risk of 30-day readmission associated with different lengths of hospital stay (early, usual, and late discharge) among patients discharged home following TEVAR and open thoracic aortic aneurysm (TAA) repair. For thoracic endovascular aneurysm repair (TEVAR), early discharge length of stay (LOS) was defined as 1 to 2 days, normal discharge LOS was 3 to 7 days, and late discharge LOS was >8 days. For open TAA repair, early discharge LOS was defined as a 3 to 6 days, normal discharge LOS was 7 to 10 days, and late discharge was >11 days. * P < .05 for trend in readmission rate between patients discharged early, normal, and late for each procedure.
Table III.
Risk-adjusted odds of readmission among patients undergoing thoracic endovascular aneurysm repair (TEVAR) and open thoracic aortic aneurysm (TAA) repair
| Variable |
TEVAR
|
Open TAA repair
|
||
|---|---|---|---|---|
| Adjusted OR | 95% CI; P value | Adjusted OR | 95% CI; P value | |
| LOS | ||||
| Late discharge | Ref | Ref | Ref | Ref |
| Usual discharge | 0.66 | 0.48–0.92; P < .05 | 0.86 | 0.73–1.02; P = .08 |
| Early discharge | 0.57 | 0.38–0.85; P < .01 | 0.70 | 0.57–0.85; P < .01 |
| Age | ||||
| 65–70 years | Ref | Ref | Ref | Ref |
| 71–80 years | 0.96 | 0.70–1.31; P = .78 | 1.16 | 1.02–1.31; P < .05 |
| ≥81 years | 1.28 | 0.91–1.81; P = .16 | 1.24 | 1.01–1.51; P < .05 |
| Male gender | 0.91 | 0.71–1.15; P = .42 | 1.01 | 0.91–1.13; P = .77 |
| Black race | 0.98 | 0.57–1.68; P = .94 | 1.00 | 0.75–1.32; P = .97 |
| Emergent procedure | 0.95 | 0.72–1.27; P = .75 | 1.07 | 0.93–1.24; P = .34 |
| Charlson comorbidity score | ||||
| 0–1 | Ref | Ref | Ref | Ref |
| 1–2 | 1.10 | 0.90–1.36; P = .34 | 1.10 | 0.90–1.36; P = .36 |
| >2 | 1.38 | 1.08–1.75; P < .01 | 1.30 | 1.10–1.53; P < .01 |
| Complication | ||||
| Renal failure | 1.85 | 1.24–2.76; P < .01 | 1.11 | 0.88–1.42; P = .37 |
| Pulmonary failure | 1.76 | 1.18–2.63; P < .01 | 1.02 | 0.88–1.17; P = .80 |
| Spinal cord ischemia | 2.22 | 0.96–5.15; P = .06 | 1.02 | 0.47–2.21; P = .95 |
| Stroke | 0.90 | 0.52–1.57; P = .72 | 0.92 | 0.66–1.29; P = .63 |
CI, Confidence interval; LOS, length of stay; OR, odds ratio.
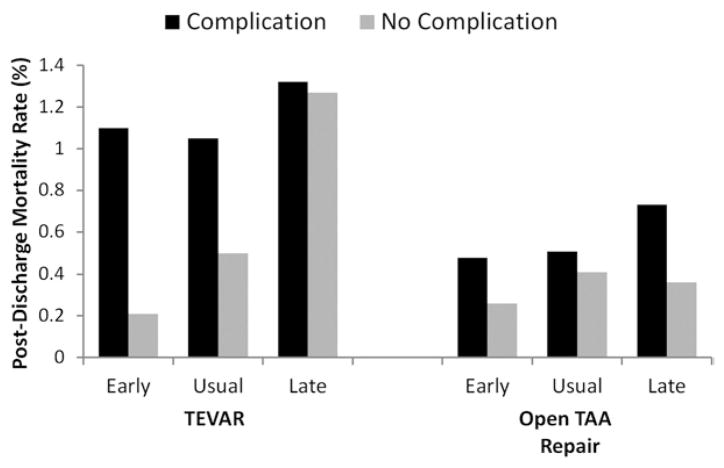
Postdischarge 30-day mortality rates were next compared among patients within the three LOS cohorts, stratified by whether they had experienced a major complication. As shown in Fig 3, there was no significant difference in postdischarge mortality among patients discharged early as compared with late following TEVAR either with complications (0.2% vs 1.2%; P = .2) or without complications (1.0% vs 1.3%; P = .7). This finding was also consistent for patients undergoing open TAA repair who were discharged home early versus late, regardless of whether they experienced any complication (0.5% vs 0.7%; P = .8) or no complication (0.3% vs 0.4%; P = .7). Further, there was no significant overall difference in 30-day postdischarge mortality (0.6% vs 0.4%; P = .2) among patients undergoing TEVAR compared with those undergoing open TAA repair.
Fig 3.
Risk of 30-day postdischarge mortality associated with different lengths of hospital stay (early, usual, and late discharge) among patients discharged home following thoracic endovascular aneurysm repair (TEVAR) and open thoracic aortic aneurysm (TAA) repair. For TEVAR, early discharge length of stay (LOS) was defined as 1 to 2 days, normal discharge LOS was 3 to 7 days, and late discharge LOS was >8 days. For open TAA repair, early discharge LOS was defined as a 3 to 6 days, normal discharge LOS was 7 to 10 days, and late discharge was >11 days.
Total hospital charges as well as Medicare adjusted payments are shown in Table IV for the three patient LOS cohorts that underwent TEVAR and open TAA repair. Overall, hospital costs ranged from between $13,429 and $285,269 for patients undergoing TEVAR, to between $26,325 and $333,274 for patients undergoing open TAA repair. For patients discharged home early, total hospital charges were found to be significantly lower when compared with patients discharged after normal or long LOS (P < .001). This finding held for the entire cohort, as well as in individual analyses for both TEVAR and open TAA repair (P < .001 for trend).
Table IV.
Average hospital charges and Medicare payments for beneficiaries who underwent thoracic endovascular aneurysm repair (TEVAR) and open thoracic aortic aneurysm (TAA) repair between 2000 through 2007
| LOS tertiles | TEVAR
|
Open TAA repair
|
||||
|---|---|---|---|---|---|---|
| Hospital charges (mean) | Medicare payments (mean) | Ratio (payment/charges) | Hospital charges (mean) | Medical payments (mean) | Ratio (payment/charges) | |
| Early discharge | $58,667 | $23,207 | 0.40 | $ 73,061 | $29,508 | 0.40 |
| Usual discharge | $80,851 | $26,395 | 0.33 | $ 92,699 | $32,520 | 0.35 |
| Late discharge | $128,478 | $33,225 | 0.26 | $136,480 | $38,960 | 0.29 |
LOS, Length of stay.
Adjusted Medicare payments were also lower for patients discharged home early following TEVAR and open TAA repair (P < .001 for trend). However, the proportion of payments received relative to hospital costs for both procedures was significantly increased for patients that were discharged home early compared with both the normal and late discharge groups (P < .001 for trend). Overall, the total charges for TEVAR ($84,270 vs $96,736; P < .001) and Medicare payments received for TEVAR ($26,883 vs $33,071; P < .001) were significantly lower compared with open TAA repair.
DISCUSSION
Many hospitals have adopted “fast-track” clinical pathways to reduce LOS. The intent of these pathways is to expedite hospital discharge following uncomplicated vascular surgery, as a means to contain hospital costs.1,2,8 The financial gains and clinical incentives associated with these pathways are negated, however, if a high percentage of patients discharged early require subsequent readmission to manage untreated complications. Our study demonstrates that Medicare beneficiaries discharged home early following open thoracic aneurysm repair or TEVAR were associated with the lowest 30-day readmission rates. Moreover, patients discharged early following these high-risk vascular procedures were not found to have a higher mortality rate once they left the hospital. These improvements in clinical outcomes associated with early discharge were achieved while hospital costs were significantly lowered. Together, these data suggest that early discharge is safe and cost-effective for patients with uncomplicated hospital courses following TEVAR and open TAA repair.
Improving the efficiency of in-hospital care for patients following routine and high-risk vascular surgery has been a target of both payers and purchasers as a means to contain healthcare costs.9,10 Medicare payments are directly impacted by the average or geometrical LOS for each surgical DRG and adjusted for case mix index.11 While early discharge to a skilled nursing facility is not rewarded within these payment systems, there is a financial incentive for patients to be discharged home before the average LOS for any given DRG. As such, early discharge clinical pathways following common surgical procedures have been promoted as a means for healthcare systems to save costs. These types of clinical pathways have already been found to be safe and cost-effective for a number of other large magnitude surgical procedures, including colon resections and coronary artery bypass surgery.12,13 Our study is the first to demonstrate similar benefits for patients undergoing endovascular and open TAA repair, two high-risk vascular surgery procedures where readmission commonly occurs. Early discharge following these high-risk vascular procedures not only contained hospital costs but was also associated with a significant higher proportion of reimbursed Medicare payments.
Critics of programs intended to create more cost-effective and efficient postoperative care argue that poorly designed or executed efforts at early discharge may ultimately be achieved at the expense of patient safety, quality of care, and/or adequacy of treatment. This is a dilemma that vascular surgeons and hospital administrators alike must confront in contemporary medicine, particularly as our health care system moves toward a capitated or bundled payment structure.14 One potential solution endorsed by the Institute of Medicine has been to standardize inhospital care pathways and enact process measures that reduce variability in clinical decision making.15 Over the past decade, CMS’s Surgical Care Improvement Project, the Leapfrog Group and the National Quality Forum have all promoted hospital compliance with evidence-based process measures designed for surgical patients as a means to achieve standardization of care.2,16,17 Prior research has shown that hospital compliance with evidence-based process measures significantly improves mortality rates among patients undergoing open abdominal aortic aneurysm repair.18,19 While the correlation between standardization of hospital care and patient outcomes such as LOS or readmission following vascular surgery have not been formally evaluated, our data nevertheless show that these outcomes have improved over time (Table I).5 In particular, endovascular procedures such as TEVAR have benefited from standardization of devices and implantation techniques, which have likely led to less device-related and access-related complications that would require longer LOS or increase the risk of subsequent readmission. Policymakers and clinician-scientists need to put the interactions between care pathways and readmission “under the microscope” to more fully understand the relationship of early discharge to readmission risk.
Future efforts directed at evaluating perioperative care for vascular surgery patients will need to be comprehensive and include traditional outcome measures such as mortality and complications, as well as focus more attention on quality measures such as LOS and readmission. Readmission following vascular surgery procedures will soon become a performance measure that hospital payers use to evaluate quality and determine reimbursement. The Patient Protection and Affordable Care Act has created a financial penalty for hospitals with excessive and preventable 30-day readmissions that is projected to include vascular surgery patients within several years.5,6 These changes to health care policy, however, carry a risk that providers will merely keep their patients in the hospital longer to reduce the risk of complications that may lead to readmissions. As such, it is important to evaluate the correlation between readmission risks and LOS and explore the tradeoff that these quality measures will engender. This includes a more thorough understanding of the factors that lead to excessive LOS for patients that otherwise have uncomplicated postoperative courses.
Using LOS as an outcome or performance measure following vascular surgical procedures, however, is potentially confounded by multiple factors. First, LOS can serve as a proxy for hospital resource utilization and reflect how efficiently a hospital allocates staff time, space, equipment, and additional considerations per patient. This may help explain why early discharge was highly correlated with hospital costs in our study (Table IV). Second, hospitals that regularly transfer patients to skilled nursing facilities will have reduced LOS. For this reason, we limited our analysis to patients discharged home following TAA repair. Third, it is impossible to know how closely the incidence of complications is related to the length of time spent recovering in the hospital following complex vascular surgery. For example, an extended LOS following surgery may be due to a complication, or a long LOS may have increased a patient’s exposure to various inpatient risks such as medication errors and hospital infections that allowed a complication to occur. Finally, the quality of postoperative care in hospitals that consistently keep patients longer may be ultimately compromised by hospital congestion and its effect on efficiency.
Our study has several other important limitations. First, it is a retrospective, observational study, which does not allow the temporal cause and effect between early discharge and readmission to be shown and that may be potentially confounded by unmeasured patient and hospital variables. While we controlled for major complications and discharge location, it is possible that other factors that influence LOS were not measured. Moreover, while readmissions and mortality are accurately captured by CMS Medicare Provider Analysis and Review, these types of administrative datasets are not as sensitive for identifying complications and cannot discriminate the clinical severity of each complication. Other common postoperative complications may have occurred, such as surgical site infections or thrombo-embolic events, which affected LOS but were not accurately captured. Hospitals that discharge patients home early after TAA repair may be systematically different than hospitals that allow patients a longer recovery time, including differences in resources for discharge planning, transition care, and availability of home care services. We do not know whether they had systems in place to encourage early discharge or whether there was variability in LOS that was random and not systematic. Finally, our cost analysis was limited to inpatient cost, but there may have been differences in outpatient-related expenses for patients discharged early that offset any aforementioned gains.
CONCLUSIONS
In conclusion, our study demonstrates that Medicare beneficiaries with an uncomplicated postoperative course could be discharged home early following open thoracic aneurysm repair or TEVAR with an acceptably low likelihood of being readmitted. These data support the safety of early-discharge programs in vascular surgery patients, even after high-risk procedures. As readmission following vascular surgery becomes more heavily scrutinized, it is clear that patient-level risk-adjustment models will be needed to more accurately assess this quality-outcome measure.
Footnotes
Author conflict of interest: none.
Presented at the Fortieth Annual Meeting of the Society for Clinical Vascular Surgery, Las Vegas, Nev, March 16, 2012.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
AUTHOR CONTRIBUTIONS
Conception and design: BB, PG, RP, DG, JC, DS
Analysis and interpretation: BB, PG, LT, DS
Data collection: BB, PG, LT, DS
Writing the article: BB, PG, RP, MF, DG, JL, DH
Critical revision of the article: BB, PG, DS
Final approval of the article: BB, PG, RP, LT, MF, DG, JL, DH
Statistical analysis: BB, PG, LT
Obtained funding: PG, DS
Overall responsibility: BB
References
- 1.Muehling B, Schelzig H, Steffen P, Meierhenrich R, Sunder-Plassmann L, Orend KH. A prospective randomized trial comparing traditional and fast-track patient care in elective open infrarenal aneurysm repair. World J Surg. 2009;33:577–85. doi: 10.1007/s00268-008-9892-2. [DOI] [PubMed] [Google Scholar]
- 2.Murphy MA, Richards T, Atkinson C, Perkins J, Hands LJ. Fast track open aortic surgery: reduced postoperative stay with a goal-directed pathway. Eur J Vasc Endovasc Surg. 2007;34:274–8. doi: 10.1016/j.ejvs.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 3.Collier PE. Do clinical pathways for major vascular surgery improve outcomes and reduce cost? J Vasc Surg. 1997;26:179–85. doi: 10.1016/s0741-5214(97)70177-0. [DOI] [PubMed] [Google Scholar]
- 4.MedPAC. [Accessed April 13, 2012];Hospital acute inpatient services payment system. Available at: http://www.medpac.gov/documents/MedPAC_Payment_Basics_11_hospital.pdf.
- 5.Brooke BS, De Martino RR, Girotti M, Dimick JB, Goodney PP. Developing strategies for predicting and preventing readmissions in vascular surgery. J Vasc Surg. 2012;56:556–62. doi: 10.1016/j.jvs.2012.03.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CRS. [Accessed April 13, 2012];Medicare provisions in the Patient Protection and Affordable Care Act. Available at: http://www.ncsl.org/documents/health/MCProv.pdf.
- 7.Berenson RA, Paulus RA, Kalman NS. Medicare’s readmissions-reduction program–a positive alternative. N Engl J Med. 2012;366:1364–6. doi: 10.1056/NEJMp1201268. [DOI] [PubMed] [Google Scholar]
- 8.Brustia P, Renghi A, Fassiola A, Gramaglia L, Della Corte F, Cassatella R, et al. Fast-track approach in abdominal aortic surgery: Left subcostal incision with blended anesthesia. Interact Cardiovasc Thorac Surg. 2007;6:60–4. doi: 10.1510/icvts.2006.137562. [DOI] [PubMed] [Google Scholar]
- 9.Fry DE, Pine M, Jones BL, Meimban RJ. Comparative effectiveness and efficiency in peripheral vascular surgery. Am J Surg. 2011;201:363–7. doi: 10.1016/j.amjsurg.2010.08.025. discussion: 367–8. [DOI] [PubMed] [Google Scholar]
- 10.Patterson RB, Whitley D, Porter K. Critical pathways and cost-effective practice. Semin Vasc Surg. 1997;10:113–8. [PubMed] [Google Scholar]
- 11.Centers for Medicare and Medicaid Services (CMS) HHS. Medicare program; Changes to the hospital inpatient prospective payment system for acute care hospitals and fiscal year 2010 rates; and changes to the long-term care hospital prospective payment system and rate years 2010 and 2009 rates. Final rules and interim final rule with comment period. Fed Regist. 2009;74:43753–4236. [PubMed] [Google Scholar]
- 12.Cowper PA, DeLong ER, Hannan EL, Muhlbaier LH, Lytle BL, Jones RH, et al. Is early too early? Effect of shorter stays after bypass surgery. Ann Thorac Surg. 2007;83:100–7. doi: 10.1016/j.athoracsur.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Hendren S, Morris AM, Zhang W, Dimick J. Early discharge and hospital readmission after colectomy for cancer. Dis Colon Rectum. 2011;54:1362–7. doi: 10.1097/DCR.0b013e31822b72d3. [DOI] [PubMed] [Google Scholar]
- 14.Mechanic RE. Opportunities and challenges for episode-based payment. N Engl J Med. 2011;365:777–9. doi: 10.1056/NEJMp1105963. [DOI] [PubMed] [Google Scholar]
- 15.Institute of Medicine. To err is human: Building a safer health system. Washington DC: National Academies Press; 2000. [PubMed] [Google Scholar]
- 16. [Accessed April 13, 2012];Surgical Care Improvement Project. Available at: http://www.jointcommission.org/surgical_care_improvement_project/
- 17.National Quality Forum. [Accessed April 12, 2012];NQF in the Quality Landscape. 2012 Available at: http://www.qualityforum.org. [Google Scholar]
- 18.Brooke BS, Dominici F, Pronovost PJ, Makary MA, Schneider E, Pawlik TM. Variations in surgical outcomes associated with hospital compliance with safety practices. Surgery. 2012;151:651–9. doi: 10.1016/j.surg.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brooke BS, Perler BA, Dominici F, Makary MA, Pronovost PJ. Reduction of in-hospital mortality among California hospitals meeting leapfrog evidence-based standards for abdominal aortic aneurysm repair. J Vasc Surg. 2008;47:1155–6. doi: 10.1016/j.jvs.2008.01.021. discussion: 1163–4. [DOI] [PubMed] [Google Scholar]