Abstract
The toxicodynamic relationship between the number and size of pulmonary microemboli resulting from uniformly sized, rigid polystyrene microparticles (MPs) administered intravenously and their potential effects on pulmonary gas exchange was investigated. CD-1 male mice (6–8 wk) were intravenously administered 10, 25 and 45 μm diameter MPs. Oxygen hemoglobin saturation in the blood (SpO2) was measured non-invasively using a pulse oximeter while varying inhaled oxygen concentration (FIO2). Resulting data were fit to a physiologically based non-linear mathematical model that estimates 2 parameters: ventilation-perfusion ratio (VA/Q) and shunt (percentage of deoxygenated blood returning to systemic circulation). The number of MPs administered prior to a statistically significant reduction in normalized VA/Q was dependent on particle size. MP doses that resulted in a significant reduction in normalized VA/Q one day post-treatment were 4,000, 40,000 and 550,000 MPs/g for 45, 25 and 10 μm MPs, respectively. The model estimated VA/Q and shunt returned to baseline levels 7 days post-treatment. Measuring SpO2 alone was not sufficient to observe changes in gas exchange; however, when combined with model-derived VA/Q and shunt early reversible toxicity from pulmonary microemboli was detected suggesting that the model and physical measurements are both required for assessing toxicity. Moreover, it appears that the MP load required to alter gas exchange in a mouse prior to lethality is significantly higher than the anticipated required MP dose for effective drug delivery. Overall, the current results indicate that the microemboli-based approach for targeted pulmonary drug delivery is potentially safe and should be further explored.
Keywords: Pulmonary gas exchange, ventilation-perfusion ratio, shunt, passive pulmonary targeting, rigid microparticle
Introduction
The current study focuses on assessing the feasibility of a microemboli-based pulmonary drug delivery approach by determining the maximum tolerated MP dose that could be intravenously administered before a significant physiologic change in pulmonary gas exchange would occur. Changes in the estimated ventilation-perfusion ratio (VA/Q) and shunt (i.e., pulmonary gas exchange) were investigated following intravenous administration of rigid MPs in order to assess the toxicodynamics of a microemboli-based drug delivery system. Currently there are a variety of commonly used techniques or indices used by intensive care clinicians to assess pulmonary gas exchange including: PaO2/FIO2, arterial-venous difference, nitrogen washout, multiple inert gas elimination (MIGET) and ventilation-perfusion scans (Rowe et al., 2010). Each method has its limitations since they cannot be performed in mice or they require complex techniques and/or costly equipment. Moreover, while measurements are possible of the individual components of VA/Q, namely alveolar ventilation and cardiac output, they may be influenced by physiologic conditions and experimental protocols. In addition, interpretation of the ratio of VA/Q may be complicated by disease status. However, the physiologic importance of a VA/Q shift in both magnitude and direction cannot be understated.
The current analysis was performed using a relatively new technique, originally developed and validated for use in humans, that estimates 2 parameters (VA/Q and shunt) through a non-linear physiologically based mathematical modeling algorithm. This technique is based on the use of a pulse oximeter to measure SpO2 while varying the percentage of oxygen inhaled (FIO2) (Sapsford and Jones, 1995; Kjaergaard et al., 2001; Karbing et al., 2007). The technique was specifically established to demonstrate that alterations consistent with clinical pathophysiologic changes could be determined by mathematical modeling and that those alterations could be determined by estimating PaO2 from the non-invasive measurement of SpO2 rather than from direct invasive measurement of PaO2 by arterial sampling.
We previously developed and reported on a size targeted intravenously injected rigid polystyrene MP based drug delivery system that was effective in treating primary lung cancer in an orthotopic rat model by providing sustained therapeutic concentrations of camptothecin locally in the lung (Chao et al., 2010; Kutscher et al., 2010). However, one concern with using an injectable MP approach for lung targeting is the potential for microemboli to adversely affect pulmonary gas exchange. Therefore, we sought to determine the maximum MP dose that could be safely administered without causing a significant change in pulmonary gas exchange.
Given the wide number of mouse models of disease, the common usage of mice in toxicity studies, and the development of a pulse oximeter designed for mice, the application of the modeling technique to mice is timely and important. In the current report, the usefulness of this technique for monitoring the effects of pulmonary capillary microembolism and its applicability to estimating VA/Q and shunt in mice is presented for the first time. The technique provides a method that is more sensitive to physiologic changes than simply measuring the SpO2 while breathing room air and, because it is non-invasive, each animal is able to serve as its own control providing a method for studying disease progression, disease model development, and/or therapeutic intervention in the future. Finally, the application of the model to assess the feasibility of the microembolic drug delivery approach is discussed.
Materials and Methods
Animals
Male CD-1 mice (6–8 wks, ~30g) were purchased from Charles River Laboratories (Wilmington, MA). Mice were fed a standard mouse diet, had free access to water and were housed in a room with a 12-hour light–dark cycle for at least 1 week before the study. All animal studies were performed in AAALAC accredited animal facilities under approved protocols from the Rutgers University Animal Use and Care Committee.
Methods
Preparation of MPs for IV injection
Polystyrene MPs of various sizes (diameters equal to 10, 25 and 45 μm) were purchased from Polysciences Inc. (Warrington, PA). MPs were washed 5 times using 0.1% Tween™ 80 in PBS followed by centrifugation for 5 min at 600g. MPs were re-suspended in a final volume (5 mL/kg) of 0.1% Tween™ 80 in PBS. MPs were fully suspended in solution by vortexing immediately prior to tail vein injection to conscious mice. MP suspensions were counted on a hemocytometer to confirm MP dose.
The 10 μm MP doses were 150,000, 200,000, 250,000, 300,000, 350,000, 400,000, 450,000, 500,000, 550,000, 600,000, 650,000, 700,000, 750,000 and 800,000 MPs/g. The 25 μm MP doses were 12,000, 15,000, 18,000, 21,000, 30,000, 40,000 and 50,000 MPs/g. The 45 μm MP doses were 2,000, 4,000, 6,000 and 8,000 MPs/g. The vehicle control animals were administered 0.1% Tween™ 80 in PBS at 5 mL/kg.
Determination of pulmonary gas exchange (VA/Q and shunt)
Free breathing mice were anesthetized by isoflurane (1.5%) in air using an EZ-3500 Multi-Animal Anesthesia System (Euthanex Corp., Palmer, PA). Upon anesthesia induction, animals were transferred to a single nose cone breather unit and placed in a supine position on a water heated surgical bed to maintain body temperature under anesthesia. Mixtures of gas were delivered by blending nitrogen and oxygen and the oxygen concentration was measured using a MaxO2 Oxygen Analyzer (Maxtec Inc., Salt Lake City, UT). The oxygen analyzer was calibrated using compressed breathing air at the beginning of each day. Arterial hemoglobin oxygen saturation (SpO2) in the blood was monitored on the mouse’s thigh using a MouseOx® pulse oximeter (STARR Life Sciences, Oakmont, PA) at 1 and 3 day pre-MP exposure and 1, 3, 5 and 7 day post-MP exposure. Initially the lower portion of the SpO2 vs. FIO2 curve was defined by decreasing FIO2 sequentially from 21 to 18, 16, 14, 12 and 10% O2. After a rest period of 3 minutes, breathing 21% O2, we then increased FIO2 to 24, 28, and 32% O2. Animals were maintained at each FIO2 for 2 min or until the SpO2 readings stabilized. The SpO2 vs. FIO2 data points were analyzed with a computer algorithm lung model previously defined in humans (Sapsford and Jones, 1995) as described below.
To determine the pulmonary gas exchange function of the lung, several parameters were measured or derived. Measurement of the PaO2 can take place through arterial blood gas sampling, or can be related by the oxygen-hemoglobin dissociation curve, which in humans can be approximated by (Severinghaus, 1979):
| (1) |
where SxO2 is the saturated oxygen hemoglobin value (%), PxO2 is the partial pressure of O2 in the blood in a particular compartment and where x is the arterial (a), venous (v) or mixed pulmonary capillary (c) compartment.
Equation 1 incorporates a simplistic model of the oxygen dissociation curve and does not take into account the effects of base excess, concentrations of methemoglobin (MetHb), carboxyhemoglobin (COHb), 2,3-DPG, or CO2 (i.e., the Haldane Effect), pH (i.e., the Bohr Effect), and temperature. Therefore PaO2 may be inaccurately calculated from the measured SpO2. However, in mice, the measurement of multiple arterial blood gasses is significantly more difficult than in larger species because the volumes of blood necessary are large relative to a mouse and locations of accessible arteries are difficult to reach (Sahbaie et al., 2006).
The standard shunt equation is as follows (Sapsford and Jones, 1995; Aboab et al., 2006):
| (2) |
where S is shunt; Qs is the amount of blood which is not oxygenated after passing through the pulmonary circulation; Qt is the total amount of blood flowing through the lungs; CxO2 is the concentration of O2 in the blood in a particular compartment. Concentration values used are per 100 mL.
The concentration of O2 in the blood stream is dependent upon the amount of O2 carrier present (hemoglobin) and the solubility of O2 in blood, which is based on the partial pressure of O2 present. The standard equation for determining the concentration of O2 in a particular blood compartment is (Aboab et al., 2006):
| (3) |
where Hb is hemoglobin concentration, which we did not measure and assumed to be 15 g/dL.
In addition, the amount of change between the CaO2 and CvO2 is referred to as the arterial-venous difference (avDO2) and is generally accepted to be 5 (Chiang, 1968; Sapsford and Jones, 1995) for healthy resting humans:
| (4) |
Assuming a constant avDO2 is a potential limitation since avDO2 can vary substantially and is dependent upon cardiac output (Q) and oxygen consumption (VO2) (Aboab et al., 2006). A near doubling of avDO2 was calculated and a decrease in cardiac output was measured in a small sample of humans (n=7) following massive pulmonary embolism with resulting arterial hypoxemia, (Jardin et al., 1979). However, a change to cardiac output would have a similar effect on three terms, avDO2, VA/Q and shunt because Q is found in the denominator of each term. If VA/Q and shunt are held constant, doubling avDO2 causes a noticeable rightward and slight downward shift of the SpO2 vs. FIO2 curve. Without measuring either oxygen consumption (VO2) or alveolar ventilation (VA) it is impossible to determine which term (avDO2 or VA/Q) is actually changing. Nevertheless multiple investigators have developed models incorporating a fixed value of 5 for avDO2 to estimate changes to VA/Q and shunt and they found that these changes are consistent with clinically evident pulmonary diseases.
Substituting equation 4 into 1 and upon rearrangement:
| (5) |
and substituting equation 3 into 5 results in:
| (6) |
PcO2 can be approximated from the amount of O2 inspired and corrected for water vapor and carbon dioxide:
| (7) |
where FIO2 is the fraction of inspired O2 (20.9% in air); Pb is barometric pressure (assumed to be 760 mmHg); PH2O is partial pressure of water vapor at 37°C (47 mmHg); PaCO2 is the partial pressure of carbon dioxide that is remaining in the respiratory dead volume; and R is the respiratory quotient which is dependent upon metabolism in the body and diet (and we assumed to be 0.9 (Zwemer et al., 2007)).
Finally, the alveolar ventilation-perfusion equation (Rahn and Fenn, 1955) is:
| (8) |
which upon rearrangement is:
| (9) |
Substitution of equations 1, 7 and 9 into 6, solving for PaO2 and back-substituting into equation 1 results in a model similar to the first one proposed by Sapsford and Jones (Sapsford and Jones, 1995), which can be viewed schematically in Figure 1.
FIGURE 1.
Model of a 1 compartment lung. PxO2 is partial pressure of O2 and CxO2 is the concentration of O2 in compartment x, where x is the arterial (a), venous (v) or mixed pulmonary capillary (c) compartment; Q[dot] is blood flow; FIO2 is the fraction of inspired O2; Shunt is the percentage of deoxygenated blood passing through the lung. Blue indicates deoxygenated blood, red indicates oxygenated blood.
Histology and Immunohistochemistry
The lung was inflated via the trachea with 3% paraformaldehyde containing 2% sucrose and immersed in fixative. After 24 h at 4 °C, the lung was transferred to 2% sucrose for an additional 24 h at 4 °C. The left lung was then embedded in OCT media and frozen until sectioning. Tissue sections (10 μm) were rehydrated in PBS, and following a citrate antigen retrieval step were blocked with 100% goat serum at room temperature for 2 h. Tissue sections were then incubated overnight at 4°C with primary rabbit affinity purified polyclonal antibodies against proliferating cell nuclear antigen (PCNA) (1:500, Abcam Cambridge, MA). Slides were then incubated for 30 min with biotinylated goat anti-rabbit secondary antibody (Vector Labs, Burlingame, CA). Antibody binding was visualized using a DAB Peroxidase Substrate Kit (Vector Labs).
Statistical analysis
The logit weighted [log(Y/(1−Y))] best fit curve parameter estimates of VA/Q and shunt and were calculated using Maple v.15 (Waterloo Maple Inc., Waterloo, ON) with the DirectSearch optimization package v.2 (Moiseev, 2011). Statistical analyses were performed using GraphPad Prism v.4.0c or GraphPad Instat v.3.1a (GraphPad Software Inc., La Jolla, CA). Experimental values are expressed as mean ± SD. The significance of a single factor in groups was tested by analysis of variance (ANOVA) at α=0.05 with a Dunnett’s post-hoc test. All the graphics in this article are generated by GraphPad Prism v.4.0c.
Results
Initially mice were analyzed for signs of distress following tail vein injection of MPs. Higher 10 μm MP loads were found to be lethal; thus after 750,000 and 800,000 MPs/g were administered 2 of 4 mice expired, while 1 of 4 expired after injection of 650,000 and 700,000 MPs/g. In some animals lethal effects of the MP were evident immediately post administration while others expired overnight.
To understand the theoretical changes in the SpO2 vs. FIO2 curve, values for shunt or VA/Q were substituted in the model; the relevant shift of the SpO2 vs. FIO2 curve is shown in Figure 2. An increase in shunt causes a downward shift of the plateau while a decrease in VA/Q causes a rightward shift of the vertical portion of the curve. It is important to note that these two parameters are independent of one another and therefore measuring SpO2 at a single fixed FIO2 is inadequate to characterize pulmonary gas exchange (Sapsford and Jones, 1995). Moreover, simply measuring the change in SpO2 of animals breathing 21% O2 between pre- and post-treatment groups did not show a statistical difference between treatment and control groups using Dunnett’s post-hoc analysis (Figure 3).
FIGURE 2.
Theoretical SpO2 vs. FIO2 curves. Values of shunt increase (0, 5, 10, 20, 30%) resulting in a downward shift. Decreased VA/Q (1.1, 1.0, 0.9, 0.8, 0.7, 0.6) resulting in a rightward shift. There is little apparent change to the shape of the curves. Dotted line indicates FIO2 of room air at sea level.
FIGURE 3.
Change of SpO2 breathing room air (FIO2 =21%) between Day −3 (pretreatment) (A) and Day 1 (post-treatment) (B). ANOVA analysis indicates P<0.05 however Dunnett’s post-hoc analysis only indicates the 8000 45 μm group as statistically different.
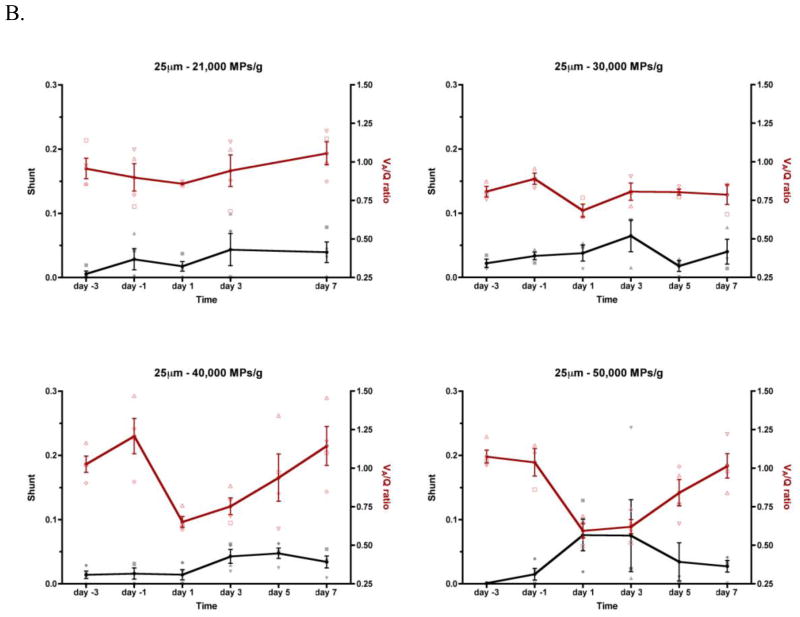
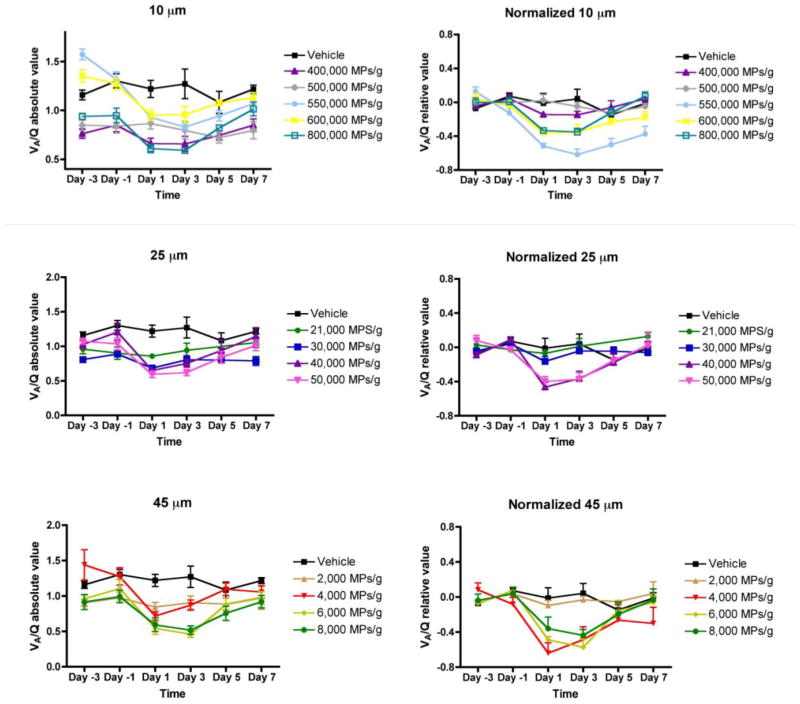
Representative SpO2 vs. FIO2 plots for animals treated with either a low or high dose of 10 μm MPs on Day −3 (i.e., pretreatment) and Day 1 are shown in Figure 4. For animals receiving low doses (e.g., 150,000 MPs/g) of MPs (Figure 4A and 4C), the raw data and best-fit curves overlap, indicating that there is little or no shift in VA/Q or shunt. In contrast, for animals receiving high doses (e.g., 550,000 MPs/g) of 10 μm MPs (Figure 4B and D), there is a statistically significant rightward shift (i.e., reduced VA/Q) but relatively little downward shift (i.e., increased shunt). All SpO2 vs. FIO2 data were fit individually using the algorithm described above. The parameters (VA/Q and shunt) were plotted vs. time as individual points for each animal with the means connected by a line (Figure 5). The temporal dependence of VA/Q and shunt is apparent. Due to inherent animal variability, animals were normalized to the average of their pretreatment measurements (Day −3 and Day −1) for both VA/Q and shunt (Figure 6). A statistically significant normalized reduction of VA/Q was observed on Days 1 and 3 compared to pretreatment (Days −3 and −1) and the magnitude of the VA/Q drop is relatively consistent across the three particle sizes (Figure 7). Interestingly, by Day 5, the animals had recovered pulmonary gas exchange relative to their pretreatment VA/Q values and by Day 7 were fully recovered. The change in shunt between Day −3 and Day 1 is only statistically significant at 40,000 and 50,000 25 μm MPs/g (45.4 and 38.9%, respectively), and most likely due to recruitment and distention of the capillary bed. This lack of visible shunt may be a result of the size of MPs administered (i.e., blockade of capillaries and pre-capillary arterioles vs. arteries), method of administration (bolus vs. infusion) or age of mice (i.e., developing vs. adult) and never reached the higher clinical values reported by MIGET or VA/Q scans observed in humans with pulmonary embolism.
FIGURE 4.
Two typical SpO2 vs. FIO2 curves of animals receiving low (150,000 10 μm MPs/g) or high (550,000 10 μm MPs/g) shown on Day −3 (pretreatment, black) and Day 1 (red). Each symbol represents the same animal on different days. The connecting lines are for clarity in the top (A, B). The best-fit lines are shown in (C, D). There is a rightward shift for the high dose treatment group, indicating a statistically significant decrease in VA/Q.
FIGURE 5.
Time course of VA/Q and Shunt for MPs of 10, 25 and 45 μm MPs from pre-treatment (Day −3 and Day −1) to post-treatment (Day 1 through Day 7). The black line represents shunt and the red VA/Q. The connected line is through the mean of the value. Error bars indicate SE. Individual VA/Q is plotted as open symbols and shunt is closed symbols. Similar shapes indicate the same animal (i.e., a red open box is animal 1’s VA/Q and a closed black box is animal 1’s shunt).
FIGURE 6.
Data grouped by MP size to look at changes in VA/Q over time. Left, raw data; Right, normalized data to the individual animal using their average VA/Q value on Day −3 and Day −1 (pretreatment).
FIGURE 7.
Changes in VA/Q (A, B) and Shunt (C, D) compared to total cross-sectional area/volume defined as Number of MPs times area/volume of an individual MP.
In humans, the physiologic shunt is <7% (Chiang, 1968) and VA/Q approximately 1 (Wagner, 2005) with a normal range of 0.8 – 1.2 (Itti et al., 2002). The average VA/Q during pretreatment (Days −3 and −1) for all animals had a mean and standard deviation of 1.07 ± 0.22 and 1.04 ± 0.28, and a range of 0.69–1.58 and 0.63–1.82, respectively. Although some of these mice had low or high VA/Q values, the mean and standard deviation are similar to those reported in healthy humans, pigs and sheep (Harris et al., 2002; Itti et al., 2002; Rizi et al., 2004).
The model did not detect shunt in any animals because there was little change in SpO2 when breathing room air. While the standard method of detecting a shunt is to administer 100% oxygen and measure the difference between SpO2, the current SpO2 values were >96% in all healthy animals and on average were >98%. After administration of MPs, and not including the noticeable outliers the average was >97%, indicating that there was only a slight decrease in SpO2 that could be attributable to either shunt or VA/Q change, but is less than the normal physiological shunt of 7% in humans. Moreover, the advantage of using the current mathematical model is that as FIO2 increases SpO2 will asymptotically approach 100% and therefore generating several data points past the point of maximum curvature will allow for the model to solve for shunt.
To check for signs of remodeling in the lung, actively dividing cells were stained for PCNA. On Day 7, animals receiving MPs, independent of size or number, exhibited markedly higher expression of PCNA throughout the lung when compared to control animals (Figure 8). Significantly greater staining was noted in cells surrounding or adjacent to MPs. Increased PCNA expression in the septal walls was also detected in animals that received high numbers of MPs. This may be a result of a nearby MP that was not visible due to the thickness of the section.
FIGURE 8.
Immunohistochemistry staining for PCNA on Day 7. Control animals have little to no staining in the septal walls of the lung (A). Injection of different sized MPs does not change PCNA localization (B, D). Injection of increased number of MPs does not change PCNA relative expression (C, D). Original magnification, 4x.
Discussion
The technique and model to assess changes in ventilation-perfusion matching and shunt were first developed in humans and require the non-invasive measurement of SpO2 using a finger pulse oximeter and the ability to change FIO2 (Sapsford and Jones, 1995). To date, this technique has not been performed in rodents due to the lack of availability of a rodent specific pulse oximeter. Sapsford and Jones’ original model was developed and used on 9 healthy, free breathing volunteers and 35 patients undergoing major surgery that were mechanically ventilated (Sapsford and Jones, 1995). Subsequently, it has been used to study pre-term infants and conscious adults with different underlying pulmonary diseases (Jones et al., 2008; Rowe et al., 2010). Although there have been improvements in the original modeling technique including incorporating PaCO2 or using multiple compartments, the original assumptions have not changed (Jones et al., 2008; Karbing et al., 2011). In the original model, the authors assumed a constant arterial-venous oxygen difference (avDO2); did not adjust for changes to the oxygen dissociation curve caused by changes to base excess, pH of the blood, which would affect SpO2 readings; and did not require patients to be on a mechanical ventilator which, by changing ventilation rate, would have made control of end tidal CO2 (PetCO2) possible (Sapsford and Jones, 1995).
The pulse oximeter used in the current studies was developed for mice and rats and validated in rats (Strohl et al., 2007). Consistent with the original Sapsford and Jones model, we assumed that a change to the calculated PaO2 from the measured SpO2 values would not have a marked effect on our estimates of VA/Q and shunt. Indeed, in order to account for changes to the oxygen dissociation curve, an arterial blood gas measurement would be required, which would be an invasive and potentially lethal procedure in a mouse and may interfere with the subsequent measurements until the animal’s blood volume returned to normal (Sahbaie et al., 2006). The animals in the current study were not placed on a mechanical ventilator nor was PetCO2 measured. This was not a requirement of the original model, and by allowing the animals to be free-breathing, they were able to adjust their PetCO2 accordingly. An advancement of the original Sapsford and Jones model put forth by Karbing et al. makes use of blood and airway concentrations of CO2 to improve the fit of the model (Karbing et al., 2011). Recent advances in rodent capable equipment now make this possible. These newer models account for PaCO2 or include a second airway compartment to describe normal vs. abnormal lung, which improves the fit of the model through the point of maximum curvature. However the newer models significantly increase the computational time while not significantly change the overall left-right placement of the curve representing VA/Q nor the position of the plateau of the curve representing shunt.
In addition, improvement in controlling the O2 concentration used would allow for faster methods of computational modeling or additional statistical analysis. By simply administering O2 at a single, fixed percentage (e.g., 15%) any detectable change to VA/Q could be quickly inferred. In fact, Jones has recommended using a number of pre-drawn curves for common VA/Q and shunts to quickly and easily determine VA/Q and shunt rather than the need for running the complete mathematical model (Rowe et al., 2010), thereby showing that an even further simplification of this model may have clinical importance. Regardless of future improvements, the current model is more than adequate for clearly demarcating the significant degree of pulmonary toxicity or loss of function.
In the current study, the model was used to assess the toxicity of microembolic events related to the use of intravenously administered lung targeted MP drug delivery system. In the case of minor microembolic events the average of the localized VA/Q ratios is reduced even though global VA and Q have not dramatically changed. This results from capillary occlusion causing a redistribution of blood flow and subsequent capillary recruitment in the apex. This difference is physiologically important since areas with increased VA/Q where maximum oxygenation has already occurred cannot overcome the decreased oxygenation of blood in areas of decreased VA/Q. Therefore the reduction in VA/Q will result in hypoxemia.
The lack of shunt detected throughout these experiments is consistent with the small size and relatively low numbers of MPs used. To date, the number of pulmonary capillary segments in a mouse is undetermined. However, using allometric scaling, a gross estimate of the number of capillary segments 8–16 μm in diameter in a 30 g mouse is ~1.34 × 108 or ~4.47 × 106 capillaries/g. This estimate is based on the assumption that the lung weight of a 70 kg human is ~650 g, a 30 g mouse has a lung weight of ~0.311 g (Lindstedt and Schaeffer, 2002), and in humans the number of capillary segments 8–16 μm in diameter is 2.8 × 1011 (Davis, 1975). Therefore, injection of 500,000 10 μm MPs/g results in only an ~11% occlusion without any statistical shift in VA/Q or shunt. The model did not detect shunt in any animals because there was little change in SpO2 when breathing room air. Although there was only a slight decrease (~1%) in SpO2 that could be attributable to either shunt or VA/Q change after administration of MPs, this is significantly less than the normal physiological shunt (~7%) in humans.
At rest, a healthy human lung uses only 30% of its capacity (Ganong, 2005), resulting in a large reserve capacity to filter out debris from the blood. Redistribution of blood flow through the underutilized capillary bed or distension of well-used capillary segments should occur well before blood is redirected through non-ventilated vessels. We believe that the redirection of blood results from active neurohumoral control or possibly passive vessel pressures. However, this remains to be investigated.
Drug delivery via inhalation predominantly targets the large airways and bronchioles due to aerodynamic limitations. As a consequence downstream vessels such as pre-capillary arterioles and capillaries are largely out of reach. Another notable disadvantage of inhalation is the high inter-patient variability due to inhaler technique and breathing capacity. An injectable lung targeting approach has the ability to bypass the large airways and target the alveolar sacs and pulmonary capillaries. However, the main concern with using an injectable particle approach is the potential for the microemboli to cause symptoms similar to pulmonary thromboembolism such as dyspnea, increased pulmonary arterial pressure and possibly even death. Using the estimated parameters (VA/Q and shunt) to detect loss of function prior to lethality has immediate applicability in terms of the determining the MP dose-safety profile for an injectable MP pulmonary delivery system. In the current studies a non-biodegradable material, PS, was used to investigate changes in pulmonary gas exchange. The advantage of PS MPs include: no degradation of the embolic material over time; tight control of embolus size; consistent delivery of MPs resulting in reproducible fixed pulmonary circulation occlusion; and the absence of erythrocyte hemolysis (Watts et al., 2012). It should be noted, however, that any injectable lung targeted delivery system for human use would need to be fabricated from biodegradable materials that are readily cleared from the body.
The results reported herein clearly demonstrate that there is a balance between the potential for pulmonary toxicity and MP dose/number administered and size. High doses of 10 μm (500,000 MPs/g) and 25 μm (30,000 MPs/g) rigid non-degradable MPs do not cause significant changes to pulmonary gas exchange. These MP doses are larger than what is needed for effective drug delivery. Larger 45 μm MPs can still be administered safely, but at a much lower dose (2,000 MPs/g). A potentially significant factor that will influence the MP dose required to treat a disease is the potency of the drug and whether it will be surface bound to the MP or entrapped inside the MP. Comparing the total surface area of 10, 25 and 45 μm MPs (i.e., number of MPs times MP surface area) prior to a significant change in VA/Q or shunt, the 10 μm MPs have ~2.7-fold and ~12.4-fold greater surface area than the 25 and 45 μm MPs, respectively. Therefore, there is a significant advantage to using smaller MPs when drugs are surface bound. If the drugs are trapped within the MP a higher drug loading capacity is possible as compared to surface attachment. The total volume of MPs administered (i.e., number of MPs times MP volume) is a good indicator of total drug loading. The total volume for the 10 μm MPs (500,000 MPs/g = ~275 mg/kg) is ~1.07 and ~2.74 fold higher than the volume for the 25 and 45 μm MPs, respectively. This suggests that 10 μm or 25 μm MPs can be used for drugs that must be trapped within the MP for reasons of a high dose requirement or stability considerations.
The current studies present a simple, clinically relevant method and mathematical model to detect changes of impaired pulmonary gas exchange that previously were unable to be determined non-invasively in mice. This method has the potential to detect toxicity of drug therapies, drug delivery systems or disease states that are ordinarily not detectable in a standard room air environment in species ranging from mice to humans. This technique also has the potential to allow for the titration of a drug with known pulmonary toxicity by measuring the subtle ventilation-perfusion changes that may occur prior to a clinically significant reduction in SpO2.
Supplementary Material
Highlights.
Murine pulmonary gas exchange after microembolization was non-invasively studied.
A physiologically based model quantified impairment of pulmonary gas exchange.
Number and size of microemboli determine severity of impaired gas exchange.
Pulmonary gas exchange returns to baseline within 7 days.
Acknowledgments
Financial support from the Parke-Davis Chair in Pharmaceutics and Controlled Drug Delivery, the National Institutes of Health through the National Cancer Institute (R01CA155061; R01CA132624), the National Institute of Environmental Health Sciences (R01ES004738; P30ES005022), and the National Institutes of Health CounterACT Program through the National Institute of Arthritis and Musculoskeletal and Skin Diseases (U54AR055073) is gratefully acknowledged. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the federal government. The National Science Foundation Integrative Graduate Education and Research Traineeship (IGERT) #0504497 and American Foundation for Pharmaceutical Education (AFPE) are acknowledged for providing graduate fellowships to Hilliard Kutscher. The National Institute of Health National Institute of Environment Health Sciences training grant “5T32ES007148 - Training in Environmental Toxicology” is acknowledged for providing a graduate fellowship to Christopher Massa. We would like to thank Drs. Howard Stone, Robert Prud’homme, Harlan Kutscher and Andrew Gow for their assistance and fruitful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aboab J, Louis B, Jonson B, Brochard L. Relation between PaO2/FIO2 ratio and FIO2: a mathematical description. Intensive Care Med. 2006;32:1494–1497. doi: 10.1007/s00134-006-0337-9. [DOI] [PubMed] [Google Scholar]
- Chao P, Deshmukh M, Kutscher HL, Gao D, Rajan SS, Hu P, Laskin DL, Stein S, Sinko PJ. Pulmonary targeting microparticulate camptothecin delivery system: anticancer evaluation in a rat orthotopic lung cancer model. Anticancer Drugs. 2010;21:65–76. doi: 10.1097/CAD.0b013e328332a322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang ST. A nomogram for venous shunt (Qs-Qt) calculation. Thorax. 1968;23:563–565. doi: 10.1136/thx.23.5.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA. Particulate Radiopharmaceuticals for Pulmonary Studies. In: Subramanian G, Rhodes BA, Cooper JF, Sodd VJ, editors. Radiopharmaceuticals. Society of Nuclear Medicine; New York: 1975. pp. 267–281. [Google Scholar]
- Ganong WF. Review of medical physiology. McGraw-Hill Medical; New York; London: 2005. [Google Scholar]
- Harris RS, Willey-Courand DB, Head CA, Galletti GG, Call DM, Venegas JG. Regional VA, Q, and VA/Q during PLV: effects of nitroprusside and inhaled nitric oxide. J Appl Physiol. 2002;92:297–312. doi: 10.1152/jappl.2002.92.1.297. [DOI] [PubMed] [Google Scholar]
- Itti E, Nguyen S, Robin F, Desarnaud S, Rosso J, Harf A, Meignan M. Distribution of ventilation/perfusion ratios in pulmonary embolism: an adjunct to the interpretation of ventilation/perfusion lung scans. J Nucl Med. 2002;43:1596–1602. [PubMed] [Google Scholar]
- Jardin F, Gurdjian F, Desfonds P, Fouilladieu JL, Margairaz A. Hemodynamic factors influencing arterial hypoxemia in massive pulmonary embolism with circulatory failure. Circulation. 1979;59:909–912. doi: 10.1161/01.cir.59.5.909. [DOI] [PubMed] [Google Scholar]
- Jones JG, Bakewell SE, Heneghan CP, Jones SE, Snape SL. Profound hypoxemia in pulmonary patients in airline-equivalent hypoxia: roles of VA/Q and shunt. Aviat Space Environ Med. 2008;79:81–86. doi: 10.3357/asem.2179.2008. [DOI] [PubMed] [Google Scholar]
- Karbing DS, Kjaergaard S, Andreassen S, Espersen K, Rees SE. Minimal model quantification of pulmonary gas exchange in intensive care patients. Med Eng Phys. 2011;33:240–248. doi: 10.1016/j.medengphy.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Karbing DS, Kjaergaard S, Smith BW, Espersen K, Allerod C, Andreassen S, Rees SE. Variation in the PaO2/FiO2 ratio with FiO2: mathematical and experimental description, and clinical relevance. Crit Care. 2007;11:R118. doi: 10.1186/cc6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaergaard S, Rees SE, Nielsen JA, Freundlich M, Thorgaard P, Andreassen S. Modelling of hypoxaemia after gynaecological laparotomy. Acta anaesthesiologica Scandinavica. 2001;45:349–356. doi: 10.1034/j.1399-6576.2001.045003349.x. [DOI] [PubMed] [Google Scholar]
- Kutscher HL, Chao P, Deshmukh M, Singh Y, Hu P, Joseph LB, Reimer DC, Stein S, Laskin DL, Sinko PJ. Threshold size for optimal passive pulmonary targeting and retention of rigid microparticles in rats. J Control Release. 2010;143:31–37. doi: 10.1016/j.jconrel.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstedt SL, Schaeffer PJ. Use of allometry in predicting anatomical and physiological parameters of mammals. Laboratory animals. 2002;36:1–19. doi: 10.1258/0023677021911731. [DOI] [PubMed] [Google Scholar]
- Moiseev SN. DirectSearch optimization package, version 2. Maple application centre; 2011. [Google Scholar]
- Rahn H, Fenn WO. A graphical analysis of the respiratory gas exchange, the O2-CO2 diagram. American Physiological Society; Washington: 1955. [Google Scholar]
- Rizi RR, Baumgardner JE, Ishii M, Spector ZZ, Edvinsson JM, Jalali A, Yu J, Itkin M, Lipson DA, Gefter W. Determination of regional VA/Q by hyperpolarized 3He MRI. Magn Reson Med. 2004;52:65–72. doi: 10.1002/mrm.20136. [DOI] [PubMed] [Google Scholar]
- Rowe L, Jones JG, Quine D, Bhushan SS, Stenson BJ. A simplified method for deriving shunt and reduced VA/Q in infants. Arch Dis Child Fetal Neonatal Ed. 2010;95:F47–52. doi: 10.1136/adc.2009.160010. [DOI] [PubMed] [Google Scholar]
- Sahbaie P, Modanlou S, Gharagozlou P, Clark JD, Lameh J, Delorey TM. Transcutaneous blood gas CO2 monitoring of induced ventilatory depression in mice. Anesthesia and analgesia. 2006;103:620–625. doi: 10.1213/01.ane.0000229714.09553.8c. [DOI] [PubMed] [Google Scholar]
- Sapsford DJ, Jones JG. The PIO2 vs. SpO2 diagram: a non-invasive measure of pulmonary oxygen exchange. Eur J Anaesthesiol. 1995;12:375–386. [PubMed] [Google Scholar]
- Severinghaus JW. Simple, accurate equations for human blood O2 dissociation computations. J Appl Physiol. 1979;46:599–602. doi: 10.1152/jappl.1979.46.3.599. [DOI] [PubMed] [Google Scholar]
- Strohl KP, Baekey D, Dase S, Hete B. Validation of the MouseOx® Arterial Oxygen Saturation Measurements. 2007. [Google Scholar]
- Wagner PD. Ventilation-Perfusion Relationships. In: Hamid Q, Shannon J, Martin J, editors. Physiologic basis of respiratory disease. BC Decker, Inc; Hamilton: 2005. pp. 165–184. [Google Scholar]
- Watts JA, Lee YY, Gellar MA, Fulkerson MB, Hwang SI, Kline JA. Proteomics of microparticles after experimental pulmonary embolism. Thromb Res. 2012;130:122–128. doi: 10.1016/j.thromres.2011.09.016. [DOI] [PubMed] [Google Scholar]
- Zwemer CF, Song MY, Carello KA, D’Alecy LG. Strain differences in response to acute hypoxia: CD-1 versus C57BL/6J mice. J Appl Physiol. 2007;102:286–293. doi: 10.1152/japplphysiol.00536.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.