Abstract
The aims of this study were to evaluate the physiological stress and anxiety responses in children with autism following completion of a standardized, social-evaluative stressor (Trier Social Stress Test-Child version), document the relationship between verbal ability, stress, and anxiety, and determine the association between stress and anxiety in children with autism and typical development. Results demonstrated the Trier Social Stress Test-Child version to be a benign stressor for children with autism. Lower verbal ability in children with autism did not predict salivary cortisol or anxiety responses. There was a lack of association between stress and anxiety for both groups, highlighting the importance of considering these terms as separate constructs. Clinical implications and the limited utility of the Trier Social Stress Test–Child version in evaluating psychosocial stress in autism are discussed.
Children with autism experience a higher incidence of anxiety, particularly social anxiety, than children with other developmental disorders (e.g. Specific Language Impairment: Gillott et al., 2001) or typical development (e.g. Gillott et al., 2001; Sutton et al., 2005). A recent review revealed that 11–84% of children and adolescents with autism spectrum disorders suffer from anxiety, identifying age, diagnosis, degree of social impairment, and cognitive ability as factors that contribute to the presence and severity of anxiety (White et al., 2009a). Social Anxiety Disorder is particularly prevalent, with 29.2% of children with autism (ages 10–14) meeting diagnostic criteria for this disorder (Simonoff et al., 2008).
Considering the high incidence of social anxiety amongst individuals with autism, as well as the inherent limitations of parent- and self-report measures of anxiety in this and other clinical groups, the utility of neuroendocrine markers of stress have been recognized as they provide an objective criterion that can be compared with concurrently reported anxiety and behavioral observations. Cortisol, a hormone released from the adrenal following Limbic-Hypothalamic-Pituitary-Adrenal (LHPA) activation, has been used as a biomarker of the endocrine stress response under various conditions to examine contextual factors that may elicit anxiety in children with autism spectrum disorders (e.g. Corbett et al., 2006, 2008; Jansen et al., 2000, 2003; Lopata et al., 2008; Richdale and Prior, 1992). These studies indicate that children with autism demonstrate significant variability in the diurnal rhythm of cortisol (Corbett et al., 2006, 2008), a pattern of hyperresponsivity to acute stress rather than chronic hyperarousal (Tordjman et al., 1997), and abnormalities in the negative feedback system following activation of the LHPA axis (Hoshino et al., 1987). In addition, children with ASDs vary substantially in their cortisol responses but do not differ as a whole from typically developing children in their response to acute nonsocial stress (mock-MRI; Corbett et al., 2008; but see Corbett et al., 2006 for evidence of variability), anticipation of repeated exposure to nonsocial stress (mock-MRI; Corbett et al., 2008), or physical stress (exercise; Jansen et al., 2003). While previous research utilizing physiological and nonsocial stressors contributes to our understanding of the neuroendocrine responses to stress in children with autism, it does not provide insight into how they respond following interactions that require communication and social skills.
In two studies limited by the exclusion of typically developing comparison groups, children with autism appeared to demonstrate a heightened stress response under naturalistic conditions in which they were required to interact socially (Lopata et al., 2008; Richdale and Prior, 1992). Recently, Corbett and colleagues (2010) provided evidence that playground interaction with typically developing peers stimulates increased cortisol in children with autism as a function of age and social engagement, while their typically developing counterparts do not exhibit this response. In contrast, a sequence of studies utilizing the Trier Social Stress Test – Child Version (TSST-C; Buske-Kirschbaum et al., 1997), a standardized laboratory-based psychosocial stress test known to activate the LHPA axis in typically developing children, revealed that children with autism showed only a slight elevation in cortisol (Jansen et al., 2003) while children with an autism-like condition (Multiple Complex Developmental Disorder), who concurrently met DSM-IV criteria for Pervasive Developmental Disorder – Not Otherwise Specified, demonstrated a blunted cortisol response (Jansen et al., 2000).
The relative, albeit insignificant, increase in cortisol following the TSST-C found by Jansen and colleagues (2003) contradicted their hypothesis that severe social impairment would preclude children with autism from recognizing the stressful nature of the task. It is noteworthy that the children with autism exhibited significantly increased cortisol relative to typically developing children in response to the control condition, during which they interacted freely with the experimenter. The observations in both conditions were attributed to hyper-responsivity of the LHPA axis. In addition, the authors reported a significant positive correlation between communication impairment and peak cortisol value for the children with autism following the TSST-C, suggesting a role for communication ability in the stress response for this group.
Although the studies performed by Jansen and colleagues (2000, 2003) lay important groundwork for our understanding of psychosocial stress in autism, there are limitations to the interpretations that can be drawn due to deviations between their experimental protocol and the standardized TSST-C (e.g. evaluative committee seated outside of view). In addition, the cortisol response to stress was evaluated in participants currently undergoing treatment with antipsychotic medication, which is presently known to influence basal cortisol levels in children by flattening the morning-to-evening slope (Hibel et al., 2007). Rather than measuring basal cortisol levels, a control condition was employed that arguably placed similar demands on the participants, requiring them to be removed from their regular routine and interact socially with an experimenter, playing games or talking. Thus, the overall findings reported provide preliminary understanding of social stress in children with autism while simultaneously posing questions for further inquiry.
Future replication and extension of the work presented by Jansen and colleagues (2000, 2003) may allow further interpretation of these findings in the context of our present understanding of acute stress in autism. Based on their previous findings, the present study sought to clarify the contribution of cognitive factors, particularly communication ability, to physiological stress following completion of this task.
TSST-C and Cognition
Though performance of the TSST-C is cognitively demanding, and Jansen and colleagues provided preliminary evidence suggesting that communication impairment may be correlated with cortisol response to the TSST-C, the present authors know of only one study to date that directly examined the contribution of cognition on the stress response following completion of this task (Fiocco et al., 2007). Fiocco and colleagues determined that typically developing adults with lower verbal fluency performance displayed higher levels of cortisol, relative to their more verbally fluent peers, following completion of the adult version of this task. Despite increased physiological stress, no group differences on subjective ratings of stress were found.
It is presently unknown if lower verbal ability heightens the stress response to this task similarly in children. If this were the case, it might be expected that children with autism would find this task particularly stressful given previous research demonstrating lowered performances on tests of verbal fluency (e.g., Verte et al., 2005). Therefore, in addition to social impairment and social anxiety, verbal ability is a factor that warrants consideration in its contribution to the stress response in children with autism and typical development following performance of the TSST-C.
Current Study
The TSST-C offers a standardized method of evaluating social stress while requiring the completion of tasks that employ verbal, executive functioning skills. The purpose of the current investigation was to examine the anxiety and physiological stress responses of children with autism following the completion of this protocol, while also evaluating the contribution of two verbal abilities that are relevant to communication and performance of the TSST-C, verbal fluency and verbal working memory, as predictor variables for both anxiety and stress. This study will also determine the association between anxiety and stress in response to the standardized protocol. The intent is to replicate and extend the previous findings reported by Jansen and colleagues (2000, 2003) utilizing more stringent diagnostic and exclusionary criteria as well as adhering more closely to the original stress protocol designed by Buske-Kirschbaum and colleagues (1997).
Hypotheses
It is predicted that children with autism will demonstrate an attenuated cortisol response to the TSST-C and report a similar level of state anxiety, relative to typically developing children. A positive correlation between stress and anxiety is expected for typically developing children though not for children with autism. Additionally, it is predicted that children with autism will demonstrate lower verbal ability than typically developing children and that differences between the groups in verbal ability will predict both the stress and anxiety responses to the TSST-C for each group.
METHOD
Participants
Two groups, each comprised of 15 male participants between the ages of 8 and 12 years, completed the study. For the autism group, diagnosis was determined by interview with the parent, classification score of autism on the ADOS (10 or greater for the communication-social interaction composite score), and meeting the diagnostic criteria for Autistic Disorder (American Psychiatric Association, 2000). Exclusionary criteria for this group included a full scale intelligence score (FSIQ) lower than 70 on the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999), the presence of a comorbid neurological (e.g. seizures) or genetic disorder (e.g. Fragile X Syndrome), and the use of medication known to influence the secretion of cortisol (e.g. antipsychotic medication). Based on the Pubertal Development Scale, participants were enrolled who had not formally entered puberty by using a cut-off score of greater than 5 based on ratings in each of three categories: voice, pubic hair, and facial hair. Enrollment in the typically developing group required, in addition to the preceding criteria, the absence of a diagnosed learning disability, neurodevelopmental disorder, and/or psychiatric disorder.
Group differences for age, intelligence, and pubertal development were assessed using independent two sample t-tests (see Table 1). The groups were similar with regard to age [t(28)=−0.396, p>0.05] and onset of puberty [t(26)=−0.697, p>0.05]. Group differences were found for intelligence: FSIQ [t(28)=8.63, p<0.001]. Therefore, the present study used FSIQ as a covariate in statistical analyses.
Table 1.
Demographics
| Information | High Functioning Autism | Typical Development |
|---|---|---|
|
| ||
| M(SD) | M(SD) | |
| WASIb | 92.77(12.29) | 124.87(9.17) |
| ADOS (Communication + SI) | 14.07(3.99) | N/A |
| Age | 9.77(1.26) | 9.55(1.65) |
| Pubertal Development Scale | 3.46(0.88) | 3.27(0.59) |
Note. WASI: Wechsler Abbreviated Scale of Intelligence. ADOS: Autism Diagnostic Observation Scale.
p<0.05.
p<0.01.
Measures
Autism Diagnostic Observation Schedule (ADOS; Lord et al., 1999)
The ADOS is designed to solicit communication and social interaction through an interactive, semi-structured series of activities while the examiner documents observed language and stereotypical/repetitive behaviors. In conjunction with additional testing and clinical interview, the ADOS can aid the diagnosis of an autism spectrum disorder.
Pubertal Development Scale (PDS; Petersen et al., 1988)
The PDS is a six-item self-report measure of pubertal development with scores ranging from 3–12. We enrolled participants who had not formally entered puberty, defined as a score of 5 based on ratings in each of three categories: voice, pubic hair, and facial hair.
State-Trait Anxiety Inventory for Children (STAIC; Spielberger, 1973)
The STAIC is a 20-item self-report questionnaire designed to measure anxiety in children. There are two forms, one to provide a measure of acute anxiety (State form) and one to measure anxiety as a stable, individual difference trait in tendency to respond to situations with acute anxiety (Trait form). Both forms were used in the current study.
Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999)
The WASI is a short and reliable instrument that can be used to obtain an estimate of general intellectual ability for individuals ages 6–89. It contains norms that determine an estimate of Full Scale IQ based on four or two subtests. The two subtest version (Vocabulary and Matrix Reasoning) of the WASI was used in the current study.
NEPSY: Narrative Memory (NEPSY; Korkman et al., 1998)
The NEPSY is a battery of tests designed to examine neuropsychological development in children, ages 3–12. Participants completed the Narrative Memory subtest, a short story recall task that can be used to assess auditory short term memory in both free recall and cued conditions.
Delis-Kaplan Executive Function System: Verbal Fluency Test (DKEFS; Delis et al., 2001)
The DKEFS Verbal Fluency Test is a measure of word-productivity, containing three conditions (i.e. letter fluency, category fluency, and category switching) during which the individual is required to generate as many words as possible in 60 seconds.
TSST-C (Buske-Kirschbaum et al., 1997)
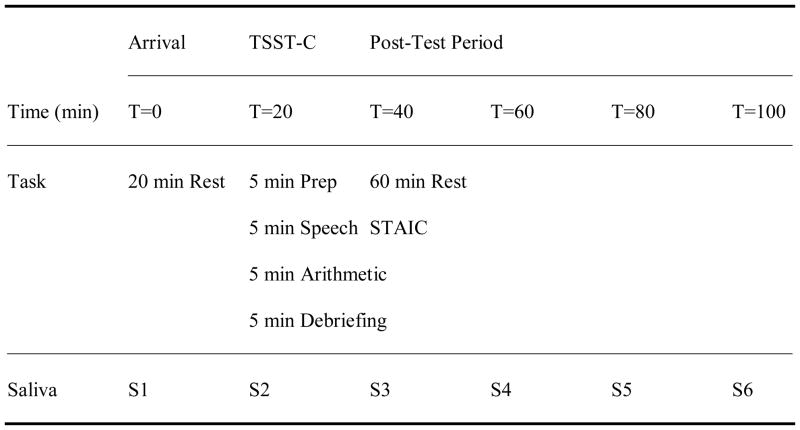
The TSST-C is a standardized psychosocial stress protocol known for reliably activating the LHPA axis in a controlled, laboratory setting. The 20-minute task is subdivided into four components: introduction and speech preparation, speech delivery, serial subtraction, and debriefing (see Figure 1). Participants undergoing the procedure are escorted to an examination room with two committee members sitting behind a table, wearing lab coats, and holding clipboards. The two committee members remain affectively neutral throughout the procedure, regardless of the participant’s performance. Next to the committee members is a videocamera, and there is a standing microphone in the center of the room. After listening to the introduction to a short story, the participant is provided 5 minutes to prepare an ending to the story. He must then deliver the ending to the story as a speech in front of the committee who will, purportedly, be judging his performance relative to the performances of other children. If the participant finishes his story before the end of 5 minutes, he is instructed to continue speaking as he still has time remaining. Following the speech delivery period, the participant is introduced to the serial subtraction task (i.e. subtracting 7’s from 758). Calculation errors result in resuming the task with the initial number. When the serial subtraction period has ended, the two committee members warmly congratulate the participant for his performance. Additionally, the committee debriefs the participant, informing him that they were not truly judging his performance in comparison with other children.
Figure 1. Experiment Procedure.
Note. TSST-C: Trier Social Stress Test – Child version. T: Time. STAIC: State-Trait Anxiety Inventory for Children. S: Sample.
Procedure
Approval for the current study was obtained from the Institutional Review Board at the university where the research was conducted. Informed written consent was obtained from parents in addition to verbal assent from children (and written assent from children who were 12 years old) for each participant prior to enrollment. Participants were recruited through a subject tracking database, IRB approved flyers distributed locally, and community events.
Home Sampling
Each participant collected (1.00 ml) saliva samples at home which estimated his diurnal rhythm of cortisol and provided a baseline from which to compare salivary cortisol on the afternoon of the experiment, as described previously (e.g. Corbett et al., 2006).
Experiment (TSST-C)
Upon arrival, the participant and his parental guardian were escorted by a research assistant (referred to as “the Greeter”) to the testing room, and a saliva sample was collected (see Figure 1). After a 20-minute resting period, a second saliva sample was taken, and the participant was escorted by a different research assistant to the examination room where he was given instructions for the experimental task (TSST-C). Following completion of the TSST-C, the participant was reunited with his parental guardian and the Greeter in the initial testing room for a 1-hour resting period. During this hour, the participant completed the STAIC and provided saliva samples at 20-minute intervals. In total, six salivary cortisol samples were collected. Following completion of the experiment session, participants were thanked and given compensation in the form of gift cards.
Cortisol Storage and Assays
As previously described (e.g. Corbett et al., 2006, 2008), samples were refrigerated, and assay procedures were modified to accommodate overall lower levels of cortisol found in human saliva relative to plasma. Specifically, standards were diluted to concentrations ranging from 2.76 to 345 nmol/L, sample volume was increased to 200 μl, and incubation times were extended to 3 hours. Serial dilution of samples indicated that the modified assay displayed a linearity of 0.98 and a least detectable dose of 1.3854 nmol/L. The absolute value for each sample was above the least detectable dose. Intra- and inter-assay coefficients of variation were 3.91 and 5.26, respectively.
Data Analyses
Demographics and Neuropsychological Measures
Group differences on age, level of pubertal development, and intelligence were calculated using independent two sample t-tests (see Table 1). Because intelligence (FSIQ) differed significantly between the groups, it was used as a covariate for the univariate analyses of covariance (ANCOVAs) when determining group differences on the neuropsychological measures.
Cortisol
Since cortisol is known to be highly right skewed (e.g. Corbett et al., 2006; Richdale and Prior, 1992; Tordjman et al., 1997), a log transformation (base e) was conducted to attain approximate normality. ANCOVA was performed to determine if the baseline cortisol values (mean afternoon, experiment arrival, and 20 minutes post-arrival) were comparable at each measurement between the two diagnostic groups. Then, to determine physiological response (change in cortisol) following performance of the TSST-C, a maximum-minus-minimum, or “change score,” was computed as the difference between each participant’s maximum cortisol level (0 minutes post-TSST-C or 20 minutes post-TSST-C) and minimum cortisol level (experiment arrival or 20 minutes post-arrival).
In order to evaluate the validity of the minimum cortisol value (or experimental baseline) used in the change score, mean afternoon cortisol (averaged across the six home sampling days) was compared to both cortisol upon arrival and cortisol 20 minutes following arrival using paired sample t-tests, and a Bonferroni correction was applied.
Once the change score to identify cortisol response was computed, ANCOVA was performed to determine group differences in their physiological responses to the stressor. One-sample t-tests were used to determine if the cortisol response was significantly different from zero (i.e. no change) for each of the diagnostic groups.
Verbal Ability
Step-wise regression models were used to determine if verbal ability predicted cortisol response or state anxiety. Prior to interpreting the models, the scaled scores of the verbal ability variables (Narrative Memory, Phonemic Fluency, Category Fluency, Category Switching Fluency, Category Switching Total Switching Accuracy) were checked for multicollinearity. Because the variables were highly correlated, a cumulative verbal ability score was computed and used as the independent variable.
Anxiety
ANCOVA, with intelligence as the covariate, was used to evaluate differences between diagnostic groups on self-reported, acute anxiety following the TSST-C (STAIC: State form) as well as trait anxiety (STAIC: Trait form). Within-group differences were also evaluated for acute anxiety (STAIC: State form) and general anxiety (STAIC: Trait form) using paired sample t-tests.
Stress and Anxiety
To determine the association between physiological stress and state anxiety, Pearson correlation coefficients were calculated both for the overall sample and for each group separately. Correlations were performed using the following variables: cortisol response, STAIC: State Form, and STAIC: Trait Form. Partial correlations (controlling for FSIQ) were also performed using these same variables.
RESULTS
Neuropsychological Measures
Group differences on the neuropsychological measures were evaluated using ANCOVAs, with intelligence (FSIQ) as the covariate (see Table 2). The children with autism and those with typical development did not differ significantly from each other on Phonemic Total [F(1,26)=0.174, p>0.05], Category Total [F(1,26)=3.774, p>0.05], or Category Switching Total [F(1,26)=2.600, p>0.05]. Performance differed significantly between the groups on Total Switching Accuracy [F(1,26)=10.249, p<0.01] and Narrative Memory [F(1,26)=5.416, p<0.05].
Table 2.
Neuropsychological Test Performance
| Information | High Functioning Autism | Typical Development |
|---|---|---|
|
| ||
| M(SD) | M(SD) | |
| DKEFS Verbal Fluency | ||
| Letter Fluency | 7.31(3.15) | 10.73(3.08) |
| Category Fluency | 6.92(4.05) | 11.47(2.88) |
| Category Switching (Total Correct) | 6.23(4.76) | 11.33(2.82) |
| Category Switchingb (Total Switching Accuracy) | 4.38(2.76) | 11.47(2.23) |
| NEPSY Narrative Memorya | 3.69(3.90) | 11.93(2.60) |
Note. DKEFS: Delis-Kaplan Executive Function System.
p<0.05.
p<0.01.
Cortisol
There were no significant differences found between the groups for mean afternoon cortisol [F(1,27)=0.002, p>0.05], cortisol upon arrival [F(1,27)=0.505, p>0.05], or cortisol 20 minutes after arrival [F(1,27)=0.495, p>0.05]. Difference variables were computed, and paired sample t-tests revealed that the differences did not deviate significantly from zero when analyzed at both the sample and group levels.
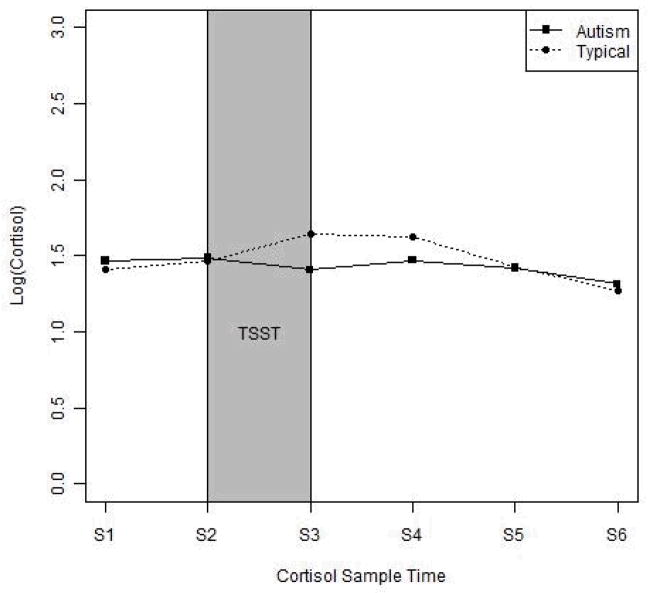
Using ANCOVA, it was determined that cortisol response to the TSST did not differ significantly between the diagnostic groups [F(1,27)=0.140, p>0.05]. However, one-sample t-tests revealed that the cortisol response for the typically developing children differed significantly from zero [t(14)=2.703, p<0.05] while the cortisol response for the children with autism did not [t(14)=1.443, p>0.05] (see Figure 2).
Figure 2. Cortisol Response.
Note. nmol/L: nanomoles per liter. S: Sample. TSST: Trier Social Stress Test – Child version.
Verbal Ability
ANCOVA determined that the verbal ability composite score differed significantly between the two groups [F(1,27)=6.732, p<0.05], and a general linear model was used to test for an interaction between diagnosis and the composite score with cortisol response as the dependent variable. The interaction term was not significant [F(1,5)=2.194, p>0.05], nor was the resulting main effects model, which included diagnosis and the verbal ability composite score as predictor variables [F(2,27)=0.978, p>0.05]. The least significant variable, the verbal ability composite [t(27)=−0.185, p>0.05] was removed, and the final main effects model was not significant [F(1,28)=1.991, p>0.05]. Similarly, a general linear model was used to test for an interaction between diagnosis and the verbal ability composite score with state anxiety as the dependent variable. The interaction term was not significant [F(1,5)=0.175, p>0.05], nor was the resulting main effects model, which included diagnosis and the verbal ability composite score as predictor variables [F(2,25)=0.638, p>0.05]. The least significant variable, the verbal ability composite [t(25) −0.857, p>0.05] was removed, and the final main effects model was not significant [F(1,26)=0.547, p>0.05].
Anxiety
Group differences on trait and state anxiety were evaluated using ANOVA as well as ANCOVA, with intelligence as the covariate (see Table 3). Using ANOVA, significant group differences were found for trait anxiety [F(1,26)=10.658, p<0.01] in which children with autism reported significantly greater trait anxiety than typically developing children. No significant differences were found between groups for state anxiety following performance of the TSST [F(1,26)=0.547, p>0.05.]. Because FSIQ differed significantly between the two groups, ANCOVA was performed to determine group differences on trait and state anxiety with FSIQ as the covariate. Results indicated no differences between groups for trait anxiety [F(1,25)=1.342, p>0.05] or state anxiety [F(1,25)=0.002, p>0.05], once intelligence was controlled.
Table 3.
Self-Reported Anxiety
| Information | High Functioning Autism | Typical Development |
|---|---|---|
|
| ||
| M(SD) | M(SD) | |
| STAIC | ||
| Trait Anxietyc | 38.77(4.94) | 32.07(5.80) |
| State Anxiety | 32.00(3.51) | 30.80(4.84) |
Note. STAIC: State-Trait Anxiety Inventory for Children.
p<0.05.
p<0.01.
p<0.01, w/o controlling for FSIQ (p=n.s, when group difference in FSIQ controlled).
Paired sample t-tests were used to determine differences within each group on state versus trait anxiety on the STAIC. The children with autism reported significantly higher trait anxiety than state anxiety [t(12)=−4.467, p<0.01] while the typically developing children reported similar levels of state and trait anxiety [t(14)=−0.872, p>0.05].
Stress and Anxiety
Pearson correlation coefficients were calculated to determine the association between physiological stress and self-reported anxiety. Cortisol response was not correlated with state anxiety at the sample (r=−0.153, p>0.05) or group levels (autism: r=0.246, p>0.05; typical development: r=−0.292, p>0.05), nor was it correlated with trait anxiety at the sample (r=−0.119, p>0.05) or group levels (autism: r=−0.126, p>0.05; typical development: r=0.061, p>0.05). For the sample as a whole, there was a significant positive correlation between state anxiety and trait anxiety (r=0.380, p<0.05), indicating that high levels of trait anxiety were associated with high levels of acute anxiety following performance of the TSST-C. However, this association was not significant when a partial correlation was conducted, controlling for FSIQ (r=0.350, p>0.05). At the group level, the association between state and trait anxiety was not significant (autism: r=0.197, p>0.05; typical development: r=0.453, p>0.05).
DISCUSSION
Neuropsychological Functioning
The present study compared children with autism to typically developing children on several tasks of verbal ability. Despite lower performances on verbal switching and story recall, there were no statistically significant relationships between stress or anxiety and these cognitive skills.
Stress and Anxiety Responses
Given that the TSST-C tasks were more cognitively demanding for children with autism, it might be expected that the stress and anxiety responses in this group would be heightened; however, they demonstrated less of a stress response and reported a similar level of anxiety relative to their typically developing peers. While the typically developing children demonstrated a modest increase in cortisol relative to baseline, the children with autism maintained a relatively stable level of cortisol throughout the experiment and subsequent rest period and reported a level of state anxiety that was significantly lower than their usual level of trait anxiety. Irrespective of the differences in the methodology employed by Jansen and colleagues (2000, 2003), the stress response exhibited by the children with autism in their work is consistent with the present findings.
The TSST-C is a well-validated measure of psychosocial stress in typically developing children (e.g. Buske-Kirschbaum et al., 1997; Buske-Kirschbaum et al., 2003), which is corroborated by the current results; however, it is noteworthy that the typically developing children in the current study exhibited a more modest stress response than has been reported by prior authors (e.g., Buske-Kirschbaum et al., 1997). While the original study by Buske-Kirschbaum and colleagues (1997) reported a larger percentage of increase in free cortisol (nmol/l) in typically developing children, differences in their sample, particularly with respect to gender (mixed sex vs. male only) and age (9–14 vs. 8–12), likely account for the relatively larger cortisol response portrayed by their data. Nonetheless, an important caveat to the current findings is that the absence of a significant stress response in the children with autism, particularly in comparison to the modest stress response exhibited by typically developing children, requires that associations between cortisol and other variables be interpreted with caution.
Despite the utility of the TSST-C as a reliable and valid social stress protocol in typically developing children, the current findings as well as others (Corbett et al., 2010; Jansen et al., 2000, 2003; Richdale and Prior, 1992), suggest that it may not be a relevant paradigm to elicit social stress in children with autism. Research on social cognition in autism has documented abnormalities in social perception (e.g. Schultz, 2005) and decreased accuracy in detecting social threat (Krysko and Rutherford, 2009). Given that the evaluators in the TSST-C remain affectively neutral throughout the task, it is likely difficult for children with autism to detect the component of social-evaluative threat in this experimental protocol. As such, the TSST-C may have limited utility in eliciting social stress in children with autism as opposed to children with typical development or medical conditions in which social perception is not compromised (e.g. Asthma: Buske-Kirschbaum et al., 2003). This explanation is consistent with evidence from the anxiety literature portraying a trend for high functioning children with autism to report higher levels of anxiety as they become increasingly aware of their own social challenges (White et al., 2009a) and also supports the robust stress responses observed in consideration of age and social engagement (Corbett et al., 2010; Lopata et al., 2008; Richdale and Prior, 1992). Furthermore, the discrepancy between the levels of state and trait anxiety reported by the children with autism may convey that this structured interpersonal interaction was less threatening than natural, everyday interactions.
Researchers observing an attenuated stress response in individuals with anxiety-based psychiatric disorders following the TSST have considered whether this may be a habituated response to social stress (e.g. Panic Disorder: Petrowski et al., 2010). Perhaps, even upon initial exposure, the threatening elements of the TSST may not be perceived as novel to an individual with a history of repeated, acute panic attacks in situations that are perceived to be uncontrollable.
However, previous studies evaluating the stress response during interpersonal interaction do not support this argument for children with autism. If this were the case, the significant stress responses demonstrated following classroom integration (Richdale and Prior, 1992) and social interaction with unfamiliar peers (Corbett et al., 2010; Lopata et al., 2008) would not be observed. The significant stress responses demonstrated in these studies, in contrast to the absence of a statistically significant stress response in the current study, highlight the importance of considering the contextual factors within social paradigms.
The standardized TSST-C protocol utilized in the current study provided participants with concrete social roles, a defined task, and consistent social feedback whereas naturally occurring social interactions are dynamic, spontaneous, and continuously evolving. The literature on executive functioning in autism has demonstrated that children with autism have more difficulty on “open-ended” tasks, performing optimally on tasks that provide clearly defined structure with less reliance on the need to decipher implicit socio-communicative demands from an experimenter (White et al., 2009b). Thus, it is possible that varying the nature of the social interaction contributed to the discrepant findings between studies evaluating social stress in different contexts. Future research protocols with varying levels of structured social interactions, differing both in the structure of social roles (e.g. teacher-student versus peer-peer) and in the structure of the performed activity (e.g. free play versus a card game with clearly outlined rules) could address the credibility of this explanation.
Limitations
The relatively modest cortisol response in the group of typically developing children and the absence of a cortisol response in the children with autism impose statistical constraints on the ability to draw more firm interpretations regarding the association between cortisol response and other variables. In addition, while a significant cortisol response was not observed in the group of children with autism as a whole, there were individuals who demonstrated significantly increased stress (see Figure 3). Variability in the rhythm and response of the LHPA axis has been well-documented in children with autism (e.g. Corbett et al, 2006, 2008), and perhaps variability in the present sample contributed to a mean cortisol response that was not statistically significant. Future research with a larger sample size may allow further differentiation of the group with autism to evaluate variables related to the presence or absence of a stress response. In addition, the absence of a clinical comparison group limits the interpretations that can be drawn from the resulting data. In particular, the inclusion of children with social anxiety disorder would contribute to our understanding of how social anxiety influences the physiological response to the TSST-C in children who do not have autism.
Figure 3. Cortisol Variability.
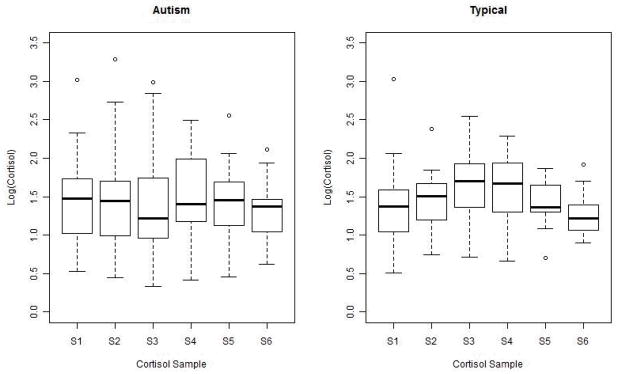
Note. nmol/L: nanomoles per liter. S: Sample.
Clinical Implications
The current study supports the discrimination between stress and anxiety as separate constructs for both children with autism and typical development, rather than referring to stress and anxiety as synonymous. This distinction corroborates previously reported findings in both children and adults (Fiocco et al., 2007; Jansen et al., 2000).
Furthermore, although many remain skeptical of the utility of self-report measures in children with autism (e.g., White et al., 2009a), it is notable that the children with autism in this study utilized the STAIC to report two significantly different states of emotion. One emotion was their high, trait level of anxiety, and the other was a lower, acute level of anxiety following performance of the TSST-C. In the absence of a significant physiological stress response following the TSST-C, it could be argued that the children with autism demonstrated emotional insight when completing this self-report measure. Although this reasoning is compelling, the statistical analyses utilized in the current study do not support a relationship between self-reported anxiety and physiological stress. Nonetheless, the children with autism demonstrated an ability to subjectively differentiate between two different levels of the same emotion. This finding is clinically relevant in that it yields evidence that children with autism have the underlying skills to be taught how to distinguish between two intensities of a single emotion. Thus, they may have sufficient emotional insight to engage in psychotherapy for anxiety (e.g., Cognitive Behavioral Therapy).
Directions for Future Research
While the current study highlights the social context of the experimental paradigm in eliciting physiological stress in children with autism, future research investigating the etiology of the observed physiological responses to stress is critical as multiple underlying causes could lead to the attenuated cortisol response. It is certainly possible that children with autoimmune disorders (e.g. Atopic Dermatitis; Asthma) and adults with Panic Disorder exhibit a similar attenuated cortisol response to this task as children with autism due to varying etiologies. A recent review by Kudielka and colleagues (2009) outlined a number of variables associated with the cortisol response to psychosocial stress, including biomedical (e.g. sex hormones), genetic (e.g. glucocorticoid receptor gene polymorphisms), and psychological (e.g. behavioral stress management training; social dominance) factors. Future research delineating the contributions of each of these domains in autism and other disorders would broaden our understanding of the mechanisms underlying observed cortisol responses and provide the groundwork for establishing efficacious treatment to restore proper functioning of the LHPA axis.
Summary and Conclusion
The current study evaluated the physiological stress and anxiety responses in children with high functioning autism and typical development following completion of a well-validated, reliable social stress protocol. Overall, the present study revealed insight into one aspect of emotional and physiological functioning in children with autism: the response to acute psychosocial stress. The task for future research is to further unveil the pathophysiology of autism and its variable manifestations in individuals with different subtypes of the disorder (e.g. Sutton et al., 2005) as well as varying (social and nonsocial) situations within a single individual (Corbett et al., 2010; Lopata et al., 2008) with the goal of providing tangible recommendations for individuals with autism and their families.
Acknowledgments
The authors would like to thank the children and families who participated in this study for their devoted time and inspiration. In addition, Ms. Lanni is grateful for the valuable insight and contributions provided by G. Len Burns, Ph.D., Paul Kwon, Ph.D., and Paul Strand, Ph.D., as members of her doctoral dissertation advisory committee. A portion of this research was completed in fulfillment of Ms. Lanni’s dissertation project.
Funding. This work was supported by a National Institute of Health (NIH) Career Development Award [grant number 5K08NMH072958] and NIH Award [grant number MINH R01 MH085717] to Blythe A. Corbett.
BIBLIOGRAPHY
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: APA; 2000. Text Revision (DSM-IV-TR) [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Wustmans A, et al. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine. 1997;59(4):419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, von Auer K, Krieger S, et al. Blunted cortisol responses to psychosocial stress in asthmatic children: A general feature of atopic disease? Psychosomatic Medicine. 2003;65(5):806–810. doi: 10.1097/01.psy.0000095916.25975.4f. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Mendoza S, Abdullah M, et al. Cortisol circadian rhythms and response to stress in children with autism. Psychoneuroendocrinology. 2006;31(1):59–68. doi: 10.1016/j.psyneuen.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Mendoza S, Wegelin JA, et al. Variable cortisol circadian rhythms in children with autism and anticipatory stress. Journal of Psychiatry & Neuroscience. 2008;33(3):227–234. [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Schupp C, Simon D, et al. Elevated cortisol during play is associated with age and social engagement in children with autism. Molecular Autism. 2010;1:1–13. doi: 10.1186/2040-2392-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (DKEFS) – Examiner’s Manual. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Fiocco AJ, Joober R, Lupien SJ. Education modulates cortisol reactivity to the Trier Social Stress Test in middle-aged adults. Psychoneuroendocrinology. 2007;32(8–10):1158–1163. doi: 10.1016/j.psyneuen.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Gillott A, Furniss F, Walter A. Anxiety in high-functioning children with autism. Autism. 2001;5(3):277–286. doi: 10.1177/1362361301005003005. [DOI] [PubMed] [Google Scholar]
- Hibel LC, Granger DA, Cicchetti D, et al. Salivary biomarker levels and diurnal variation: Associations with medications prescribed to control children’s problem behavior. Child Development. 2007;78(3):927–937. doi: 10.1111/j.1467-8624.2007.01041.x. [DOI] [PubMed] [Google Scholar]
- Hoshino Y, Yokoyama F, Watanabe M, et al. The diurnal variation and response to dexamethasone suppression test of saliva cortisol level in autistic children. Japanese Journal of Psychiatry and Neurology. 1987;41(2):227–235. doi: 10.1111/j.1440-1819.1987.tb00406.x. [DOI] [PubMed] [Google Scholar]
- Jansen LM, Gispen-de Wied CC, Van der Gaag RJ, et al. Unresponsiveness to psychosocial stress in a subgroup of autistic-like children, multiple complex developmental disorder. Psychoneuroendocrinology. 2000;25(8):753–764. doi: 10.1016/s0306-4530(00)00020-2. [DOI] [PubMed] [Google Scholar]
- Jansen LM, Gispen-de Wied CC, van der Gaag RJ, et al. Differentiation between autism and multiple complex developmental disorder in response to psychosocial stress. Neuropsychopharmacology. 2003;28(3):582–590. doi: 10.1038/sj.npp.1300046. [DOI] [PubMed] [Google Scholar]
- Krysko KM, Rutherford MD. A threat-detection advantage in those with autism spectrum disorders. Brain and Cognition. 2009;69(3):472–480. doi: 10.1016/j.bandc.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp S. NEPSY – A Developmental Neuropsychological Assessment. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- Kudielka BM, Hellhammer DH, Wust S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34(1):2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Lopata C, Volker MA, Putnam SK, et al. Effect of social familiarity on salivary cortisol and self-reports of social anxiety and stress in children with high functioning autism spectrum disorders. Journal of Autism & Developmental Disorders. 2008;38:1866–1877. doi: 10.1007/s10803-008-0575-5. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, et al. Autism Diagnostic Observation Schedule – WPS. Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- Petersen AC, Crockett L, Richards M, et al. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Petrowski H, Herold U, Joraschky P, et al. A striking pattern of cortisol non-responsiveness to psychosocial stress in patients with Panic Disorder with concurrent normal cortisol awakening responses. Psychoneuroendocrinology. 2010;35:414–421. doi: 10.1016/j.psyneuen.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Richdale AL, Prior MR. Urinary cortisol circadian rhythm in a group of high-functioning children with autism. Journal of Autism & Developmental Disorders. 1992;22(3):433–447. doi: 10.1007/BF01048245. [DOI] [PubMed] [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: The role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience. 2005;23(2–3):125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, et al. Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47(8):921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory for Children. Palo Alto, CA: Consulting Psychologists Press; 1973. [Google Scholar]
- Sutton SK, Burnette CP, Mundy PC, et al. Resting cortical brain activity and social behavior in higher functioning children with autism. Journal of Child Psychology and Psychiatry. 2005;46(2):211–222. doi: 10.1111/j.1469-7610.2004.00341.x. [DOI] [PubMed] [Google Scholar]
- Tordjman S, Anderson GM, McBride PA, et al. Plasma beta-endorphin, adrenocorticotropin hormone, and cortisol in autism. Journal of Child Psychology and Psychiatry. 1997;38(6):705–715. doi: 10.1111/j.1469-7610.1997.tb01697.x. [DOI] [PubMed] [Google Scholar]
- Verte S, Geurts HM, Roeyers H, et al. Executive functioning in children with autism and Tourette Syndrome. Development and Psychopathology. 2005;17(2):415–445. doi: 10.1017/s0954579405050200. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- White SW, Oswald D, Ollendick T, et al. Anxiety in children and adolescents with autism spectrum disorders. Clinical Psychology Review. 2009a;29:216–229. doi: 10.1016/j.cpr.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SJ, Burgess PW, Hill EL. Impairments on “open-ended” executive function tests in autism. Autism Research. 2009b;2(3):138–147. doi: 10.1002/aur.78. [DOI] [PubMed] [Google Scholar]