Abstract
Coordinated interactions between signaling networks govern the balance of cell fate decisions in human embryonic stem cells. In this issue, Singh et al. (2012) report that PI3K/Akt signaling switches Activin/Smad activity between pro-self-renewal and pro-differentiation by regulating ERK and GSK3β/β-catenin signaling.
Human embryonic stem cells (hESCs) hold great promise in regenerative medicine. Understanding the detailed signaling network that dictates hESC self-renewal and directed differentiation will undoubtedly contribute to future applications. Several extrinsic signaling pathways have been implicated in maintaining hESC pluripotency, including Activin/Nodal, fibroblast growth factor (FGF), insulin-like growth factor (IGF), and Wnt signaling (Pera and Tam, 2010). However, these signaling pathways are often studied in isolation and in undefined medium conditions. Thus, how these signaling pathways, as a network, coordinate to influence hESC fate in a defined context is unclear. In the current issue of Cell Stem Cell, Singh et al. (2012) employed a chemically defined medium to examine the combinatorial input of signaling pathways that govern hESC fate determination.
In this defined medium, only three types of recombinant growth factors were included to maintain hESC self-renewal: Heregulin (H), Activin A (A) and IGF-1 (I). Heregulin and IGF-1 are known potent activators of PI3K/Akt signaling, whereas Activin A activates Smad2/3, which function to regulate transcription in the nucleus. The authors found that omission of Heregulin and IGF-1 from the medium (-HI medium) or blockage of PI3K/Akt activity induced the expression of mesendoderm genes such as Brachyury, Eomes, Goosecoid and MixL1, whereas self-renewal could be maintained by expressing constitutively active Akt (caAkt) in hESCS cultured in −HI medium. Interestingly, simultaneous blockage of Activin/Smad signaling also abolished induction of these mesendoderm genes in the −HI condition. As Activin/Smad2/3 signaling is required for expression of the pluripotency gene Nanog, these results suggest that robust PI3K/Akt activity collaborates with Activin/Smad signaling to maintain hESC self-renewal, while weak PI3K/Akt activity switches the function of Activin/Smad to promote differentiation.
The authors further investigated the possible downstream effectors of PI3K/Akt. They observed that inhibition of PI3K/Akt signaling enhanced phosphorylation of ERK at T202 and Y204 and GSK3β at S9, indicating ERK activation and GSK3β inactivation, respectively. Likewise, addition of Heregulin and IGF-1 or ectopic expression of caAkt in the −HI medium blocked ERK and GSK3β phosphorylation. Thus, PI3K/Akt suppresses ERK activity but activates GSK3β. In agreement, inhibition of ERK activity attenuated mesendoderm marker gene expression and retained Nanog expression in the −HI medium, suggesting a negative role for ERK in maintenance of hESC self-renewal. Consistent with a previous report (Ding et al., 2005), the authors showed that Raf/MEK/ERK signaling negatively controls GSK3β activity. As Akt can directly bind to and negatively regulate Raf activity via phosphorylation (Rommel et al., 1999), these data reveal that PI3K/Akt promotes hESC self-renewal by interfering with Raf/MEK/ERK signaling, leading to enhanced GSK3β activity.
GSK3β is a critical component of the canonical Wnt pathway, and the inactivation of GSK3β leads to accumulation of β-catenin in the nucleus and drives the transcriptional regulation of target genes. Thus, the observed inactivation of GSK3β by PI3K/Akt inhibition and ERK activation may lead to activation of β-catenin. Indeed, PI3K/Akt inhibition enhanced the transcriptional activity of β-catenin, and this effect could be blocked by inhibition of ERK activity. These results suggest that PI3K/Akt and ERK regulate hESC fate determination by influencing the activities of the Wnt signaling mediators GSK3β and β-catenin. Activation of β-catenin was previously reported to sustain self-renewal in hESCs (Sato et al., 2004). However, Singh et al. (2012) found that blockage of GSK3β by the pharmacological inhibitor BIO induced mesendoderm gene expression under the +HAI condition. This result could be explained by the dose effect of BIO: Low BIO dose stabilizes Myc, which contributes to self-renewal while high BIO dose promotes β-catenin-mediated expression of differentiation genes.
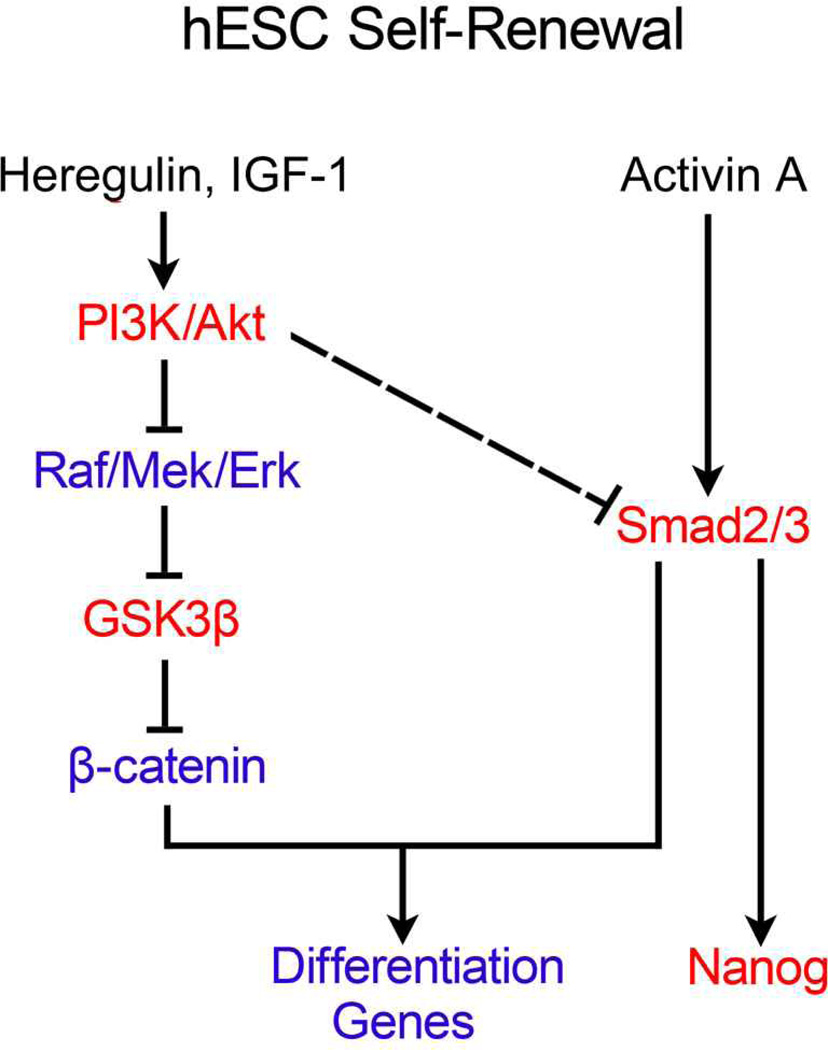
Finally, the authors tested whether β-catenin affects the outcome of Activin/Smad2/3 signaling. They found that Activin/Smad2/3 signaling is required for the BIO-induced mesendoderm gene expression, and ectopic expression of an active form of Smad3 can enhance MixL1 promoter reporter activity, indicating that Smad2/3 may induce mesendoderm gene expression in collaboration with β-catenin. Based on their findings, the authors proposed a model for maintenance of hESC self-renewal (Figure 1). In this model, Heregulin and IGF-1 synergistically activate PI3K/Akt signaling and thus suppress Raf/MEK/ERK activity, leading to high GSK3β activity and low β-catenin activity; the low β-catenin activity does not allow the moderate level of Activin/Smad2/3 activity to initiate mesendoderm gene expression and thereby maintains hESCs in their self-renewal state.
Figure 1. Heregulin and IGF-1 via PI3K/Akt and Activin via Smad2/3 cooperate to maintain self-renewal of hESCs.
Red color indicates the activated state of signaling molecules whereas blue color indicates the repressive state. The dashed line indicates the regulation with unclear mechanisms.
This study has convincingly linked the PI3K/Akt pathway to GSK3β/β-catenin signaling and revealed the collaboration between β-catenin and Smad activities in controlling hESC fate determination, but it also raised some interesting questions. Functions of ERK were previously regarded to be quite different in mouse and human ESCs. A number of laboratories have demonstrated a pro-differentiation role for ERK in mESCs (Li et al., 2012; Ying et al., 2008). However, in hESCs, ERK was thought to act as a pro-self-renewal signal. (Armstrong et al., 2006; Li et al., 2007). One possible explanation for this discrepancy could be that different culture conditions dictate different signal requirements to maintain hESC self-renewal. In the defined medium of the present study, the two robust PI3K/Akt stimulators Heregulin and IGF-1 keep the ERK activity low and maintain the capacity for self-renewal in cooperation with Activin/Smad2/3. However, in other culture media containing multiple undefined growth factors, high ERK activity may promote self-renewal in collaboration with signals other than Activin. This idea is supported by a recent study showing that inhibition of ERK activity renders hESCs more sensitive to BMP-induced differentiation (Na et al., 2010). Although the present study showed that PI3K/Akt switches Activin/Smad2/3 activity from pro-differentiation to pro-self-renewal mainly via inhibition of β-catenin activity, the contribution of direct regulation of Smad2/3 activity by PI3K/Akt remains to be determined. It is also unclear how PI3K/Akt inhibition leads to enhanced Smad2/3 activity. Whether loss of function of β-catenin substitutes the requirement of PI3K/Akt activation in maintenance of hESC self-renewal also awaits investigation. As Smad2/3 can interact with the β-catenin/TCF complex to drive gene transcription, it would be interesting to investigate how Smad2/3 collaborates with β-catenin to stimulate expression of differentiation genes but not pluripotency genes in hESCs. Moreover, systematic investigation of the expression pattern of downstream target genes in response to different combinatorial signal inputs will also shed light on the underlying mechanisms of hESC cell fate determination.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ye-Guang Chen, Email: ygchen@tsinghua.edu.cn.
Xiao-Fan Wang, Email: wang0011@mc.duke.edu.
References
- Armstrong L, Hughes O, Yung S, Hyslop L, Stewart R, Wappler I, Peters H, Walter T, Stojkovic P, Evans J, et al. Hum Mol Genet. 2006;15:1894–1913. doi: 10.1093/hmg/ddl112. [DOI] [PubMed] [Google Scholar]
- Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, Bartholomeusz G, Li Y, Pan Y, Li Z, et al. Mol Cell. 2005;19:159–170. doi: 10.1016/j.molcel.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Li J, Wang G, Wang C, Zhao Y, Zhang H, Tan Z, Song Z, Ding M, Deng H. Differentiation. 2007;75:299–307. doi: 10.1111/j.1432-0436.2006.00143.x. [DOI] [PubMed] [Google Scholar]
- Li Z, Fei T, Zhang J, Zhu G, Wang L, Lu D, Chi X, Teng Y, Hou N, Yang X, et al. Cell Stem Cell. 2012;10:171–182. doi: 10.1016/j.stem.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Na J, Furue MK, Andrews PW. Stem Cell Res. 2010;5:157–169. doi: 10.1016/j.scr.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Pera MF, Tam PP. Nature. 2010;465:713–720. doi: 10.1038/nature09228. [DOI] [PubMed] [Google Scholar]
- Rommel C, Clarke BA, Zimmermann S, Nunez L, Rossman R, Reid K, Moelling K, Yancopoulos GD, Glass DJ. Science. 1999;286:1738–1741. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- Singh AM, Reynolds D, Cliff T, Ohtsuka S, Mathyses A, Sun Y, Menendez L, Kulik M, Dalton S. Cell Stem Cell. 2012;10 doi: 10.1016/j.stem.2012.01.014. ? - ? [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]