Abstract
Human gene therapy has made substantial progress since the initiation of the first clinical trials 20 years ago. Here, we summarized important applications of gene transfer protocols in the treatment of various human diseases using different viral vectors. Recent successful trials on the treatment of ocular diseases and inherited immune deficiencies are particularly encouraging and have raised hopes that human gene therapy as a standard treatment option will finally become a reality. While immune responses and insertional mutagenesis pose obstacles for this novel form of molecular medicine, continuous progress suggests that a wider range of diseases can be treated with gene therapy in the future.
Introduction
With advances in molecular cloning, gene transfer emerged in the late 1980s as a potentially revolutionary new form of molecular medicine, holding promise for novel therapies for many genetic and acquired diseases. After two decades of ups and downs, Science journal, in its December 18th, 2009 issue, considered the “return of gene therapy” as one of the major scientific breakthroughs in 2009. Similarly, Time magazine, reporting on recent high-impact publications in clinical gene therapy, asked the question: “Is gene therapy finally ready for prime time?”
The concept of gene therapy for genetic disease is very simple: a functional copy of the defective gene is introduced to replace the missing function. In principle, this can be accomplished using ex vivo gene transfer to cells that had been removed from the patient. The genetically modified cells are subsequently re-introduced to the patient’s body. Alternatively, the vector carrying the functional gene copy is directly injected into the body to achieve in vivo gene transfer. The first clinical trials were initiated in 1990 and published in 1995, summarizing ex vivo gene transfer to umbilical cord blood cells or to autologous T lymphocytes of children with severe combined immune deficiency (SCID) due to mutations in the adenosine deaminase (ADA) gene (Blaese et al., 1995; Bordignon et al., 1995; Kohn et al., 1995). In these early studies, gene transfer protocols based on a murine retroviral vector were inefficient and unable to transduce hematopoietic stem cells (HSC), thereby limiting extent and duration of gene transfer. Since 1990, >1500 clinical trials have been approved worldwide utilizing viral and non-viral vectors and targeting various organs, cell types, and diseases.
The first decade of clinical gene therapy sought to establish safety of this novel treatment modality and to gain first experience in patients. In the late 1990s, superior gene transfer protocols and vectors were rapidly developed, and numerous successful treatments in small and large animal models of human diseases were reported. In the clinic, the dawn of the new millennium brought the first successes as well as setbacks. Immune responses and insertional mutagenesis emerged as main hurdles for gene therapy. A violent innate immune response to an intravenously delivered adenoviral vector caused the death of a patient with a rare metabolic disorder in 1999 (Somia and Verma, 2000). In 2000, the first clearly successful gene therapy was reported. Ex vivo retroviral gene transfer of the γc-chain (common to several cytokine receptors) to autologous HSC (CD34+ bone marrow cells) (Figure 1) cured boys with X-linked SCID (Cavazzana-Calvo et al., 2000). However, 4 of 10 children in this French trial and 1 of 10 children in a similar trial in the UK developed leukemia (Hacein-Bey-Abina et al., 2008). Leukemic T cell clones showed integration near the LMO2 T cell oncogene (Hacein-Bey-Abina et al., 2003). It is likely that the combination of the growth advantage of the gene-corrected T cells and the activation of LMO2 was responsible for this outcome. Retroviral vectors integrate randomly into the host genome, but show a preference for transcriptionally active genes, and they contain sequences that are prone to activating nearby genes encoded by the host chromosome.
Figure 1.
Ex vivo gene transfer to bone marrow-derived CD34 hematopoietic stem cells (HSCs) of a patient with inherited severe immune deficiency. Autologous HSCs are cultured and infected with a recombinant retroviral or lentiviral vector carrying a functional copy of the defective gene (for example, ADA, gamma-c, or ABCD1). The gene-corrected cells are then injected back into the patient. For some protocols, the patients may receive mild myeloablation prior to infusion of the HSCs.
Successful Treatment of ADA-SCID
Unlike the experience with X-SCID, retroviral transduction of HSC continues to show high therapeutic efficacy in ADA-SCID without the development of leukemia (Aiuti et al., 2009; Aiuti et al., 2002). Eight of ten children have essentially been cured in an Italian trial. For the past 8 years, these pediatric patients no longer required enzyme replacement therapy, they respond well to vaccination, and they live normal lives, attending school instead of living as “bubble” boys or girls. The protocol included mild bone marrow conditioning with busulfan, and traditional ADA enzyme replacement therapy was halted in order to increase engraftment of gene-modified cells and provide some growth advantage over non-modified cells, in which toxic purine metabolites accumulate. Gene engraftment of multipotent HSCs resulted in correction of multiple hematopoietic lineages in a polyclonal fashion. The ADA transgene was expressed in lymphocytes and red blood cells, thereby leading to systemic detoxification and restoration of the immune function.
Lentiviral Vectors -- Encouraging First Clinical Experiences
The newest viral vectors in clinical trial are HIV-based lentiviral vectors, in which all HIV genes have been deleted. Unlike murine gamma retroviral vectors, these retroviruses do not require cell division for gene transfer and integration into the host genome and can therefore transduce dividing and non-dividing target cells. Furthermore, higher levels of gene transfer to HSCs and transgene expression in hematopoietic progenitor cells have been achieved, thereby facilitating therapy. These features, for the first time, make gene therapy for globin disorders such as β-thalassemia or sickle cell disease a possibility (May et al., 2000; Pawliuk et al., 2001). First results from an ongoing French gene therapy trial on lentiviral gene transfer for β-thalassemia are promising in that one treated patient has not required red blood cell transfusions for the past 16 months (Kaiser, 2009). Again, gene transfer was performed ex vivo to HSCs. However, a concern has emerged as well: in spite of very low-efficiency of gene transfer, approximately 10% of the patient’s blood cells contain viral integrants in the HMGA2 gene, apparently resulting in increased gene expression and a growth advantage. Thus far, this does not seem to have caused adverse effects in the patient. Interestingly, in an earlier trial, which showed transient efficacy in two subjects with a rare immune deficiency called chronic granulomatous disease (CGD), murine retroviral HSC gene transfer caused expansion of myeloid clones (Ott et al., 2006). CGD is characterized by lack of functional phagocytic cells due to mutations in genes required for oxidase function in these cells, thereby reducing the immune system’s ability to fend off microbial pathogens. Viral integration upregulated expression of genes that seemed to favor proliferation of cells that are beneficial to therapy. However, efficacy was subsequently lost because of transgene silencing and activation of endogenous EVI1 gene, causing genomic instability (Stein et al., 2010).
Any of these events linked to changes in endogenous gene expression upon viral integration were unexpected, and therefore underscore that much remains to be learned about the effects of insertion of viral sequences and therapeutic expression cassettes into host genomes. In the long-run, development of site-specific integration systems is desirable.
Nonetheless, the first successful use of lentiviral gene transfer to HSC was recently reported for X-linked adrenoleukodystrophy (ADL), a devastating lipid storage disorder in boys that results in demyelination of neurons in the brain. Gene transfer halted the progressive brain damage in two 7-year old patients (Cartier et al., 2009). It is possible that benefits from the use of integrating vectors in many cases and diseases will out-weigh risks associated with insertional mutagenesis, in particular as vectors are being developed with reduced impact on cellular gene expression and as these risks are being better understood. Furthermore, non-integrating lentiviral vectors have also been developed recently, but their long-term safety and efficacy remains to be evaluated.
Adeno-associated Viral (AAV) Vectors for the Treatment of Inherited Blindness
AAV vectors are derived from a non-pathogenic parent virus with a single-stranded DNA genome. High efficiency of in vivo gene transfer to numerous cell types, development of a large arsenal of viral capsids with different tropisms, and low innate/inflammatory responses to viral particles have made these vectors highly attractive. Numerous investigations have yielded impressive results on the correction of various genetic diseases in mice, dogs, non-human primates, and other animal models (Warrington and Herzog, 2006). For example, hepatic coagulation factor IX gene transfer resulted in greater than eight years of stable correction of hemophilia B in canines and was also shown to induce immune tolerance to factor IX and several other therapeutic proteins (LoDuca et al., 2009; Niemeyer et al., 2009). However, similar therapeutic expression in humans was not achieved because of pre-existing antibodies against the capsid of this particular serotype (AAV2), or was transient because of a CD8+ T cell response to the viral capsid (Manno et al., 2006).
In a gene therapy trial for Parkinson’s disease (PD) with AAV2 vectors, some clinical efficacy was observed (Kaplitt et al., 2007). At the 2009 annual meeting of the American Society of Gene and Cell Therapy, Muramatsu and colleagues reported results from a more recent trial in Japan. Six patients with mild- to late-stage PD treated with AAV2 vectors showed marked improvement in motor functions six-months post-treatment. In ongoing clinical trials, AAV gene transfer to the brains of patients with Parkinson’s disease has shown promising results. Partial restoration of dopamine synthesis combined with L-dopa administration can ameliorate disease. Furthermore, a tricistronic lentiviral vector encoding all genes required for dopamine synthesis has been successfully tested in nonhuman primates (Jarraya et al., 2009).
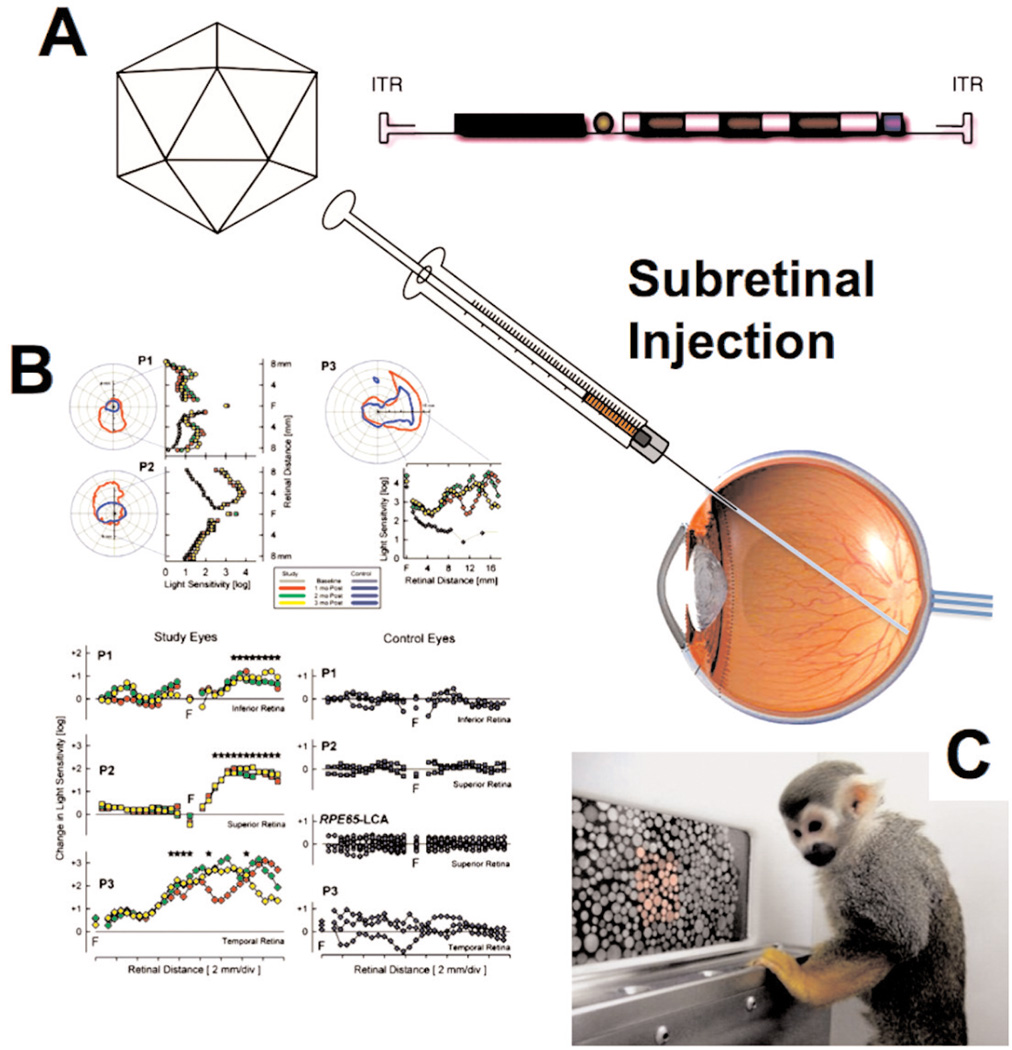
In 2008, three groups (two in the US and one in the UK) reported success in patients using the AAV2 vector (Bainbridge et al., 2008; Cideciyan et al., 2008; Maguire et al., 2008). Local (subretinal, an immune privileged site) administration of a vector expressing RPE65 (retinal pigment epithelium-specific 65 kD protein) led to gain of light sensitivity and, in some cases, of vision, in patients with Leber’s congenital amaurosis (LCA). This rare form of inherited blindness is caused by progressive loss of rod and cone function due to the lack of the RPE65 isomerase, which is required to form light sensitive pigment. Proof of concept with this gene therapy approach had initially been achieved in animals in 2001, when vision was restored in a Briard dog, named Lancelot (Acland et al., 2001). Gain of light sensitivity was documented at the site of retinal gene transfer (Figure 2), and patients gained vision as evidenced by behavior correlates such as the ability to walk through a maze (Cideciyan et al., 2008). A one-year follow-up showed no diminution in any of the clinical parameters (Cideciyan et al., 2009). A subsequent trial in children showed that the extent of visual improvement achieved by the gene therapy is age-dependent (Maguire et al., 2009). Earlier intervention, when degeneration of photoreceptors has progressed less, results in substantial gains in ambulatory vision. Most impressively, four children (8–11 years old) are now able to play sports and attend school without the use of learning aids. Another recent study, demonstrating correction of color-blindness in squirrel monkeys by ocular therapy with an AAV2 vector (Figure 2C), provides a glimpse of future therapies to come (Mancuso et al., 2009).
Figure 2.
In vivo gene transfer for ocular diseases. A. An adeno-associated viral (AAV) vector is injected into the subretinal space using a surgical procedure. B. Gene transfer of RPE-65 to the retina restored light sensitivity in patients with Leber’s congenital amaurosis (LCA) (from Proc Natl Acad Sci USA 105(39):15112-7, 2008; © 2008 by The National Academy of Sciences of the USA). C. A similar protocol for AAV-mediated L-opsin corrected color blindness in squirrel monkeys.
More Advances in the Foreseeable Future
There are many more promising gene therapy protocols in clinical trials or in the pre-clinical pipelines. Existing protocols that encountered obstacles are being modified to overcome identified hurdles. For example, AAV vector capsids are being engineered to be more resistant to neutralizing antibodies in the human population, to transduce target cells more efficiently, and to show enhanced tropism to specific tissues (Zhong et al., 2008). One exciting development is the ability to transfer a gene to cardiac and multiple skeletal muscles, which holds promise for the treatment of muscular dystrophies and lysosomal storage disorders (Asokan et al., 2009). Some gene transfer protocols may include transient immune suppression or modulation to prevent adaptive responses against capsid or transgene derived antigen (Sack and Herzog, 2009). Such approaches are not unlike cell and organ transplantation or protein replacement therapies, which are also often rejected by the immune system. Future vectors may also include microRNA-regulated expression cassettes to detarget expression from professional, bone marrow-derived antigen presenting cells (Brown et al., 2006). With regard to degenerative muscle disorders, a recent study accomplished robust improvements of muscle growth and strength in nonhuman primates using AAV-mediated follistatin gene transfer (Kota et al., 2009).
In summary, human gene therapy has become a reality, clinical gene therapy is here to stay, and more successful protocols are expected to emerge in the near future. Treatment of ocular diseases and of inherited immune deficiencies are particularly successful. The most severe hurdles that have been encountered are immunemediated rejection of the therapy or complications arising from insertional mutagenesis (especially following gene transfer to stem cells). Success in a wider range of diseases will depend on solutions to these problems.
Contributor Information
Roland W. Herzog, Department of Pediatrics, University of Florida College of Medicine, 2033 Mowry Road, Gainesville, Florida 32610, USA
Ou Cao, Department of Pediatrics, University of Florida College of Medicine, 2033 Mowry Road, Gainesville, Florida 32610, USA
Arun Srivastava, Departments of Pediatrics and Molecular Genetics & Microbiology, University of Florida College of Medicine, 2033 Mowry Road, Gainesville, Florida 32610, USA
References
- Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, Pearce-Kelling SE, Anand V, Zeng Y, Maguire AM, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28(1):92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L, Scaramuzza S, Andolfi G, Mirolo M, Brigida I, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360(5):447–458. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- Aiuti A, Slavin S, Aker M, Ficara F, Deola S, Mortellaro A, Morecki S, Andolfi G, Tabucchi A, Carlucci F, et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296(5577):2410–2413. doi: 10.1126/science.1070104. [DOI] [PubMed] [Google Scholar]
- Asokan A, Conway JC, Phillips JL, Li C, Hegge J, Sinnott R, Yadav S, DiPrimio N, Nam HJ, Agbandje-McKenna M, et al. Reengineering a receptor footprint of adeno-associated virus enables selective and systemic gene transfer to muscle. Nat Biotechnol. 2009;28(1):79–82. doi: 10.1038/nbt.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Blaese RM, Culver KW, Miller AD, Carter CS, Fleisher T, Clerici M, Shearer G, Chang L, Chiang Y, Tolstoshev P, Greenblatt JJ, Rosenberg SA, Klein H, Berger M, Mullen CA, Ramsey WJ, Muul L, Morgan RA, Anderson WF. T lymphocyte-directed gene therapy for ADA-SCID: initial trial results after 4 years. Science. 1995;270(5235):475–480. doi: 10.1126/science.270.5235.475. [DOI] [PubMed] [Google Scholar]
- Bordignon C, Notarangelo LD, Nobili N, Ferrari G, Casorati G, Panina P, Mazzolari E, Maggioni D, Rossi C, Servida P, et al. Gene therapy in peripheral blood lymphocytes and bone marrow for ADA- immunodeficient patients. Science. 1995;270(5235):470–475. doi: 10.1126/science.270.5235.470. [DOI] [PubMed] [Google Scholar]
- Brown BD, Venneri MA, Zingale A, Sergi Sergi L, Naldini L. Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat Med. 2006;12(5):585–591. doi: 10.1038/nm1398. [DOI] [PubMed] [Google Scholar]
- Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, Vidaud M, Abel U, Dal-Cortivo L, Caccavelli L, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326(5954):818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, Selz F, Hue C, Certain S, Casanova JL, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288(5466):669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- Cideciyan AV, Aleman TS, Boye SL, Schwartz SB, Kaushal S, Roman AJ, Pang JJ, Sumaroka A, Windsor EA, Wilson JM, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci U S A. 2008;105(39):15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL, Windsor EA, Conlon TJ, Sumaroka A, Roman AJ, et al. Vision 1 year after gene therapy for Leber’s congenital amaurosis. N Engl J Med. 2009;361(7):725–727. doi: 10.1056/NEJMc0903652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, Clappier E, Caccavelli L, Delabesse E, Beldjord K, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118(9):3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, Lim A, Osborne CS, Pawliuk R, Morillon E, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302(5644):415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- Jarraya B, Boulet S, Ralph GS, Jan C, Bonvento G, Azzouz M, Miskin JE, Shin M, Delzescaux T, Drout X, et al. Dopamine gene therapy for parkinson’s disease in a nonhuman primate without associated dyskinesia. Sci Transl Med. 2009;1(2):2ra4. doi: 10.1126/scitranslmed.3000130. [DOI] [PubMed] [Google Scholar]
- Kaiser J. Gene therapy. Beta-thalassemia treatment succeeds, with a caveat. Science. 2009;326(5959):1468–1469. doi: 10.1126/science.326.5959.1468-b. [DOI] [PubMed] [Google Scholar]
- Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet. 2007;369(9579):2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- Kohn DB, Weinberg KI, Nolta JA, Heiss LN, Lenarsky C, Crooks GM, Hanley ME, Annett G, Brooks JS, el-Khoureiy A, et al. Engraftment of gene-modified umbilical cord blood cells in neonates with adenosine deaminase deficiency. Nat Med. 1995;1(10):1017–1023. doi: 10.1038/nm1095-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota J, Handy CR, Haidet AM, CL M, Eagle A, Rodino-Klapac LR, Tucker D, Shilling CJ, Therlfall WR, Walker CM, et al. Follistatin gene delivery enhances muscle growth and strength in nonhuman primates. Sci Transl Med. 2009;1(6):6ra15. doi: 10.1126/scitranslmed.3000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoDuca PA, Hoffman BE, Herzog RW. Hepatic gene transfer as a means of tolerance induction to transgene products. Curr Gene Ther. 2009;9(2):104–114. doi: 10.2174/156652309787909490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F, Mingozzi F, Bennicelli JL, Ying GS, Rossi S, et al. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374(9701):1597–1605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso K, Hauswirth WW, Li Q, Connor TB, Kuchenbecker JA, Mauck MC, Neitz J, Neitz M. Gene therapy for red-green colour blindness in adult primates. Nature. 2009;461(7265):784–787. doi: 10.1038/nature08401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Ozelo MC, Hoots K, Blatt P, Konkle B, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12(3):342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- May C, Rivella S, Callegari J, Heller G, Gaensler KM, Luzzatto L, Sadelain M. Therapeutic haemoglobin synthesis in beta-thalassaemic mice expressing lentivirusencoded human beta-globin. Nature. 2000;406(6791):82–86. doi: 10.1038/35017565. [DOI] [PubMed] [Google Scholar]
- Niemeyer GP, Herzog RW, Mount J, Arruda VR, Tillson DM, Hathcock J, van Ginkel FW, High KA, Lothrop CD., Jr Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy. Blood. 2009;113(4):797–806. doi: 10.1182/blood-2008-10-181479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U, Glimm H, Kuhlcke K, Schilz A, Kunkel H, et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006;12(4):401–409. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- Pawliuk R, Westerman KA, Fabry ME, Payen E, Tighe R, Bouhassira EE, Acharya SA, Ellis J, London IM, Eaves CJ, et al. Correction of sickle cell disease in transgenic mouse models by gene therapy. Science. 2001;294(5550):2368–2371. doi: 10.1126/science.1065806. [DOI] [PubMed] [Google Scholar]
- Sack BK, Herzog RW. Evading the immune response upon in vivo gene therapy with viral vectors. Curr Opin Mol Ther. 2009;11(5):493–503. [PMC free article] [PubMed] [Google Scholar]
- Somia N, Verma IM. Gene therapy: trials and tribulations. Nat Rev Genet. 2000;1(2):91–99. doi: 10.1038/35038533. [DOI] [PubMed] [Google Scholar]
- Stein S, Ott MG, Schultze-Strasser S, Jauch A, Burwinkel B, Kinner A, Schmidt M, Krämer A, Schwäble J, Glimm H, Koehl U, Preiss C, Ball C, Martin H, Göhring G, Schwarzwaelder K, Hofmann WK, Karakaya K, Tchatchou S, Yang R, Reinecke P, Kühlcke K, Schlegelberger B, Thrasher AJ, Hoelzer D, Seger R, von Kalle C, Grez M. Genomic instability and myelodysplasia with monosomy 7 consequent to EVI1 activation after gene therapy for chronic granulomatous disease. Nat Med. 2010;16(2):198–204. doi: 10.1038/nm.2088. [DOI] [PubMed] [Google Scholar]
- Warrington KH, Jr, Herzog RW. Treatment of human disease by adeno-associated viral gene transfer. Hum Genet. 2006;119(6):571–603. doi: 10.1007/s00439-006-0165-6. [DOI] [PubMed] [Google Scholar]
- Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M, Herzog RW, Zolotukhin I, Warrington KH, Jr, Weigel-Van Aken KA, et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci U S A. 2008;105(22):7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]