Abstract
Biomaterial scaffolds have been extensively used to deliver growth factors to induce new bone formation. The pharmacokinetics of growth factor delivery has been a critical regulator of their clinical success. This review will focus on the surface interactions that control the non-covalent incorporation of growth factors into scaffolds and the mechanisms that control growth factor release from clinically relevant biomaterials. We will focus on the delivery of recombinant human bone morphogenetic protein-2 from materials currently used in the clinical practice, but also suggest how general mechanisms that control growth factor incorporation and release delineated with this growth factor could extend to other systems. A better understanding of the changing mechanisms that control growth factor release during the different stages of preclinical development could instruct the development of future scaffolds for currently untreatable injuries and diseases.
Keywords: Tissue Engineering, Drug delivery, Biomaterials, Ceramic/polymer composites, Hydrogels
1. Introduction
One of the most challenging research areas in drug delivery today is how to effectively induce new bone formation for fracture healing and bone fusion. During bone healing multiple soluble signaling molecules, insoluble extracellular matrix molecules, and cells interact to direct the formation of functional new tissue. Many of the molecules that have been used to induce bone healing in adults have been inspired by normal tissue developmental programs. Growth factors, which are soluble proteins that stimulate cell growth and differentiation, have emerged as a broadly applicable tool to induce bone formation. Bone morphogenetic proteins (BMPs) have been the most effective growth factors at orchestrating new bone formation in humans by recapitulating the different stages of bone development (1-3). To induce bone formation, it has often been necessary to deliver growth factors in scaffolds that retain their activity at the implant site. We must have a mechanistic understanding growth factor incorporation into and release from scaffolds to realize their therapeutic potential.
1.1 Fundamental properties of growth factors for bone healing
Approaches in drug delivery to promote bone healing are increasingly leveraging knowledge of the body's endogenous regenerative capabilities. Marshall Urist first described the osteoinductive capabilities of demineralized bone in 1965 (4). Building on this discovery, Wozney and colleagues sequenced the gene for BMP-2 which facilitated the production of recombinant human BMP-2 (rhBMP-2) using genetic engineering techniques (5). Since then, several different growth factors have been used to induce bone healing including BMP-2, BMP-7 (5), insulin-like growth factors (IGFs) (6), transforming growth factor beta (TGF-β) (7), platelet derived growth factors (PDGFs) (8), fibroblast growth factors (FGFs) (9), growth and differentiation factors (GDFs) (9), stromal derived factors (SDFs) (10), and vascular endothelial growth factor (VEGF) (11). To date BMP-2, BMP-7 (12), and PDGF-BB (13) have been approved by the Food and Drug Administration (FDA) for orthopedic indications. Growth factors orchestrate two important roles during new bone formation. First, they recruit endogenous stem cells from adjacent tissues into scaffolds. Secondly, they direct the differentiation of recruited cells into bone tissue. The balance between growth factor release and retention could be a critical regulator of the efficacy of growth factor-based treatments for bone regeneration as BMPs have been involved in inflammation (14), systemic iron balance (15), antibody formation (16), deleterious effects on the central and peripheral nervous system (17), and oncogenesis (18). Therefore, it will be crucial to understand the fundamental physiochemical properties of growth factors to enhance their safe and effective delivery.
Non-covalent incorporation of growth factors into scaffolds has been extensively explored for scientific and pragmatic reasons. Non-covalent intermolecular interactions have included electrostatic interactions, hydrophobic interactions, hydrogen bonding and Van der Waals forces. An understanding of the physiochemical properties rhBMP-2 has contributed to the mechanisms that control its non-covalent incorporation into scaffolds. rhBMP-2 has a measured isoelectric point of >8.5 (19) and a theoretical isoelectric point of 9.16 and thus has a positive charge around physiological pH (20). Once expressed in mammalian cells, rhBMP-2 has been characterized as a dimer consisting of two glycosylated rhBMP-2 monomers, has a molecular weight of approximately 30kD, and has limited solubility at physiological conditions due to its hydrophobic exterior surface. rhBMP-2 for therapeutic applications has been produced in Chinese Hamster Ovary (CHO) cells and has contained a mixture of 3 different N-terminal sequences with varying charges. This mixture has enabled the possibility of 6 different rhBMP-2 isoforms with different electrostatic properties (21). Significantly, rhBMP-2′s N-terminal region contains the heparin binding domain that plays a critical role in growth factor binding to the extracellular matrix (22). The dimensions of rhBMP-2 have been modeled as 2.5 × 3.5 × 7nm which results in a 20nm2 footprint for side-on adsorption to biomaterials which controls the upper limit of rhBMP-2 that could be adsorbed directly on a scaffold (23). Changes in pH have the potential to induce protein aggregation (24) that could reduce protein bioactivity or enable unintended biologic side effects (25). rhBMP-2 has been typically lyophilized with excipients so that when reconstituted in a defined volume of water it has formed 1.5-4 mg/ml solutions in 5mM glutamic acid buffer pH 4.5 (2.5% glycine, 0.5% sucrose, 0.01% polysorbate 80, 5mM NaCl, 5mM glutamic acid) (26). Increasing the buffer's ionic strength from 0 to 0.15M dramatically decreased its solubility, but further increasing the ionic strength to 0.5M increased rhBMP-2 solubility. Increasing the ionic strength above 0.5M induced rhBMP-2 precipitation (27). If CHO-derived rhBMP-2 is reconstituted in phosphate buffer at pH 7.4 its maximum solubility is 30μg/ml. Therefore, if reconstituted at therapeutic concentrations and physiologic pH, rhBMP-2 precipitates to form microparticles that erode over time (27). Also, when rhBMP-2 has been adsorbed in collagen sponges, a common carrier for rhBMP-2, the pH shifts higher which could affect protein aggregation (28). In one illustrative study, when the pH of a 0.75mg/ml rhBMP-2 solution was shifted from pH 4.5 to pH 6.5 the size of rhBMP-2 aggregates shifted from 0.1um to 0.1-2um and also increased the fraction of aggregated rhBMP-2 (29). Taken together, these studies indicate that it will be important to consider the physicochemistry of growth factors when designing scaffolds for controlled delivery applications (Figure 1).
Figure 1.
Description of key BMP-2 properties that instruct the design of scaffolds for controlled delivery applications. Crystal structures of monomeric BMP-2 with its basic residues highlighted (top left) and acidic residues highlighted (top right) (PDB: 3BMP). The physicochemistry of BMP-2 has been an important consideration for understanding its delivery from scaffolds (middle). Varying the pH and ionic strength of the buffer has had a significant impact on BMP-2 solubility (bottom).
1.2 Purpose and scope of review
The purpose of this review is to highlight the surface interactions that control growth factor incorporation and release from scaffold materials that have published release data from both in vitro and vivo environments. Understanding the mechanisms that control growth factor-material interactions in these systems could help improve the safety, efficacy, and cost effectiveness of current bone regeneration techniques. Furthermore, these mechanisms could enable future techniques for the delivery of multiple growth factors, site-specific bone regeneration approaches, and covalent tethering approaches. Relevant examples from the literature, especially approaches that are similar to current clinical techniques will be discussed due to their relevance to human health. Although cell-based therapies, metal caged-based fixation techniques, and electrically-mediated bone healing have all shown promise to promote bone healing they are out of the scope of this review. Please see excellent reviews by Ward (30) and Basset (31)for more detailed analyses of how these approaches can be used to promote bone healing.
2. Mechanisms that control growth factor incorporation into scaffolds
It is clear that the physiochemical and biological properties of growth factors motivate the need to design scaffolds that maintain growth factor bioactivity and enhance growth factor retention at implant sites. In this section we will review the mechanisms that control non-covalent growth factor incorporation into scaffolds. Extensively characterized and emerging approaches to incorporate growth factors into scaffolds will be highlighted to suggest techniques that could address current limitations in growth factor delivery.
2.1 Growth factor adsorption to solid scaffolds
Scaffolds for bone regeneration must fulfill key requirements that have been closely tied to their growth factor binding and releasing properties. To be clinically relevant, scaffolds would ideally fill the appropriate space, be composed of biocompatible and osteoconductive materials, match the mechanical properties at the implant site, possess a 3-D structure that addresses cell metabolic requirements, be efficiently manufactured, allow for fixation into the surgical site, degrade over a relevant time frame, and facilitate cell migration into the construct (32). Materials with unique physiochemical properties have been tested for bone healing applications including autograft bone (33), allograft bone (34), xenograft bone tissue (35), tissue derived-proteins (36), recombinant proteins (37), ceramics (38), synthetic polymers (39), and calcium phosphate bone cements (40). A common observation has been that regardless of material choice, scaffolds that retain greater concentrations of encapsulated growth factors have proven more effective at promoting bone regeneration (21, 41, 42). This section will highlight studies that have explored the fundamental mechanisms that control growth factor incorporations into scaffolds including non-specific growth factor adsorption and specific intermolecular interactions to maximize growth factor retention.
Growth factor adsorption to scaffolds has been tuned by employing materials with different physiochemical properties. Growth factors placed in the presence of a scaffold have been transported to the material surface through diffusion, flow, or thermal convection where they bind via a thermodynamically favorable process. Material properties that have been varied to control protein adsorption include surface wettability (43), roughness (44), surface charge, charge density (45), and functional groups (46). In addition to material properties, solution properties such as ionic strength (47) and other proteins in the media (48) have influenced the mass of growth factor adsorption onto biomaterials. Adjacent proteins have also effected the conformation and orientation of adsorbed growth factors (49). Mechanisms that have mediated how different surface, solution, and protein properties have interacted to control protein adsorption and bioactivity have been protein-specific. Generally, increasing electrostatic attraction between rhBMP-2 and scaffolds has increased rhBMP-2 incorporation into scaffolds. Several methods have been used to increase the electrostatic attraction between rhBMP-2 and scaffolds including increasing the number of charged moieties in the scaffold, decreasing the ionic strength of the media, increasing the pH of the media to maximize the charge on rhBMP-2, and increasing the incubation time before implantation (26, 50). In this review we will focus on the mechanisms that control rhBMP-2 because it has been the most widely used growth factor for bone healing in the clinic (Figure 2).
Figure 2.
Schematic representation of BMP-2 adsorption to ceramic scaffolds with the properties that generally have increased the mass of BMP-2 adsorbed to its surface (PDB: 3BMP).
Ceramics such as bone-derived minerals, hydroxyapatite, tricalcium phosphate, or combinations of the two have been used for scaffold-carriers of rhBMP-2 due to their osteoconductive properties and their environmentally sensitive binding capabilities. For example, exposing 3ml of biphasic calcium phosphate to 2.1ml of 3mg/ml rhBMP-2 resulted in a binding efficiency of 86±9.8% of the initial growth factor (51). Molecular simulations and experimental results have indicated that rhBMP-2 adsorption to hydroxyapatite involves electrostatic attraction and hydrogen bonding (52). Growth factor adsorption to ceramics has proceeded in a time-dependent process (53, 54). In one study, greater than 80% of the total rhBMP-2 adsorbed during first minutes of exposure to hydroxyapatite. However, rhBMP-2 did not reach its equilibrium bound concentration until after 4 hours of incubation (55). Growth factor adsorption has also been modulated by the properties of its buffer. In one study, decreasing the pH from 8.4 to 7.0 increased the rate of rhBMP-2 adsorption to hydroxyapatite. Decreasing the pH could have ionized rhBMP-2, which contains a region with a high concentration of basic residues, and enabled rhBMP-2 to hydrogen bond more readily to the hydroxyapatite surface (55). The affinity of rhBMP-2 for hydroxyapatite has been measured to be 2.4−230*10−5 M which was similar to common model proteins like bovine serum albumin (55). This relatively low affinity interaction may limit the ability of these ceramics to retain growth factors in scaffolds in vivo and motivates the need to characterize how other proteins modulate growth factor adsorption.
To maintain protein bioactivity before implantation, growth factors have been reconstituted with carrier proteins that could alter protein adsorption. Of the different proteins that adsorb to the surface of biomaterials, albumin has the greatest concentration in the blood and it has been studied both for its properties as a carrier for growth factors in solution and for its interactions with biomaterials (56). In an illustrative study, calcium phosphate scaffolds were left untreated or coated with rat serum albumin and exposed to rhBMP-2. rhBMP-2 had a loading efficiency of 96±4% for both albumin treated and untreated scaffolds which suggested that rhBMP-2 and albumin bound to calcium phosphate scaffolds through different mechanisms (57). These results suggest that understanding the physiochemical properties of growth factors and other molecules in their environment will be crucial for developing new scaffolds for bone tissue engineering applications.
Protein adsorption has also served as a versatile mechanism to non-covalently include growth factors for therapeutic applications. For example, growth factors have been adsorbed on FDA approved polymers such as poly(lactide-co-glycolide) (PLG) (58) and poly(caprolactone) (PCL) (59). For PLG microspheres, increasing the hydrophilicity, decreasing the pI, and exposing the microspheres to multiple rounds of rhBMP-2 adsorption increased the mass of rhBMP-2 adsorbed on their surface. Microspheres formed from similarly charged PLG polymers with different molecular weights did not change the mass of rhBMP-2 adsorped (60). These results suggested that rhBMP-2 may interact with synthetic polymers through a variety of non-covalent interactions.
Although synthetic polymeric materials provide a pragmatic approach to incorporate growth factors into scaffolds, they are not osteoconductive on their own. To increase the bone-regenerating capability researchers have developed techniques to coat scaffold surfaces with osteoconductive bone-like mineral layers that bind osteoinductive growth factors and peptides (61-66). For example, polymeric Orthocord sutures have been coated with hydroxyapatite-like mineral by incubating them in modified simulated body fluid for 7 days. Then, the mineralized sutures were incubated with bFGF for 4 hours which enabled stable bFGF incorporation on the suture even after 5 passages through sheep infraspinatus tendon (65). These results demonstrated that mineral coatings can facilitate growth factor adsorption to polymeric materials and maintain their integrity even after extensively handling. This finding could be significant as resistance to changes in material properties during surgery has been indicated as a requirement to increase the user-friendliness of devices (67).
Researchers have expanded on this fundamental understanding of the mechanisms that control growth factor incorporation into scaffolds by developing nanostructured materials to increase their binding capacity. Nanostructured materials have increased the surface area of materials and the number of available non-covalent interactions between the surface and protein (68). In one study, rhBMP-2 was adsorbed to either amorphous glass mica ceramic or a ceramic with nano-pores. Both substrates were amino- or epoxy- functionalized using silane linkers. Amino-functionalized surfaces adsorbed more rhBMP-2 than epoxy functionalized surfaces: up to a concentration of 96 ng/cm2 rhBMP-2. This concentration was significantly higher than the 67 ng/cm2 bound to unstructured silica ceramic. However, it was unclear whether rhBMP-2 binding to these substrates was mediated through electrostatic interactions with the amino-group or hydrophobic interactions with the alkyl moieties of the linker molecule (69). In one recent study rhBMP-2 adsorbed with high affinity to propyl- and hexyl- alkyl groups presented on titanium surfaces, but not in hydrogel materials (70). Separately, chemical vapor deposition (CVD) techniques have been used to form nano-crystalline diamond surfaces with hydrogen- and oxygen- terminal groups. In these studies oxygen-terminated surfaces interacted with rhBMP-2 with higher affinity than hydrogen terminated surfaces. This observation could be attributed to the geometry of rhBMP-2 bound to the surface, hydrogen bonding, and increased van-der-Waals interactions. The interaction between rhBMP-2 and these surfaces had a binding constant near to K = 109 which was similar to the affinity of specific receptor-ligand interactions (71). CVD techniques might be broadly applicable during scaffold fabrication processes due their ability to functionalize complex 3-D architectures (72). CVD was used to explore the role surface hydrophobicity on rhBMP-2 adsorption by forming nanostructured surfaces from vertically aligned carbon nanotube arrays that were either superhydrophilic or superhydrophobic using plasma-enhanced CVD. The rhBMP-2 solutions spread on the superhydrophilic surfaces which enabled interaction with the material. Alternatively, the superhydrophobic surfaces limited rhBMP-2 molecules from interacting with the nanotubes by limiting the surface area of the fluid on the substrate (73). These results indicated that maximizing the surface area of scaffolds increased growth factor binding through a combination of non-covalent mechanisms.
2.2 Incorporation into sponge and hydrogel scaffolds
Adsorbing growth factors into dehydrated hydrophilic polymer networks has been the most commonly used approach to incorporate growth factors into scaffolds. Therefore, understanding the mechanisms that control this incorporation is critical to instruct the design of future biomaterial scaffolds for bone healing applications (74-76). Collagen sponges were identified as a potential scaffold carrier for rhBMP-2 because of collagen's prevalence in natural bone and its high rhBMP-2 binding capacity. Collagen is composed of 3 helix-forming polypeptide chains with a combined molecular weight of approximately 300kDa. Absorbable collagen sponges used for rhBMP-2 delivery were initially developed for hemostatic applications. The sponges have been produced by freeze drying a dispersion of bovine Achilles tendon collagen and then crosslinking and sterilizing the sponge using a chemical protocol (26). Collagen sponges have been reconstituted to form hydrogels which have measured rhBMP-2 loading efficiencies of up to 0.135mg rhBMP-2/ml collagen scaffold after extensive mechanical manipulation (26). These high binding efficiencies have enabled their use in bone fusion and fracture repair that required supraphysiological rhBMP-2 concentrations.
Many factors have been implicated in modulating the incorporation efficiency of rhBMP-2 into collagen hydrogels. Increasing the collagen concentration in the hydrogels, increasing the pI of rhBMP-2, decreasing the degree of formaldehyde induced crosslinking, increasing the incubation time prior to implantation, increasing the NaCl concentration to 20mM, neutral pH conditions, and decreasing the concentration of rhBMP-2 during adsorption has increased rhBMP-2′s incorporation efficiency into collagen-based scaffolds. Sterilizing the collagen hydrogels with ethylene oxide gas decreased the binding efficiency of hydrogels. Also, if the pH or ionic strength is increased too high then rhBMP-2 has precipitated rather than undergone incorporation into collagen scaffolds. rhBMP-2 precipitated when the chloride concentration was raised above 20mM and the pH was adjusted to be greater than 6.45 (26). This result could be significant because many growth factor release studies have been conducted in pH 7.4 phosphate buffered saline (PBS) which contains greater than 100mM NaCl (77). Critically, collagen sponges have measured binding capacities of less than 0.01 mg rhBMP-2/mg collagen at pH 3 and 4, 0.02 mg rhBMP-2/mg collagen at pH 4.5, 0.1mg rhBMP-2/mg collagen at pH 5.2, and 0.18 mg/mg at pH 6.5 (26). Physiological pH in blood plasma has a pH of 7.3–7.5 and fracture healing sites have a slightly more acidic pH of 7.2 during the early stages of fracture healing which could influence protein precipitation (78). Solutions applied to collagen sponges have undergone rapid evaporation because of their high surface area. For example, 5% of the solution has been measured to evaporate over the course of 15 minutes and 27% of the solution evaporated over the course of 120 minutes which could increase the local rhBMP-2 concentration and lead to precipitation (79). From these studies it is clear that many of the variables that controlled rhBMP-2 in solution also control its incorporation into collagen sponges and therefore could extend to other hydrophilic materials (Figure 3).
Figure 3.
Schematic representation of BMP-2 incorporation into a dried polymer network that swells to form a hydrogel and properties that generally increase the mass of BMP-2 incorporated into the hydrogel network (PDB: 3BMP).
The mechanisms that have been have used to control growth factor incorporation into collagen-based scaffolds have extended to other hydrogels. Hydrogels based on gelatin (80), poly(lactide ethylene oxide fumarate) (81), fibrin (82), and dextran (83) among others have been used to deliver growth factors. Gelatin has been commonly used as hydrogel carrier for growth factors because it is derived from the hydrolysis of collagen, the most abundant protein in bone. Gelatin contains a partially reformed triple-helix structure of collagen and forms hydrogel networks when reconstituted at 35°C. Although rhBMP-2 has a positive charge at physiologic pH, it has a stronger binding affinity for basic gelatin as compared to acidic gelatin (84). This is the opposite trend observed for TGF-β1 which would not adsorb onto similarly charged basic hydrogels (80). Specific intermolecular interaction studies demonstrated that rhBMP-2 has increasing binding affinities for acidic gelatin, basic gelatin, and heparin, respectively. The weakness of interaction between rhBMP-2 and gelatin has indicated that binding was a modulated physiochemical forces instead of ionic binding (85). Therefore, it is possible that rhBMP-2 interacts with basic gelatin primarily through non-covalent interactions where electrostatic attraction plays a minor role.
Non-covalent mechanisms have also been used to incorporate growth factors into dynamic hydrogels that possess greater temporal control over growth factor release than static hydrogel networks. For example, we have developed mechanisms to form dynamic protein-based hydrogels that have been processed into disks and microspheres. These dynamic hydrogels have been used to trigger the release of growth factors in response to specific biochemical ligands (50, 86-91). In these studies we demonstrated that increasing the concentration of negatively charged calmodulin in dynamic hydrogel disks increased the mass of VEGF adsorbed into hydrogel networks (86). Also, when rhBMP-2 (pI =8.5-9.2) or VEGF (pI = 8.5) were incubated in the presence of calmodulin-based microspheres more rhBMP-2 was adsorbed into the hydrogel networks which suggested that electrostatic attraction was a key regulator of the interaction between growth factors and non-collagen based protein hydrogels (50). Together these results suggested that the affinity of rhBMP-2 for different hydrophilic polymers could be tuned by including molecules that bind to rhBMP-2 with varying combinations of different non-covalent interactions.
Combinations of non-covalent interactions are used in the body to sequester growth factors in the extracellular matrix with high affinity. Researchers have harnessed these interactions to develop scaffolds that sequestered growth factors through high-affinity non-covalent interactions (92). For example, scaffolds that contained the proteoglycan heparin have bound rhBMP-2 with high affinity through the growth factor's N-terminal heparin binding domain (22, 93, 94). In one approach, hyaluronic acid-based hydrogels with pendant heparin groups were formed to sequester rhBMP-2. Hyaluronic acid-heparin hydrogel microspheres adsorbed almost twice as much rhBMP-2 compared to unmodified hyaluronic acid hydrogel microspheres (95). In a separate approach, hyaluronic acid was covalently modified with the proteoglycan perlecan. Perlecan has been demonstrated to bind BMP-2, localize in cartilage, and has mediated endochondral ossification. Perlecan modified microspheres also adsorbed almost twice as much rhBMP-2 compared to unmodified microspheres (96). To sequester endogenous heparin and growth factors, Hudalla and colleagues formed biomaterials that presented a heparin-binding peptide. These growth factor sequestering surfaces enhanced the osteogenic differentiation of human mesenchymal stem cells without the addition of exogenous growth factors (97). Similar high throughput biomaterial surfaces have been engineered to screen for peptides that specifically interact with growth factors and stem cells (98). This approach of sequestering endogenous growth factors could accelerate the clinical applicability of growth factor mediated-bone healing therapies due to the high upfront cost of growth factors (99). Heparin mimetic materials have been formed by adding sulfate groups to hyaluronic acid. In these studies, sulfated hyaluronic acid bound BMP-4 with a significantly increased affinity compared to unmodified hyaluronic acid (100). These studies highlight the broad applicability of using well-characterized intermolecular interactions to incorporate growth factors into scaffolds. Future work will be necessary to characterize the selectivity of these approaches in vivo as proteoglycans bind multiple proteins with varying affinities (101).
The non-covalent intermolecular interactions that mediate the formation of helical coiled-coils and β-sheets have been harnessed to incorporate growth factors into hydrogels. Coiled-coil peptides used to incorporate growth factors into scaffolds have been derived from collagen (102) and leucine zipper peptide domains (103). The stability of these interactions has been tuned by varying the temperature, ionic strength, and pH surrounding the hydrogel (104). In one illustrative example, a model protein was engineered with a pair of peptides at the N- and C-termini that formed coiled-coil helices with complementary peptides immobilized to the hydrogel network. When the engineered protein was added to the media bathing the hydrogel, the coiled-coil interactions enabled a specific protein incorporation efficiency of 30% (105). β-sheet forming peptides have also been used to form uniquely stable non-covalent bonds in hydrogel networks. β-sheet forming peptides have formed from cooperative non-covalent interactions containing alternating cationic, hydrophobic, and anionic amino acid residues. Hydrogels formed from β-sheets have maintained their network structure even after exposure to high temperatures, high salt concentrations, and denaturing chemicals (106). Fusion proteins of growth factors with the RAD16 β-sheet forming peptide have also been formed to incorporate growth factors into hydrogel networks including the growth factor stromal cell derived factor-1 (SCF-1). RAD16-SCF-1 incorporated into RAD16-based hydrogels with 8 fold greater efficiency than unmodified SCF-1 and maintained its original loading efficiency after 32 washes. Although these specific intermolecular interactions have not yet been used to deliver growth factors in the clinic they have great potential due to their specific intermolecular interactions.
2.3 Incorporation into composite scaffolds
Composite materials that combine the osteoinductive properties of ceramics with the high growth factor binding properties of hydrogels have been explored as scaffolds for bone healing applications. In composite materials, increasing the material surface area before adding the growth factor solution and increasing the mineral phase concentration have enhanced the incorporation of growth factors into scaffolds. In one illustrative study, composite chitosan-calcium phosphate microsphere-based scaffolds were formed using a co-precipitation approach. Lyophilizing the materials increased the surface area of the microspheres 200 fold and the surface area of the scaffolds 4 fold compared to materials that were air-dried. This increased surface area translated to increased rhBMP-2 loading efficiencies. Composite microspheres and composite scaffolds had 200% and 40% greater growth factor loading capacities over air dried materials, respectively. Also, composite materials had a greater rhBMP-2 binding capacity than chitosan-based materials without a ceramic component (107). Similar composite materials have been explored in clinical trials for rhBMP-2 mediated spine fusion (108). This composite material was formed from hydroxyapatite:β-tricalcium phosphate biphasic ceramic particles suspended in a collagen hydrogel. When composite collagen/ceramic particle composite scaffolds were exposed to 1.05ml of 3mg/ml rhBMP-2 they adsorbed 96.8±10.5% of the total rhBMP-2 which was statistically greater than the fraction adsorbed into ceramic-free collagen hydrogels (51). These materials have the added advantage of being compression resistant for load-bearing applications and were easier to handle for users (67).
3. From growth factor incorporation to release in vitro
It is evident that the physiochemical properties of growth factors, scaffolds, and their environment all contribute to the non-covalent incorporation of growth factor into scaffolds. How non-covalent interactions also control growth factor release in vitro will be discussed, with a focus on materials that have been translated to in vivo applications. Understanding these mechanisms will be crucial for the development of future growth factor delivery applications as in vitro tests are an important component of the preclinical development process.
3.1 Disruption of non-covalent bonds in vitro
Growth factors have been released from scaffolds when they: 1) desorbed from the scaffold, 2) when they remained bound to scaffolding material that degraded, or 3) failed to interact with the scaffold. Growth factor release from scaffolds has then proceeded via diffusion, convective fluid flow, or a combination of flow regimes. Desorption is the opposite process of adsorption, in which growth factors detach and release from the scaffolds. Protein desorption has occurred over much longer time frames compared to adsorption unless the protein has been perturbed by an environmental change (109). Adsorption has been generally thought of as an irreversible process because of the number of non-covalent bonds that need to be disrupted for a protein to be released from a surface. The kinetics of growth factor release from scaffolds in vitro has been controlled by the types of non-covalent interactions between the material and growth factor, the scaffold's environment, and scaffold degradation. Even in a single material scaffold, a combination of non-covalent interactions have controlled rhBMP-2 release because many materials interact with growth factors through electrostatic attraction, hydrophobic interactions, and other non-covalent interactions (110). Protein diffusivity in hydrogel networks has been controlled by the hydrogel mesh size. The mesh size of hydrogel networks has been tuned by varying the polymer concentration in the hydrogel network, the crosslinking density, and the solubility of the polymer chains (111). Protein transport in solid scaffolds has been controlled by the scaffold permeability which has been directly related to pore size and scaffold porosity (112). It is clear from these studies that varying a single material parameter, like polymer concentration, can effect both protein desorption and diffusion in scaffolds. Therefore, it has been a challenge to delineate all of the factors that control growth factor release from scaffolds.
Growth factor release from collagen-based scaffolds that have been used with rhBMP-2 has proceeded in 3 distinct phases: 1) An initial burst release which has been followed by, 2) some period of sustained release, and in some scaffolds 3) a period in which no growth factor is released at all. The initial burst phase of protein release could be attributed to protein that does not interact with the scaffold or surface bound protein that rapidly diffuses away (113). Minimizing the initial burst release could be of significance as some of the serious side effects of rhBMP-2 treatment including inflammatory and osteoclastic activity have occurred early after implantation (114, 115). The sustained release period has been implicated in the recruitment of stem cells to the bone healing site (116). There has been debate over whether surface bound BMP-2 can promote osteoblast differentiation. For example, mutant BMP-2 molecules that could reversibly bind to titanium surfaces were able to induce osteogenic gene expression, whereas mutant BMP-2 engineered irreversibly bind to titanium did not induce osteogenic gene expression in C2C12 cells (117). However, numerous studies have demonstrated that irreversibly adsorbed or covalently immobilized native rhBMP-2 was able to induce osteogenic gene expression (69). Each phase could have a significant effect on bone healing and therefore it is important to understand the mechanisms that control growth factor release for the development of future bone healing scaffolds. Significantly, scientists have developed materials that have eliminated the burst release (118) or delayed the burst release for a period of time (86). These materials could be used to delineate how the 3 phases of growth factor release observed in currently clinically used scaffolds modulate bone healing.
3.1.1 Disruption of electrostatic interactions in vitro
Increasing the magnitude of the electrostatic interactions between growth factors and the scaffold has decreased the initial burst release. During an illustrative study, bovine serum albumin (BSA) (pI = 5.8) released significantly faster than VEGF (pI = 8.5), which released significantly faster than BMP-2 (pI = 8.5-9.2) from negatively charged hyaluronic acid hydrogels (20). These results suggested that increasing the electrostatic attraction between proteins and the scaffold could increase their retention in vitro. This trend could be significant for bone healing applications because CHO-derived rhBMP-2 has 3 different isoforms which could affect the protein charge and release from scaffolds (21). A common technique that has been used to increase the electrostatic attraction between growth factors and scaffolds has been to increase the concentration of charged polymers in hydrogel networks. This approach has been used to increase growth factor retention in collagen (119) and calmodulin (86) hydrogel networks. This could be an attractive approach to limiting growth factor release as the concentration of polymer incorporated into hydrogel networks has restricted growth factor diffusion out of hydrogel networks. However, increasing polymer concentrations have produced solutions with high viscosities and have limited the processing of these materials. Alternatively, rhBMP-2 has undergone rapid release when incorporated into bioinert scaffolds like poly(ethylene glycol) (PEG) (120), poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) hydrogels (PEO-PPO-PEO or Pluronics) (121), and alginate hydrogels (Figure 4) (122). These studies demonstrated that increasing the electrostatic attraction between growth factors and scaffolds plays a key role in controlling the burst release from scaffolds in vitro and motivates the need for specific intermolecular interactions for the inhibition of the burst release.
Figure 4.

Results of studies that characterized how electrostatic attraction has controlled protein release from hydrogels. A) Increasing the electrostatic attraction between proteins with different isoelectric points (pI) and hyaluronic acid hydrogels decreased the protein release rate (20) with permission. B) Increasing the collagen concentration in hydrogel networks increased the interactions with a model protein, interferon (IFN), and increased its in vitro retention (119) with permission. C) Protein release from uncharged polymer networks including PEG networks formed from PEG-diacrylate (PEGDA) has proceeded rapidly in vitro (120) with permission.
Electrostatic attraction between scaffolds and rhBMP-2 has been enhanced by harnessing specific intermolecular interactions to limit the burst release of growth factors (94, 123). Incorporating heparin in hyaluronic acid hydrogel microspheres has significantly increased rhBMP-2 retention in a concentration-dependent manner. Specifically, unmodified hyaluronic acid microspheres, microspheres with a low concentration of heparin, and microspheres with a high heparin concentration released 15%, 5%, and less than 5% of their rhBMP-2 over 24 hours in PBS (95). Similar results have been observed when perlecan was covalently immobilized in hyaluronic acid-based hydrogel microspheres. In these studies, unmodified hyaluronic acid microspheres released 43% of their rhBMP-2 in 24 hours, whereas perlecan-modified hyaluronic acid microspheres released only 10% in the first 24 hours (123). In a separate approach including heparin-functionalized PLG microspheres decreased the initial burst release of rhBMP-2 from fibrin hydrogels to less than 10% of the total in PBS (94). Alternatively, collagen sponge-based scaffolds have released 35% of their encapsulated rhBMP-2 in 24 hours in vitro (39). Together, these studies have suggested that maximizing attraction between rhBMP-2 and scaffolds with specific intermolecular interactions may be more effective at minimizing the burst release of growth factors from hydrogels when compared to non-specific electrostatic interactions. These studies also suggest that including components of growth factors' natural environment may be able to better model growth factor transport in vivo.
Including components of the in vivo milieu during in vitro studies has increased the release rate of growth factors from scaffolds. For example, gelatin scaffolds retained 92% of their rhBMP-2 after 24 hours in PBS with 1% BSA (124) and only 65% in protein-free simulated body fluid (39). Similar observations have been made for rhBMP-2 release from calcium phosphate carriers in vitro. For example, rhBMP-2 release from calcium phosphate scaffolds was increased when performed in a complex biologic fluid (fetal calf serum) in comparison to release in a simple buffered solution (PBS). Specifically, calcium-phosphate scaffolds released 22±2% during the first 24 hours of release in serum and 6±2% in PBS (57). This observation could be significant as interactions of rhBMP-2 in a scaffold with a hematoma could influence growth factor release (125). During the course of wound healing and bone formation, scaffolds have been exposed to different pH conditions, molecules, and cells (78) which motivates the need for the development of high throughput systems to better mimic growth factor release in vivo. Several platforms could be used to screen for environmental conditions that control growth factor release including high-throughput hydrogel arrays (126-128) and microfluidic platforms (129). Furthermore, the general mechanisms derived from these studies could be applied to other important macromolecules including therapeutic nucleic acids and viruses (130).
Although many environmental factors could affect protein release in vivo, the most commonly included component in vitro has been other proteins. In a representative study, albumin-treated calcium-phosphate scaffolds released 30±1% and untreated scaffolds released 22±2% of their rhBMP-2 during the first 24 hours of release in serum. Alternatively, after 24 hours in PBS only 4±2% rhBMP-2 was released from albumin treated scaffolds and 4±1% rhBMP-2 was released from untreated scaffolds. After the first day of release in serum both treated and untreated scaffolds released rhBMP-2 at similar release rates with a residence half-life of 6±1 days for treated scaffolds and 7±1 days for untreated scaffolds. Alternatively, 11±5% rhBMP-2 was released from albumin treated scaffolds and 6±2% rhBMP-2 was released from untreated scaffolds. These results suggested that the albumin treatment may have blocked some of the high affinity binding sites for rhBMP-2 on the calcium phosphate scaffolds and that the complex mixture of charged proteins and peptides significantly increased the rhBMP-2 release rate from calcium phosphate-based scaffolds (57). Similar observations have been made for rhBMP-2 release from collagen sponge carriers in vitro. For example, 92% of rhBMP-2 was released after 1 day in PBS+ 1% BSA (124) and only 35% in protein free simulated body fluid (39). There are many proteins in the blood besides albumin and the profile of molecules present in the scaffold could change over the course of bone healing. Therefore, future in vitro tests could incorporate known components that change over time to better mimic the in vivo environment.
3.1.2 Disruption of hydrophobic interactions in vitro
Hydrophobic interactions have been an important mechanism in controlling rhBMP-2 retention in scaffolds after the initial burst period. Hydrophobic alkyl groups have mediated long term retention of rhBMP-2 on biomaterial surfaces in several studies (69, 70). In one study, equivalent masses of rhBMP-2 were adsorbed from solution to titanium surfaces functionalized with hydrophobic molecules including n-propyl trichlorosilane (PTC), 3-aminopropyl triethoxysilane (APS), and n-propyl trimethoxysilane (PTM). This result indicated that the positive charge on APS was not necessary for rhBMP-2 adsorption (70). Also, including hydrophobic polymer segments in hydrophilic polymer networks has increased the long term retention of rhBMP-2 after the initial burst release. In one study, including rhBMP-2 in a 24% PEO-PPO-PEO hydrogel dispersed in the pores of poly(propylene fumarate) (PPF) scaffolds initially decreased the retention of rhBMP-2 compared to gelatin microparticles. However, after the initial burst period there was little rhBMP-2 released over the course of the experiment (121). The stable incorporation could be attributed to hydrophobic interactions between rhBMP-2 and the hydrophobic PPO components of the PEO-PPO-PEO block-copolymer (131). These results suggested that varying hydrophobic and electrostatic interactions in hydrogel networks may allow for the tuned release of rhBMP-2 from hydrogels during different release phases.
One approach to vary rhBMP-2 release could be to form hydrogels from combinations of hydrophobic and hydrophilic polymers. Poly(N-isopropylacrylamide) (PNIPAAm)-based polymers have been used to study hydrophobic interactions with growth factors in hydrogel networks due to their temperature-induced phase transition from soluble to insoluble. To evaluate the role of hydrophobic interactions on rhBMP-2 release from scaffolds, PNIPAAm, a PNIPAAm/ethyl methacrylate copolymer (PNIPAAm/EMA), and an amine-reactive PNIPAA/N-acryloxysuccinimide copolymer (PNIPAAm/NASI) were synthesized and formed into hydrogel networks independently or as interpenetrating networks in collagen sponges. rhBMP-2 retention in PNIPAAm-based materials was initially greater than in collagen sponges but eventually released as much or more rhBMP-2 over the course of the 7 day experiment. The reaction between amines on rhBMP-2 and PNIPAAm/NASI resulted in covalent tethering of the growth factor into the hydrogel. Therefore, release from these networks could be attributed to bulk degradation of the hydrogel (39). In the absence of PNIPAAm-based polymers approximately 50% of the adsorbed rhBMP-2 was released from the collagen sponges after 3 days of release. Adding PNIPAAm-based polymers decreased the retention 8-15% after 3 days of release and the subsequent release profiles were similar to release from collagen sponges. These results could be attributed to the decreased mesh size of the collagen network in the presence of insoluble PNIPAAm polymer chains which could inhibit rhBMP-2 diffusion into the network, increase the fraction of surface bound proteins, and increased the initial burst release. Alternatively, this observation could be attributed to the decreased concentration of hydrophobic groups when the polymer was dispersed in a collagen network. Similar results were observed when APS groups, which significantly increased growth factor retention on titanium substrates, were linked into hydrogel networks and lost their ability to sequester rhBMP-2 (39). These polymers have supported the osteoinductive activity of rhBMP-2 in rats (132) and there have been many different techniques to form PNIPAAm-block copolymers with unique properties (133). Therefore, this material system could be used as a broadly applicable tool to understand the role of hydrophobic interactions in controlling growth factor release in vivo.
Hydrophobic interactions may also play an important role in controlling growth factor release from gelatin scaffolds in vitro. This conclusion has been motivated by results of in vitro experiments that have run counter to expected results if electrostatic forces were the primary mechanism that controlled release in gelatin networks. When rhBMP-2 was incorporated into gelatin hydrogel disks 60-75% of the encapsulated rhBMP-2 was initially released from both acidic and basic gelatin hydrogels. However, more rhBMP-2 was maintained in the basic hydrogels compared to the acidic hydrogels over the course of the experiment (134). rhBMP-2 release would be expected to be faster from basic hydrogels due to electrostatic repulsion. In a related study, rhBMP-2 release was characterized from acidic and basic gelatin microspheres with varying degrees of crosslinking and in different environments. rhBMP-2 retention was increased by increasing the degree of crosslinking, increasing the rhBMP-2 dose, and using basic gelatin (121). Previous research has demonstrated that increasing the degree of glutaraldehyde crosslinking has increased the surface hydrophobicity of gelatin hydrogels which could increase the hydrophobic interactions between the hydrogel and rhBMP-2 (135). However, none of these parameters modulated the fraction of rhBMP-2 retained after 24 hours in this study, which was greater than 90% (Figure 5) (121). The slow rhBMP-2 release from basic gelatin hydrogels observed in this study could also be attributed to hydrophobic interactions between rhBMP-2 molecules as the loading procedure was conducted in PBS at pH 7.4 which has induced rhBMP-2 precipitation (26). These results suggested that native collagen and denatured gelatin interact with rhBMP-2 through different mechanisms in vitro which could enable their use in different indications that require distinct release kinetics.
Figure 5.
Results of studies that compared rhBMP-2 release from acidic and basic gelatin hydrogels in vitro. A) rhBMP-2 release was faster from acidic gelatin hydrogel disks compared to basic gelatin hydrogel disks in PBS (134) with permission. rhBMP-2 release was also faster from acidic gelatin hydrogel microparticles (B) crosslinked with 10 or 40mM glutaraldehyde at low or high rhBMP-2 doses compared to basic gelatin hydrogel microparticles (C) (121) with permission.
3.2 Degradation-mediated release in vitro
Scaffold degradation has been an important mechanism in controlling release from scaffolds that strongly interact with growth factors. Generally, growth factor release from these scaffolds has been related to the rate of material degradation after the initial burst release period. For example, growth factor release from PEG-low molecular weight heparin hydrogels mirrored both the original rapid degradation of the material but released 10% less growth factor than the fractional mass of hydrogel that degraded over the course of the experiment (136). Degradation also has been a key mechanism in controlling rhBMP-2 retention in gelatin hydrogel networks. rhBMP-2 retention was significantly decreased in lightly crosslinked basic gelatin microspheres when experiments were performed in collagenase buffer. Similarly, acidic gelatin microspheres degraded rapidly and released all of their rhBMP-2. Although rhBMP-2 release from basic gelatin microspheres in collagenase buffer was slower than from acidic microspheres in the presence of collagenase, the release rate was still significantly faster than release in collagenase-free PBS. Increasing the glutaraldehyde crosslinking inhibited the ability of collagenase to degrade acidic or basic microspheres (121). In a separate material system the addition of collagenase had no significant effect on release from PPF/Pluronics scaffolds which indicated the specificity of using collagenase as a mimic of the in vivo degradation of collagen-based materials (121). The stability of native collagen has been dramatically increased by forming synthetic mimics in which the hydroxy-l-proline residues were replaced with 4(R)-fluoro-l-proline (Flp) residues (137). Also, all D stereoisomer collagen IV mimetic peptides have been formed which maintain their biological function and have been resistant to proteolysis (138). These materials could be processed into collagen-based scaffolds with enhanced stability for controlled growth factor delivery applications.
Diverse proteases have been used to direct growth factor release from biomaterial scaffolds in preclinical studies. For example, matrix metalloproteinase (MMP) cleavable peptide sequences have been covalently incorporated into hydrogel networks that enabled cell-mediated control of material degradation (139) or tethered protein release (140). Also, VEGF has been tethered into hydrogel matrices using plasmin-degradable peptide sequences (141). Protease concentration, type, and inhibitors vary over time at different bone healing sites. Furthermore, protease expression profiles deviate between patients with normal, delayed, or non-union fractures (142). Understanding the temporal expression patterns of these proteases in different bone regeneration sites could enable the design of injury-specific growth factors carriers for bone healing applications.
3.3 Convective fluid flow-mediated release in vitro
Convective fluid flow has been a key mediator of growth factor release from hydrogel networks. Mooney and colleagues demonstrated that mechanically loading alginate hydrogels induced fluid flow and triggered the release of encapsulated VEGF in vitro and in mice. In these studies increasing the strain amplitude also increased the mass of growth factor released from the hydrogel network over the course of 6 loading cycles (Figure 6A) (143). Similar mechanisms have also controlled rhBMP-2 release from collagen sponges. In one clinically relevant study, 1.4ml of 1.5mg/ml rhBMP-2 was adsorbed on a 4.4ml collagen sponge for either 15 minutes or 2 hours. The sponges were mechanically compressed to release either: 1)100μl -250μl, 2) 300μl-650μl, or 3) 750μl -1100μl of fluid. Condition 1 best mimicked the compression that has been observed during intraoperative procedures during which the rhBMP-2 laden collagen sponge has been placed in a titanium cage for spine fusion applications. After 15 minutes of adsorption this loading condition induced the release of 0.44±0.12 mg/ml rhBMP-2 which represented 4.9% of the total encapsulated rhBMP-2. Increasing the adsorption time to 120 minutes significantly decreased the fraction of rhBMP-2 released to 2.9% of the total (79). This time dependence could have significant consequences for in vivo treatments, as adsorption times reviewed in this study varied from 5 minutes (51) to overnight (80). Increasing the magnitude of compression also increased the difference in rhBMP-2 retention between the 15 minute and 120 minute adsorption protocols (79). This finding highlights the need to increase the rhBMP-2 adsorption period for applications which require larger compressions of hydrogels like during insertion into proximal femoral core defects (144).
Figure 6.
Influence of convective fluid flow on growth factor release in vitro. A) Mechanical strain (top) triggered the release of VEGF from alginate hydrogels over the course of 6 loading cycles (bottom) (143) with permission. B) Schematic representation of dynamic hydrogels in which specific biochemical ligands induced protein-conformational changes, hydrogel volume decreases, and growth factor release (87) with permission. C) Temporal control over BMP-2 release from dynamic hydrogel networks was controlled by varying the timing that hydrogels underwent trifluoperazine (TFP)-induced volume decreases (50) with permission.
Convective fluid flow has also been a critical regulator of rhBMP-2 release from composite scaffolds even though they have been more resistant to compression than collagen sponges. For example, when 2.5ml of 4.0mg/ml rhBMP-2 was adsorbed into 5ml of collagen-ceramic particle scaffolds for 5 or 60 minutes, growth factor release could be induced by mechanical loading from compression during centrifuging or in syringes. 82.19% and 89.96% were retained in the scaffolds after 5 and 60 minute adsorption times, respectively after compression in a syringe to half their initial volumes (79). In comparison, when collagen sponges were compressed with a similar loading regiment they retained 72% and 88% their incorporated rhBMP-2 after 15 and 120 minute soak times, respectively (26). After centrifugation at 1500rpm for 5 minutes, 36.09% and 56.95% of the adsorbed rhBMP-2 was retained after soaking for 5 and 60 minutes, respectively (79). These techniques could be applied in future studies to decrease the initial burst release from scaffolds for procedures with increasing mechanical manipulation. Also, it would be interesting to analyze the isoforms of rhBMP-2 released after mechanical manipulation to observe if a fraction of the rhBMP-2 loaded into composite scaffolds is preferentially retained.
Convective fluid flow has also controlled growth factor release from dynamic hydrogels. We have adsorbed rhBMP-2 into dynamic scaffolds based on calmodulin's nanometer scale protein conformational change. These rhBMP-2 laded microspheres underwent specific biochemical ligand-induced volume decreases of 76 ± 10% which induced fluid flow and triggered the release of 79.5 ± 3.0% of their encapsulated rhBMP-2 (50). The mass of triggered growth factor release from these materials was increased when they underwent larger magnitude volume changes (86). Similarly, macroporous ferrogels have undergone 70% decreases in volume when exposed to magnetic fields which triggered the release of encapsulated growth factors (Figure 6B-C) (145). In contrast to collagen, alginate, and calmodulin hydrogels which remain hydrophilic when they decrease in size, polymers like PNIPAAm undergo phase transitions from soluble to insoluble which restricts protein release through hydrophobic interactions (146). Although these dynamic materials have yet to be extensively explored in the clinic they hold great promise for applications that require temporal control over growth factor release.
5. From growth factor release in vitro to release in vivo
Non-covalent interactions have been used as a broadly applicable mechanism to incorporate growth factors into scaffolds and modulate their release in vitro. In this section we will discuss the mechanisms that modulate growth factor release in vivo. Special attention will be given to differences between release in vitro and in vivo.
4.1 Disruption of non-covalent bonds in vivo
4.1.1 Differences between release in vitro and in vivo
rhBMP-2 release has been faster in vivo than in vitro from different scaffolds with diverse physical properties. This general trend has been observed during release from collagen sponges (21, 39), gelatin hydrogels (42, 121, 147), calcium phosphate cement scaffolds (57), pNIPAAm-based scaffolds (39), PPF scaffolds, and PPF-gelatin composite scaffolds (121). As an example, 91% of the adsorbed rhBMP-2 was retained in gelatin hydrogels vitro (121)whereas 15% was retained in vivo after 7 days (147). These results could be attributed to the complexity of in vivo environments compared to the relative simplicity of in vitro release environments. After implantation biomaterials are covered in endogenous molecules through mechanisms first described by Vroman (148). The in vivo bone regenerating environment has been characterized to contain different cell types including osteoclasts that degrade ceramic scaffolds and secrete proteases that degrade polymer scaffolds. Also, the mechanical environment for each bone healing or fusion approach will vary for different indications and these loading profiles have yet to be explored for rhBMP-2 release in vitro. Furthermore, patients have different tissue architectures with different comorbidities which could affect growth factor diffusion away from scaffolds (149). Although these components have been incorporated into in vitro growth factor release studies they have not yet been combinatorially screened to find conditions that mimic in vitro environments for specific indications. Examining the mechanisms by which non-covalent interactions have controlled growth factor release in vivo will aide in the design of future in vitro test platforms and scaffolds with designed growth factor delivery properties.
4.1.2 Disruption of electrostatic interactions in vivo
Varying the degree of electrostatic attraction between growth factors and scaffolds has tuned growth factor release in vivo. One approach to changing the degree of electrostatic attraction has been to covalently modify the charged amino acid residues of growth factors. In an illustrative study, rhBMP-6 and rhBMP-2 which had similar pIs were released from scaffolds at similar rates. E. coli derived- rhBMP-2 contained the most basic N-terminal amino acids and was retained at similar or greater rates than CHO derived rhBMP-2 in both collagen and ceramic scaffolds. However, if rhBMP-2′s positively charged epsilon- amine groups were acetylated or succinylated only 0.2-2.1% was maintained in any scaffold after 3 hours of implantation compared to 13-43% for CHO derived BMP-2. Also, rhBMP-4, which lacked the basic amino acid containing N-terminal region of rhBMP-2, was released at a much faster rate than CHO derived- or E. coli derived- rhBMP-2 (21). There has been extensive research on creating engineered BMPs that overcome many of the current limitations of natural molecules that have identified how the proteins can be improved without decreasing their bioactivity (150). The same recombinant engineering techniques could be used to tune the pI of growth factors and control their release from charged scaffolds in vivo.
Increasing the surface area of negatively charged ceramic scaffolds has, in some experiments, increased the initial retention of growth factors. For example, hydroxyapatite scaffolds with a surface area of 1m2/g retained 34% of their adsorbed rhBMP-2 after 24 hours, whereas β-tricalcium phosphate scaffolds with a surface area of 4m2/g retained 49.6% of their adsorbed rhBMP-2. After this initial burst release their release rates were similar in a mouse subcutaneous site (151). Alternatively, among rat demineralized bone matrix, bovine hydroxyapatite particles, synthetic hydroxyapatite particles, tricalcium phosphate, delipidated bovine bone matrix, coral-derived hydroxyapatite, human demineralized bone matrix, human bone powder, human bone mineral, human irradiated bone chips there was no clear trend relating particle size, which would regulate the available surface area for rhBMP-2 binding, with release kinetics in rat subcutaneous sites (41). However, the second study used 4 times as concentrated rhBMP-2 solution and 20 times the initial rhBMP-2 dose during the adsorption period compared to the first hydroxyapatite/tricalcium phosphate study. Therefore, it is difficult to directly compare the results of these studies. These results motivate the need for further investigations on the role of scaffold surface area on growth factor release and standardized conditions for testing growth factor release from different materials during early preclinical studies.
Growth factor release from hydrogels has been regulated by their crosslinking density. For example, rhBMP-12 retention in hyaluronan paste, type I collagen sponges, type I/III collagen sponges, and hyaluronan sponges in rat muscle pouches varied significantly among the crosslinking density and carrier materials. Uncrosslinked hyaluronan paste released rhBMP-12 almost as rapidly as rhBMP-12 delivered in buffer alone. Alternatively, the crosslinked hyaluronan sponges retained greater than 80% and 35% the adsorbed rhBMP-12 after 1 and 7 days, respectively (152). Increasing the crosslinking density has also increased rhBMP-2 retention in basic gelatin hydrogels implanted subcutaneously in mice. For example, increasing the degree of glutaraldehyde mediated crosslinking in gelatin hydrogels increased rhBMP-2 retention from 15% to 45% of the initial rhBMP-2 after 7 days when implanted subcutaneously in mice (80). A similar trend has been observed for gelatin microspheres incorporated into PPF scaffolds as well (121). There are several different mechanisms to vary hydrogel crosslinking that have been optimized to maintain growth factor bioactivity and therefore this approach may provide a facile mechanism to vary rhBMP-2 release in vivo.
Electrostatic attraction has also played a key role in controlling growth factor release from hydrogel scaffolds in vivo. In one system, BMP-2 release had a half-life of 3.19 days in poly(ε -caprolactone)-alginate-RGD peptide scaffolds whereas BMP-2 had a half-life of 1.87 days in collagen scaffolds. The increased retention in poly(ε -caprolactone)-alginate-RGD peptide scaffolds could be attributed to increased electrostatic attraction between BMP-2 and alginate compared to collagen (116). Hydrogel network conditions can be readily varied by changing their initial polymer composition or processing conditions (153). Thus, hydrogels could represent a broadly adaptable material system for tuning growth factor release for specific indications.
rhBMP-2 retention in collagen scaffolds has been tuned by varying their electrostatic attraction and incorporating ceramic particles for load-bearing applications. In general, composite scaffolds that have contained both collagen and ceramic particles have released rhBMP-2 at similar rates to ceramic particles alone. For example, collagen-ceramic composite scaffolds released slightly less rhBMP-2 initially then released rhBMP-2 at similar rates compared to ceramic particles in a rabbit posterolateral spine fusion model over the course of 3 weeks. However, the mean resonance time between ceramics and ceramic/hydrogel composites was not significantly different (51). These results suggested that rhBMP-2 may preferentially adsorb to ceramic particles in composite scaffolds. Also, this increased retention could have physiological consequences as composite sponges containing collagen with corticocancellous chips or biphasic ceramic particles scaffolds produced significantly less ectopic bone formation in a canine cranial defect (Figure 7) (154). It is significant to note that rhBMP-2 released from collagen sponges has not been mediated by collagen sponge degradation in non-load bearing sites. In one study chemically crosslinked collagen sponges degraded over the course of 2 weeks whereas dehydroxythermally crosslinked collagen sponges degraded over the course of 1-2 months. However, rhBMP-2 release was similar from chemically and dehydroxythermally crosslinked collagen sponges after the initial 3 hour release period in rat subcutaneous sites (41). This result demonstrated that rhBMP-2 is released from collagen through desorption and diffusion in ectopic bone regeneration sites. Together, these studies motivate the need to measure the distribution of rhBMP-2 throughout collagen and composite scaffolds after adsorption.
Figure 7.
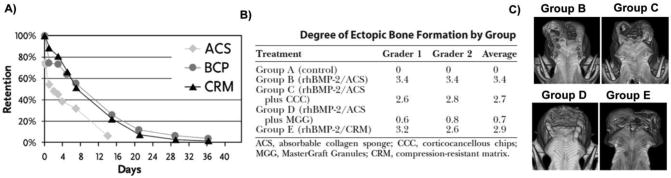
Comparison of rhBMP-2 release from absorbable collagen sponges (ACS), biphasic ceramic particles (BCP), or ACS-BCP compression resistant matrices (CRM) and its effect on ectopic bone formation. A) rhBMP-2 release has been similar from BCPs and ACS-BCP composite matrices. Alternatively, rhBMP-2 release has been faster from ACS-based materials than from BCP-containing materials (67) with permission. B-C) Decreasing the rhBMP-2 burst release by including ceramic materials with ACS also decreased ectopic bone formation in a canine cranial defect model as demonstrated by histological score and micro-computed tomography (154) with permission.
The rhBMP-2 concentration in scaffolds has the potential to modulate growth factor retention in bone healing sites. Typically μg/ml concentrations of rhBMP-2 have been included scaffolds during small animal studies and mg/ml rhBMP-2 concentrations have been employed for large animal studies. These high mg/ml concentrations could exceed the binding capacity of scaffolds and enable large burst releases of the growth factor. However, the rhBMP-2 concentration has had little effect on its retention in collagen sponges in vivo when analyzing clinically relevant concentrations in ectopic animal models. Collagen sponges exhibited similar retention when they were loaded with 0.08-2 mg/ml rhBMP-2 with approximately 70% of their encapsulated rhBMP-2 retained after 3 hours when implanted in a rat ectopic model. However, decreasing the concentration to 0.08mg/ml increased the mean residence time over the course of 2 weeks (41). In a separate study, the fraction of rhBMP-2 adsorbed into collagen hydrogels and maintained in an orthotopic rabbit site was also independent of the initial mass of rhBMP-2 (155). These results could suggest that a fraction of the rhBMP-2 has not interacted strongly with collagen sponges or is maintained in solution inside the material during the early phases of release due to their uniform fraction of burst release.
Ceramic scaffolds have generally released rhBMP-2 faster than collagen sponges during the initial stages of release. This general trend was observed when 0.4 mg/ml rhBMP-2 was adsorbed on different ceramic materials and implanted in a rat ectopic site. Synthetic ceramics, including bovine-derived hydroxyapatite particles, tricalcium phosphate, delipidated bovine bone matrix, and coral-derived hydroxyapatite retained 30-50% rhBMP-2 at the implant site after 3 hours of implantation except for the synthetic hydroxyapatite Osteograf/D® which retained only 11% of its initial rhBMP-2. Human bone-derived biomaterials, including demineralized bone matrix, thermomashed bone mineral, nondemineralized bone particles, and irradiated cancellous chips retained 18-27% of the implanted rhBMP-2 after 3 hours with significant variability among the materials during the entire release period (41). The decreased retention from human-derived materials could have been attributed to endogenous rhBMP-2 that was not removed from the materials during processing or the processing procedures modifying the human bone mineral so that it did not have as many binding sites.
Although ceramic scaffolds have released rhBMP-2 faster than collagen sponges during the initial stages of release they have generally retained a greater fraction of their rhBMP-2 after the burst phase. After the initial burst release period rhBMP-2 had a similar high retention ratio in ceramic materials except for the human bone mineral which had an increased half-life of 17.3 days. Significantly, ceramics retained greater than 20% of their rhBMP-2 after 21 days in subcutaneous rodent implant sites including calcium phosphate (57), β-tricalcium phosphate (151), and hydroxyapatite (151). Pure ceramic particle scaffolds retained 2 fold greater rhBMP-2 as compared to composite collagen-ceramic scaffolds after 21 days implantation (51). It is currently unclear how protein adsorption at implant sites has modulated rhBMP-2 release during orthopedic procedures. In one study, albumin pre-coating did not have a significant effect on rhBMP-2 retention on calcium-phosphate cement scaffolds in a rat subcutaneous implant site. Alternatively, pre-coating with albumin did affect rhBMP-2 release in vitro. After 28 days of implantation 27±5% and 19±5% of the implanted rhBMP-2 was retained in uncoated and coated disks, respectively (57). Significantly, this fraction of rhBMP-2 retained in the scaffolds was 2-3 fold lower than the fraction retained in serum or PBS in vitro (57). Although understanding how the body interfaces with scaffolds and controls growth factor release will be technically challenging, the lessons learned could be broadly applicable and applied to other therapeutically relevant biologic molecules including nucleic acids and therapeutic viruses.
4.1.3 Disruption of hydrophobic interactions in vivo
Hydrophobic interactions have controlled rhBMP-2 retention through different mechanisms when used in primarily hydrophobic constructs or when used in concert with collagen hydrogels. Low concentrations of PNIPAAm-based polymers, including polymers that conjugated with rhBMP-2, did not significantly change rhBMP-2 release kinetics when implanted with collagen sponges. Alternatively, when the polymer concentration was increased to 28.7 mg/ml there was no difference in retention after 1 day when rhBMP-2 was non-covalently incorporated into the scaffolds. Covalently immobilizing rhBMP-2 with PNIPAAm/NASI in collagen sponges did significantly increase rhBMP-2 retention at the implant sites after 5 and 9 days post implantation. These results suggested that high concentrations of hydrophobic polymers may be necessary to increase rhBMP-2 retention at implant sites when incorporated in collagen sponges. Directly injecting the hydrophobic polymers that formed hydrogels in situ resulted in clearer retention trends. Hydrophobic PNIPAAm and hydrophilic collagen sponges retained rhBMP-2 at the same rate in rat intramuscular pouches. Alternatively, PNIPAAm/EMA and PNIPAAm/NASI doubled the retention of rhBMP-2 after1day compared to collagen sponges. These materials retained 50% of their encapsulated rhBMP-2 over the course of the 9 day experiment which represented a 218-242 fold increase in rhBMP-2 retention compared to collagen sponges. Also, autoradiograms indicated that the blots with the greatest intensity were from the PNIPAAm/NASI and PNIPAAm/EMA after 1 and 5 days of implantation. The rhBMP-2 distribution in the muscle compartment spread out for all of the polymers except for PNIPAAm/NASI. The increased retention in these polymers could be attributed to the increased stability of PNIPAAm/EMA hydrogels compared to PNIPAAm hydrogels and the covalent immobilization of rhBMP-2 in PNIPAAm/NASI hydrogels (39). PNIPAAm-based polymers have exerted time and concentration-dependent cytotoxic effects on cells (156). Therefore, more bioinert polymers that undergo phase transitions may be required for human bone healing applications.
Hydrophobic interactions may have also played an important role in controlling rhBMP-2 from gelatin hydrogels in vivo. It has been suggested that rhBMP-2 has bound to basic gelatin through hydrophobic interactions during in vitro binding (85) and release studies (134). This result was suggested in vitro because negatively charged rhBMP-2 is retained more in similarly charged basic gelatin hydrogels compared to oppositely charged acidic gelatin hydrogels, which were hypothesized to undergo electrostatic repulsion with rhBMP-2 (121). High rhBMP-2 retention in basic gelatin hydrogels has also been observed when implanted subcutaneously in mice (121, 147, 157). Basic gelatin hydrogels retained more rhBMP-2 over time compared to acidic gelatin hydrogels in mouse models. For example, after 14 days acidic gelatin hydrogels retained 25% of the rhBMP-2 retained by basic gelatin hydrogels (134). These results suggested that the gelatin's rhBMP-2 binding mechanism extends from in vitro to in vivo studies and instruct the choice of basic gelatin in future bone healing approaches. For example, gelatin has been used as a scaffold carrier for adenovirus encoding BMP-2. In this system gelatin could act as a natural depot for cell produced rhBMP-2 and maintain the growth factor at the implant site after the transient transduction period ends (158). A separate potential approach would be to covalently immobilize the bone morphogenetic binding peptide (BBP) into gelatin networks. BBP is a cyclic 19 amino acid peptide that has bound to rhBMP-2 with high affinity. BBP has been used to enhance BMP-mediated bone healing and formation by increasing BMPs' stability (159, 160) and limiting their inflammatory response (161). When BBP was included in collagen sponges with adsorbed rhBMP-2 it significantly increased rhBMP-2 retention when implanted in a mouse muscle pouch (162). Together, the amenability of basic gelatin hydrogels to be modified with peptides and incorporate cells could enable new approaches in retaining growth factors for in vivo bone regeneration (39).
4.2 Degradation-mediated release in vivo
Scaffold degradation has been an important mechanism to control rhBMP-2 release from scaffolds in vivo for several carriers. For example, the release of rhBMP-2 and gelatin implant sites was linearly correlated with a slope of 0.9 and an R2 of 0.89. This result indicated that rhBMP-2 released from gelatin hydrogel disks was degradation- mediated in the subcutaneous space. Also, increasing the degree of crosslinking significantly increased rhBMP-2 retention which could limit degradation (147). In a separate study, decreasing the water content of basic gelatin hydrogels from 99.7% - 93.8% increased the half-life of rhBMP-2 retention during the burst phase 4.4 fold and the half-life of rhBMP-2 retention during the sustained release period 18 fold. This study also reported that the rhBMP-2 release rate was linearly correlated with the gelatin scaffold degradation rate. However, gelatin hydrogels with the greatest rhBMP-2 retention did not have the greatest bone mineral density in skull defects in rabbits or monkeys (157,163). Histology has demonstrated that resorption of gelatin at the surface of implants and poor cell migration into the scaffolds in vivo (42). Therefore, it is possible that at too great a gelatin concentration, cells are unable to migrate into scaffolds with high rhBMP-2 retention capabilities. One approach to address this shortcoming could be to fabricate gelatin scaffolds with channels that allow for cell migration using solid free form fabrication techniques (32). Separately, rhBMP-2 was retained in PNIPAAm-based scaffolds that had greater stability in vitro regardless if the growth factor was covalently immobilized into the hydrogel network (39). Together, these studies have indicated that degradation is an important mechanism in controlling growth factor release from scaffolds that strongly interact with growth factors.
4.3 Convective fluid flow-mediated release in vivo
Convective fluid flow has been a key mediator of protein release in vivo from scaffolds that have interacted with rhBMP-2 primarily through electrostatic attraction. In one clinically relevant example, 3.5mm core defects were made in the femoral head and neck of adult male cynomolgus monkeys and rhBMP-2 containing collagen sponges were implanted in these defects. The sponges were significantly compressed from their volume of 0.24 ml to a 0.15 ml volume in the defect (144). rhBMP-2 rapidly released from these compressed collagen sponges which could be attributed the compression-induced release of rhBMP-2 from collagen sponges observed in vitro (79). 30 minutes after implantation 47.1% ±1.5% of the initial rhBMP-2 was retained in the defect site. This release rate was faster than the release of rhBMP-2 implanted on collagen sponges when implanted subcutaneously in rats and mice when similar compression regimes were not required (41, 147). The concentration of rhBMP-2 declined over time until there was 0.5% ± 0.1% of the initial dose retained after three weeks (144). Similar amounts have been retained in unloaded rat subcutaneous sites (21) and loaded femoral defects (116). In a related study, bilateral 8 mm core decompressions were formed in the proximal femur of adult sheep and the decompressions were filled with either rhBMP-2 adsorbed on collagen sponges or injected with rhBMP-2 in solution. Immediately after injection 37% of 3mg/ml rhBMP-2 adsorbed on collagen sponges and 42% of 0.8mg/ml rhBMP-2 collagen sponges were released into the proximal femur (164). Together, these results suggest that multiple approaches may be required to restrict protein release from implant sites when compression of the scaffold is necessary.
Clinicians have used diffusion barriers to restrict growth factor release from the implant site. For example, demineralized bone matrix putty has been used to limit growth factor diffusion out of the implant site (165). This approach to restricting rhBMP-2 diffusion was similar to approaches in which rhBMP-2 was encapsulated in a calcium phosphate matrix to limit its diffusion in non-human primates (166). Scientists have reported that they had ceased using allograft and autograft with BMP-2 due to reports of resorption (165). This result suggested that using allograft of autograft materials may not be suitable as diffusion barriers to their ability to be resorbed in the presence of rhBMP-2. In a separate study, Floseal was injected around rhBMP-2 laden collagen sponges surrounded by a PEEK implant during spine fusion surgeries. Floseal is a proprietary combination of bovine-derived gelatin and human-derived thrombin. Significantly, no patient in this study developed perioperative dysphagia which required any intervention and ectopic ossification was not observed. Also, all patients had resolution of their conditions which lead to surgery. However, 68% of patients had ossification within the spinal canal and spherical cyst like structures appeared within the fusion mass in 39% of patients. They hypothesized that the ossification in the spinal canal could have been produced by stem cells located in hematomas left in the postoperative space (167). In a separate approach to restrict protein release collagen sponges were soaked with 32mg of rhBMP-2 for 5 minutes or 60 minutes. Two monkeys received 9mg rhBMP-2 adsorbed on collagen sponges and placed in titanium carriers covered with a poly(ethylene) mesh shield after implantation to prevent compression of the sponges by the surrounding muscles. Spinal fusion went to completion in both these monkeys and they had the most extensive bone formation observed (168). It may be necessary to use solid materials as diffusion barriers. In one illustrative study, coating composite materials with a layer of hydrophilic chitosan did not consistently change the magnitude of the burst release of growth factor or modulate release over the course of the experiment in vitro (107). These approaches to form diffusional barriers represent an innovative mechanism to control growth factor diffusion that have not been extensively measured in vitro or in vivo. Therefore, future research will be necessary to quantify the effect of diffusional barriers on rhBMP-2 transport.
Composite compression resistant scaffolds have also been used to reduce convective fluid-flow induced growth factor release from scaffolds. In one study, rhBMP-2 release from composite compression resistant collagen sponges containing 5:95 hydroxyapatite:tricalcium phosphate ceramic particles was measured when implanted bilaterally in a rabbit spine model. rhBMP-2 had a mean half-life of 7.51 days in the implant site in this model (51). Alternatively, rhBMP-2 had a mean half-life of 3.76 days when it was adsorbed into compressible collagen sponges and implanted in a rabbit ulna defect model (169). Although these are different implant sites with different mechanical environments these results suggest that compression resistant sponges could restrict convective fluid-flow mediated release. For example, compression resistant scaffolds have enabled the use of lower effective concentrations of rhBMP-2 to induce bone fusion in a nonhuman primate lumbar intertransverse spinal fusion model (170). Including ceramic particles in other matrix materials that retain rhBMP-2 at the implant site longer than collagen and using diffusional barriers could have the potential to decrease the effective dose even further.
5. Future Directions
5.1 Delivery of multiple factors
Bone healing is a complex process that has been controlled by multiple soluble signals. To better replicate the natural bone healing process, investigators have delivered of multiple factors involved in bone healing. A common theme in these studies is the delivery of pro-osteogenic and pro-angiogenic growth factors (171, 172). For example, rhBMP-2 has been delivered with VEGF (173), bFGF (174), and IGF-1 (175) to promote bone healing. While delivering multiple growth factors from the same scaffold could be a promising approach for future bone regeneration applications, many growth factors have approximately the same molecular weight and isoelectric point which has made it challenging to deliver growth factors from the same construct without using complex material processing techniques. Also, it might be more clinically relevant to systemically deliver molecules that are currently FDA approved for other indications that also aid in growth factor-mediated bone healing. One approach could be to systemically deliver molecules that block endogenous inhibitors of BMP signaling. For example, monoclonal antibody molecules have been used to block the inhibitory activity of sclerostin in a phase I clinical trial (176). Erythropoietin has also been identified as an important mediator of both hematopoiesis and bone formation in vivo (177). Erythropoietin has been used in the clinic to treat anemia for almost 25 years and its safety profile has been well-characterized (178). Another systemic molecule that has regulated bone metabolism is the parathyroid hormone. Intermittent administration of parathyroid hormone significantly enhanced the bone mineral density in rhBMP-2 containing composite scaffolds (179). An emerging technique for the simultaneous delivery of many factors is to genetically manipulate human mesenchymal stem cells that can evade the host immune response, produce pro-osteogenic growth factors, and participate in osteogenesis (180-182). In one illustrative study, human mesenchymal stem cells were infected with an adenovirus carrying the gene for rhBMP-2 and implanted in a collagen-ceramic composite scaffold in rat femoral defects. Significantly, these genetically modified cells exhibited the same capacity as the rhBMP-2 protein to induce bone healing. This approach to deliver growth factors could eliminate the need for supraphysiologic concentrations of growth factors currently used in the clinic (183). Together, local and systemic co-delivery of soluble factors has the potential to increase the safety and efficacy of growth factor-mediated bone healing techniques.
5.2 Developing new combination devices for difficult-healing areas
Future scaffold-growth factor combination devices will need to control growth factor release, have the appropriate form, and fixation. The use of rhBMP-7, also referred to as osteogenic protein-1 (OP-1), during long bone healing could provide lessons for treating difficult to heal areas. OP-1 consists of lyophilized rhBMP-7 on collagen granules suspended with carboxymethyl cellulose that when mixed with water forms an implantable paste. In one illustrative study on the importance of fixation, 30 patients were treated with either OP-1 or autologous bone and were stabilized with either external fixation or internal pi-plates. Two of the four patients treated with OP-1 and external fixation presented osteolysis around the bone regeneration site. The observed osteolysis lead to long bone nonunion and could be attributed to inability of the OP-1 paste to contribute to the stability of the regeneration site. The investigators then sought to improve the stability of the regeneration site with internal fixation techniques. Although bone healing proceeded in both OP-1 and autograft-treated patients with internal fixation, autograft treatments healed in 7 weeks compared to 19 weeks for OP-1-treated nonunions (184). It is also significant to note that fixation techniques with poor stability had a significant impact on the role that infections play during growth factor mediated bone regeneration (185, 186). These results suggested that healing difficult areas with growth factors could be improved by increasing the stability of the bone regeneration site and will require improved scaffold design and carriers.
Many of the approaches used in the clinic have adapted the collagen sponge carrier for rhBMP-2 with different cages and fixation devices for varying locations. The cutting of sponges to be pressed inside or around a previously established fixation device has been a commonly reported approach. However, mechanical loading can induce fluid exclusion and trigger the release of encapsulated growth factors (143). One approach that could enable the incorporation of growth factors and carriers into pre-existing devices with established fixation techniques are polymer solutions that assemble in vivo. For example, Zhang and colleagues have formed self-assembling hydrogels from the RADA16 peptide fused with pro-osteogenic peptides. These hybrid peptides self-assembled to form hydrogels that promoted stem cell differentiation and proliferation in vitro (187). Similar RADA16-based hydrogels have been used to modulate the release of bFGF, VEGF, and brain derived neurotrophic factor (BDNF) over the course of days depending upon the growth factor and presence of charged molecules in the network (188). Also, MMP-degradable peptide sequences can be included in these peptide sequences to increase the rate of cell infiltration (189). Significantly, these peptides readily formed hydrogels in PEEK cages that promoted bone formation when implanted in a rat bone defect when compared to an open cage (190). Therefore, this self-assembling peptide system could provide broad-based flexibility depending on the bone regeneration site with preexisting implants.
Although flexibility in carrier design could enable broad-based utility in diverse bone regeneration applications, to get to market devices must address a specific indication. Therefore, future growth factor-scaffold combination devices will need to be designed to meet quantitative technical demands (191). Computer-aided design has been used to form scaffolds with optimized mechanical properties to match the surrounding bone, permeability properties to address the metabolic requirements of cells, and fixation to meet mechanical and surgical needs. Solid freeform fabrication techniques are currently the most used manufacturing technique of computer-aided designed scaffolds and have even been applied in humans (32, 192). Other modules can be added to these scaffolds without affecting their form or fixation approaches. For example, Murphy and colleagues have developed approaches to increase the osteoconductivity of designed scaffolds using approaches to surface functionalize scaffolds with biomimetic hydroxyapatite (193, 194). If mineralized scaffolds do not meet the technical requirements for bone regeneration, then other biologics can be incorporated into the manufacturing process or coating processes. This approach to incorporating growth factors into scaffolds limits the need for separate carrier materials. For example, hybrid peptides have been developed that contain a mineral binding domain and a growth factor mimicking domain to induce bone regeneration (61, 63). In one relevant approach, growth factor mimicking peptides were adsorbed to mineralized PCL scaffolds formed using computer aided design techniques. The growth factor release rate could be tuned by varying the mineral content on the surface of the scaffold (195).
CVD-based approaches have also been used to functionalize scaffolds formed using computer aided design techniques. For example, PCL scaffolds were formed using computer aided design and functionalized with amine groups using CVD. Then, biotin was covalently immobilized to the scaffold and the functionalized scaffolds were incubated with a solution of avidin and biotin functionalized adenoviruses to non-covalently immobilize the adenovirus to the scaffold. Wax masking techniques enabled the selective immobilization of the adenoviruses on distinct areas of the scaffold. This technique could be used to selectively functionalize areas of the scaffold within the bone regeneration site and leave fixation points unmodified (196) or be used to pattern therapeutic adenovirus gradients (197). The adaptability of using computer aided design to form scaffolds with orthobiologic delivery, as a design parameter will be critical for the development of combination devices for specific applications.
5.3 Emerging approaches to covalently tether rhBMP-2 to scaffolds
Covalently tethering growth factors to scaffolds could enable localized growth factor delivery in specific areas of rationally designed scaffolds and limit growth factor release during bone regeneration. In covalent systems growth factors have been tethered either directly or via a linker molecule that has provided space between the scaffold and the growth factor. Once immobilized, growth factors have bound with cell surface receptors and initiated intracellular signaling pathways. The biological effects of covalent tethering has been extensively studied in cell culture systems due to their increased protein stability, length of receptor activation, receptor clustering, and co-localization of the growth factor with cells. This co-localization has been characterized to mimic how growth factors are naturally presented to cells in the body from extracellular matrices or cell surfaces. Therefore, Ito and colleagues have referred to this as “artificial juxtacrine stimulation (198).” Many proteins have been tethered to substrates including epidermal growth factor (EGF), nerve growth factor (NGF), BMPs, VEGF, FGFs, IGF-1, TGF-β1, hepatocyte growth factor (HGF), notch ligand, leukemia inhibitory factor (LIF), stem cell growth factor (SCF), interleukins, tumor necrosis factor-α (TNF-α), erythropoietin (Epo), Neurotrophin-3, Transferrin, and E-cadherin. However, chemistries used to covalently immobilize growth factors can lead to protein denaturing, inactivation, or incomplete immobilization. The techniques available to immobilize growth factors are dependent upon both the functional groups in the scaffold and the growth factor's biochemistry. Also, the concentration of growth factors incorporated is controlled by the surface area of the scaffold (199). These results suggest that approaches to covalently immobilize growth factors to scaffolds will be protein and application specific.
rhBMP2 has been covalently tethered to biomaterials to improve its stability and increase its retention in regeneration sites. For example, rhBMP-2 has been covalently tethered to succinylated type 1 atelocollagen to improve rhBMP-2 stability in complex biologic environments. Tethering rhBMP-2 to the biopolymer increased the length of osteogenic gene expression in a mouse bone marrow stem cell line compared to untethered rhBMP-2 (200, 201). In a separate approach, rhBMP-2 has been directly immobilized to PCL scaffolds that were treated to present amine groups. Less than 10% of the immobilized rhBMP-2 was released over the course of 15 days in vitro compared to 25% of adsorbed rhBMP-2 (59). Similar results were observed when rhBMP-2 was covalently tethered to PLG scaffolds using acrylate-PEG-N-hydroxysuccinimide (NHS) spacer molecules. The acrylate groups reacted with –OOH groups on PLG scaffolds treated with UV light and hydrogen peroxide and the –NHS groups reacted with primary amines on rhBMP-2. In this tethered system 90% of the rhBMP-2 was retained in the scaffold over the course of a 28 day in vitro exposure to DMEM with 10% FBS. Alternatively, 10% of adsorbed rhBMP-2 was maintained in scaffolds over the same period (202). To test if surface functional groups altered protein bioactivity, lysozyme and rhBMP-4 were covalently immobilized to functionalized titanium surfaces presenting either amine or carboxylic acid groups. Lysozyme's bioactivity was eliminated when covalently tethered to surfaces presenting amines, but maintained 60% of its bioactivity when conjugated to carboxylic acid presenting surfaces. When the procedures optimized for lysozyme were extended to rhBMP-4 these functionalized surfaces induced osteogenic gene expression in C3H10T1/2 cells even after the surfaces were washed overnight (203). In a separate titanium-based approach, surfaces were modified with polymers containing phosphonic ester groups which bind to metals and epoxy groups which react with amine groups on rhBMP-2. In these studies, only a fraction of the tethered rhBMP-2 was accessible to cells because it was tethered inside the polymer network. The accessibility of rhBMP-2 in this system could be improved by including PEG spacer molecules in the polymer to give cells better access to the growth factor (204). These approaches present the broad-based utility of covalently tethering rhBMP-2 to biomaterials through surface exposed primary amines and carboxylic acids. It will be crucial to extend the in vitro release kinetics delineated in these studies to in vivo studies to ensure their clinical safety and success.
Researchers have developed site-specific covalent modification techniques from other proteins to rhBMP-2 to improve its bioactivity and stability after processing. For example, fully folded rhBMP-2 contains no free cysteines with reactive sulfhydryl groups. Therefore, Sebald and colleagues generated 3 rhBMP-2 mutants with free cysteines at specific locations to enable site-specific conjugation of polymers to the growth factor. They demonstrated that rhBMP-2 pegylated at a mutant cysteine located near the N-terminus retained its ability to bind to its receptor and initiate intracellular signaling pathways. Alternatively, a fraction of rhBMP-2′s bioactivity was lost when it was pegylated at locations near its receptor binding site (205). The same group then adapted previously established techniques (206) to selectively tether PEG molecules to the N-terminus of rhBMP-2 using acidic pH conditions in combination with PEG molecules terminated with reactive aldehyde or NHS groups. The selective modification of the N-terminal amine groups at acidic pH conditions could be attributed to their pKa values (7.6-8.0) compared to the pKa value of ε-amines of lysine side chains (10-10.2). The reaction of rhBMP-2 with aldehyde- or NHS- terminated PEG produced mono- and di- conjugated rhBMP-2 and aldehyde -PEG had a greater reaction efficiency compared to NHS- PEG. Mono-pegylated rhBMP-2 had increased solubility compared to unmodified rhBMP-2, decreased binding affinity for its receptor, and exhibited higher bioactivity than unmodified rhBMP-2 (207). Separately, there has been extensive work developing reactive non-canonical amino acids, which could enable the development of new techniques to covalently tether rhBMP-2 into scaffolds (208). Although these site-specific covalent tethering techniques have not yet been extensively applied to scaffolds they could hold significant promise in future bone regeneration approaches.
Acknowledgments
This research was supported by NIH grants R01 DE018890 and the NIDCR T32 Tissue Engineering and Regeneration Training Program fellowship for WJK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnson EE, Urist MR, Finerman GAM. Repair of segmental defects of the tibia with cancellous bone-grafts augmented with human-bone morphogenetic protein - a preliminary report. Clin Orthop Rel Res. 1988;(236):249–57. [PubMed] [Google Scholar]
- 2.Johnson EE, Urist MR, Finerman GAM. Bone morphogenetic protein augmentation grafting of resistant femoral nonunions - a preliminary report. Clin Orthop Rel Res. 1988;(230):257–65. [PubMed] [Google Scholar]
- 3.Johnson EE, Urist MR, Finerman GAM. Resistant nonunions and partial or complete segmental defects of long bones - treatment with implants of a composite of human bone morphogenetic protein (BMP) and autolyzed, antigen-extracted, allogeneic (AAA) bone. Clin Orthop Rel Res. 1992;(277):229–37. [PubMed] [Google Scholar]
- 4.Urist MR. Bone - formation by autoinduction. Science. 1965;150(3698):893–9. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 5.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation- molecular clones and activities. Science. 1988;242(4885):1528–34. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 6.Blumenfeld I, Srouji S, Lanir Y, Laufer D, Livne E. Enhancement of bone defect healing in old rats by TGF-beta and IGF-1. Exp Gerontol. 2002;37(4):553–65. doi: 10.1016/s0531-5565(01)00215-7. [DOI] [PubMed] [Google Scholar]
- 7.Ripamonti U, Ferretti C, Teare J, Blann L. Transforming Growth Factor-beta Isoforms and the Induction of Bone Formation: Implications for Reconstructive Craniofacial Surgery. J Craniofac Surg. 2009;20(5):1544–55. doi: 10.1097/SCS.0b013e3181b09ca6. [DOI] [PubMed] [Google Scholar]
- 8.Howell TH, Fiorellini JP, Paquette DW, Offenbacher S, Giannobile WV, Lynch SE. A phase I/II clinical trial to evaluate a combination of recombinant human platelet-derived growth factor-BB and recombinant human insulin-like growth factor-I in patients with periodontal disease. J Periodont. 1997;68(12):1186–93. doi: 10.1902/jop.1997.68.12.1186. [DOI] [PubMed] [Google Scholar]
- 9.Park MS, Kim SS, Cho SW, Choi CY, Kim BS. Enhancement of the osteogenic efficacy of osteoblast transplantation by the sustained delivery of basic fibroblast growth factor. J Biomed Mater Res Part B. 2006;79B(2):353–9. doi: 10.1002/jbm.b.30549. [DOI] [PubMed] [Google Scholar]
- 10.Ratanavaraporn J, Furuya H, Kohara H, Tabata Y. Synergistic effects of the dual release of stromal cell-derived factor-1 and bone morphogenetic protein-2 from hydrogels on bone regeneration. Biomaterials. 2011;32(11):2797–811. doi: 10.1016/j.biomaterials.2010.12.052. [DOI] [PubMed] [Google Scholar]
- 11.Street J, Bao M, deGuzman L, Bunting S, Peale FV, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, van Bruggen N, Redmond HP, Carano RAD, Filvaroff EH. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. P Natl Acad Sci USA. 2002;99(15):9656–61. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Axelrad TW, Einhorn TA. Bone morphogenetic proteins in orthopaedic surgery. Cytokine Growth Factor Rev. 2009;20(5-6):481–8. doi: 10.1016/j.cytogfr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Nevins M, Camelo M, Nevins ML, Schenk RK, Lynch SE. Periodontal regeneration in humans using recombinant human platelet-derived growth Factor-BB (rhPDGF-BB) and allogenic bone. J Periodont. 2003;74(9):1282–92. doi: 10.1902/jop.2003.74.9.1282. [DOI] [PubMed] [Google Scholar]
- 14.Zara JN, Siu RK, Zhang X, Shen J, Ngo R, Lee M, Li W, Chiang M, Chung J, Kwak J, Wu BM, Ting K, Soo C. High Doses of Bone Morphogenetic Protein 2 Induce Structurally Abnormal Bone and Inflammation In Vivo. Tissue Eng Part A. 2011;17(9-10):1389–99. doi: 10.1089/ten.tea.2010.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Babitt JL, Huang FW, Xia Y, Sidis Y, Andrews NC, Lin HY. Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J Clin Invest. 2007;117(7):1933–9. doi: 10.1172/JCI31342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burkus JK, Gornet MF, Glassman SD, Slosar PJ, Rosner MK, Deckey JE, Nowak J, Hatcher BM. Blood Serum Antibody Analysis and Long -Term Follow-up of Patients Treated with Recombinant Human Bone Morphogenetic Protein-2 in the Lumbar Spine. Spine. 2011 doi: 10.1097/BRS.0b013e3182059a8c. Publish Ahead of Print: 10.1097/BRS.0b013e3182059a8c. [DOI] [PubMed] [Google Scholar]
- 17.Dmitriev AE, Lehman RA, Symes AJ. Bone morphogenetic protein-2 and spinal arthrodesis: the basic science perspective on protein interaction with the nervous system. Spine J. 2011;11(6):500–5. doi: 10.1016/j.spinee.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 18.FDA. Food and Drug Administration Executive summary for P050036 Medtronic's AMPLIFY TM rhBMP-2 Matrix Orthopaedic and rehabilitation devices advisory panel 2010 [Google Scholar]
- 19.Wozney JM. Bone morphogenetic proteins. Prog Growth Factor Res. 1989;1(4):267–80. doi: 10.1016/0955-2235(89)90015-x. [DOI] [PubMed] [Google Scholar]
- 20.Patterson J, Siew R, Herring SW, Lin ASP, Guldberg R, Stayton PS. Hyaluronic acid hydrogels with controlled degradation properties for oriented bone regeneration. Biomaterials. 2010;31(26):6772–81. doi: 10.1016/j.biomaterials.2010.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uludag H, D'Augusta D, Golden J, Li J, Timony G, Riedel R, Wozney JM. Implantation of recombinant human bone morphogenetic proteins with biomaterial carriers: A correlation between protein pharmacokinetics and osteoinduction in the rat ectopic model. J Biomed Mater Res. 2000;50(2):227–38. doi: 10.1002/(sici)1097-4636(200005)50:2<227::aid-jbm18>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 22.Ruppert R, Hoffmann E, Sebald W. Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity. Eur J Biochem. 1996;237(1):295–302. doi: 10.1111/j.1432-1033.1996.0295n.x. [DOI] [PubMed] [Google Scholar]
- 23.Laub M, Seul T, Schmachtenberg E, Jennissen HP. Molecular modelling of bone morphogenetic protein-2 (BMP-2) by 3D-rapid prototyping. Materwiss Werksttech. 2001;32(12):926–30. [Google Scholar]
- 24.Chi EY, Krishnan S, Randolph TW, Carpenter JF. Physical stability of proteins in aqueous solution: Mechanism and driving forces in nonnative protein aggregation. Pharm Res. 2003;20(9):1325–36. doi: 10.1023/a:1025771421906. [DOI] [PubMed] [Google Scholar]
- 25.Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurdo JS, Taddei N, Ramponi G, Dobson CM, Stefani M. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416(6880):507–11. doi: 10.1038/416507a. [DOI] [PubMed] [Google Scholar]
- 26.Friess W, Uludag H, Foskett S, Biron R, Sargeant C. Characterization of absorbable collagen sponges as rhBMP-2 carriers. Int J Pharm. 1999;187(1):91–9. doi: 10.1016/s0378-5173(99)00174-x. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz D. Development of an Aqueous Suspension of Recombinant Human Bone Morphogenetic Protein-2 (rhBMP-2) Thesis. 2005 http://edoc.ub.uni-muenchen.de/4431/1/Schwartz_Daniel.pdf.
- 28.Vallejo LF, Rinas U. Optimized procedure for renaturation of recombinant human bone morphogenetic protein-2 at high protein concentration. Biotechnol Bioeng. 2004;85(6):601–9. doi: 10.1002/bit.10906. [DOI] [PubMed] [Google Scholar]
- 29.Luca L, Capelle MAH, Machaidze G, Arvinte T, Jordan O, Gurny R. Physical instability, aggregation and conformational changes of recombinant human bone morphogenetic protein-2 (rhBMP-2) Int J Pharm. 2010;391(1-2):48–54. doi: 10.1016/j.ijpharm.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Ward BB, Brown SE, Krebsbach PH. Bioengineering strategies for regeneration of craniofacial bone: a review of emerging technologies. Oral Dis. 2010;16(8):709–16. doi: 10.1111/j.1601-0825.2010.01682.x. [DOI] [PubMed] [Google Scholar]
- 31.Bassett CAL. Beneficial-effects of electromagnetic-fields. J Cell Biochem. 1993;51(4):387–93. doi: 10.1002/jcb.2400510402. [DOI] [PubMed] [Google Scholar]
- 32.Hollister SJ. Scaffold Design and Manufacturing: From Concept to Clinic. Adv Mater. 2009;21(32-33):3330–42. doi: 10.1002/adma.200802977. [DOI] [PubMed] [Google Scholar]
- 33.Jones AL, Bucholz RW, Bosse MJ, Mirza SK, Lyon TR, Webb LX, Pollak AN, Golden JD, Valentin-Opran A, Tibial BMPES. Recombination human BMP-2 and allograft compared with autogenous bonegraft for reconstruction of diaphyseal tibial fractures with cortical defects -A randomized, controlled trial. J Bone Joint Surg Am. 2006;88A(7):1431–41. doi: 10.2106/JBJS.E.00381. [DOI] [PubMed] [Google Scholar]
- 34.Allen RT, Lee YP, Stimson E, Garfin SR. Bone morphogenetic protein-2 (BMP-2) in the treatment of pyogenic vertebral osteomyelitis. Spine. 2007;32(26):2996–3006. doi: 10.1097/BRS.0b013e31815cde3e. [DOI] [PubMed] [Google Scholar]
- 35.Tolli H, Kujala S, Jamsa T, Jalovaara P. Reindeer bone extract can heal the critical-size rat femur defect. Int Orthop. 2011;35(4):615–22. doi: 10.1007/s00264-010-1034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takaoka K, Koezuka M, Nakahara H. Telopeptide-depleted bovine skin collagen as a carrier for bone morphogenetic protein. J Orthop Res. 1991;9(6):902–7. doi: 10.1002/jor.1100090617. [DOI] [PubMed] [Google Scholar]
- 37.Karageorgiou V, Tomkins M, Fajardo R, Meinel L, Snyder B, Wade K, Chen J, Vunjak-Novakovic G, Kaplan DL. Porous silk fibroin 3-D scaffolds for delivery of bone morphogenetic protein-2 in vitro and in vivo. J Biomed Mater Res A. 2006;78A(2):324–34. doi: 10.1002/jbm.a.30728. [DOI] [PubMed] [Google Scholar]
- 38.Jarcho M. Calcium-phosphate ceramics as hard tissue prosthetics. Clin Orthop Rel Res. 1981;(157):259–78. [PubMed] [Google Scholar]
- 39.Gao T, Kousinioris N, Winn SR, Wozney JM, Uludag H. Enhanced retention of rhBMP-2 in vivo by thermoreversible polymers. Materwiss Werksttech. 2001;32(12):953–61. [Google Scholar]
- 40.Constantz BR, Ison IC, Fulmer MT, Poser RD, Smith ST, Vanwagoner M, Ross J, Goldstein SA, Jupiter JB, Rosenthal DI. Skeletal repair by in-situ formation of the mineral phase of bone. Science. 1995;267(5205):1796–9. doi: 10.1126/science.7892603. [DOI] [PubMed] [Google Scholar]
- 41.Uludag H, D'Augusta D, Palmer R, Timony G, Wozney J. Characterization of rhBMP-2 pharmacokinetics implanted with biomaterial carriers in the rat ectopic model. J Biomed Mater Res. 1999;46(2):193–202. doi: 10.1002/(sici)1097-4636(199908)46:2<193::aid-jbm8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 42.Kempen DHR, Lu L, Hefferan TE, Creemers LB, Maran A, Classic KL, Dhert WJA, Yaszemski MJ. Retention of in vitro and in vivo BMP-2 bioactivities in sustained delivery vehicles for bone tissue engineering. Biomaterials. 2008;29(22):3245–52. doi: 10.1016/j.biomaterials.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arima Y, Iwata H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials. 2007;28(20):3074–82. doi: 10.1016/j.biomaterials.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 44.Rechendorff K, Hovgaard MB, Foss M, Zhdanov VP, Besenbacher F. Enhancement of protein adsorption induced by surface roughness. Langmuir. 2006;22(26):10885–8. doi: 10.1021/la0621923. [DOI] [PubMed] [Google Scholar]
- 45.Gessner A, Lieske A, Paulke BR, Muller RH. Influence of surface charge density on protein adsorption on polymeric nanoparticles: analysis by two-dimensional electrophoresis. Eur J Pharm Biopharm. 2002;54(2):165–70. doi: 10.1016/s0939-6411(02)00081-4. [DOI] [PubMed] [Google Scholar]
- 46.Gessner A, Lieske A, Paulke BR, Muller RH. Functional groups on polystyrene model nanoparticles: Influence on protein adsorption. J Biomed Mater Res A. 2003;65A(3):319–26. doi: 10.1002/jbm.a.10371. [DOI] [PubMed] [Google Scholar]
- 47.Pasche S, Voros J, Griesser HJ, Spencer ND, Textor M. Effects of ionic strength and surface charge on protein adsorption at PEGylated surfaces. J Phys Chem B. 2005;109(37):17545–52. doi: 10.1021/jp050431+. [DOI] [PubMed] [Google Scholar]
- 48.Noh H, Vogler EA. Volumetric interpretation of protein adsorption: Competition from mixtures and the Vroman effect. Biomaterials. 2007;28(3):405–22. doi: 10.1016/j.biomaterials.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sadana A. Protein adsorption and inactivation on surfaces. Influence of heterogeneities. Chem Rev. 1992;92(8):1799–818. [Google Scholar]
- 50.King WJ, Toepke MW, Murphy WL. Facile formation of dynamic hydrogel microspheres for triggered growth factor delivery. Acta Biomater. 2011;7(3):975–85. doi: 10.1016/j.actbio.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Louis-Ugbo J, Kim HS, Boden SD, Mayr MT, Li RC, Seeherman H, D'Augusta D, Blake C, Jiao AP, Peckham S. Retention of I-125-labeled recombinant human bone morphogenetic protein-2 by biphasic calcium phosphate or a composite sponge in a rabbit posterolateral spine arthrodesis model. J Orthop Res. 2002;20(5):1050–9. doi: 10.1016/S0736-0266(02)00011-6. [DOI] [PubMed] [Google Scholar]
- 52.Dong X, Wang Q, Wu T, Pan H. Understanding adsorption-desorption dynamics of BMP-2 on hydroxyapatite (001) surface. Biophys J. 2007;93(3):750–9. doi: 10.1529/biophysj.106.103168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou H, Wu T, Dong X, Wang Q, Shen J. Adsorption mechanism of BMP-7 on hydroxyapatite (001) surfaces. Biochem Biophys Res Commun. 2007;361(1):91–6. doi: 10.1016/j.bbrc.2007.06.169. [DOI] [PubMed] [Google Scholar]
- 54.Turhani D, Weissenboeck M, Stein E, Wanschitz F. Exogenous recombinant human BMP-2 has little initial effects on human osteoblastic cells cultured on collagen type I coated/noncoated hydroxyapatite ceramic granules. J Oral Maxillofac Surg. 2007;65(3):485–93. doi: 10.1016/j.joms.2005.12.065. [DOI] [PubMed] [Google Scholar]
- 55.Boix T, Gomez-Morales J, Torrent-Burgues J, Monfort A, Pulgdomenech P, Rodriguez-Clemente R. Adsorption of recombinant human bone morphogenetic protein rhBMP-2m onto hydroxyapatite. J Inorg Biochem. 2005;99(5):1043–50. doi: 10.1016/j.jinorgbio.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 56.Serro AP, Bastos M, Pessoa JC, Saramago B. Bovine serum albumin conformational changes upon adsorption on titania and on hydroxyapatite and their relation with biomineralization. J Biomed Mater Res A. 2004;70A(3):420–7. doi: 10.1002/jbm.a.30096. [DOI] [PubMed] [Google Scholar]
- 57.Ruhe PQ, Boerman OC, Russel FGM, Mikos AG, Spauwen PHM, Jansen JA. In vivo release of rhBMP-2 loaded porous calcium phosphate cement pretreated with albumin. J Mater Sci Mater Med. 2006;17(10):919–27. doi: 10.1007/s10856-006-0181-z. [DOI] [PubMed] [Google Scholar]
- 58.Duggirala SS, Mehta RC, DeLuca PP. Interaction of recombinant human bone morphogenetic protein-2 with poly(d,l lactide-co-glycolide) microspheres. Pharm Dev Technol. 1996;1(1):11–9. doi: 10.3109/10837459609031413. [DOI] [PubMed] [Google Scholar]
- 59.Zhang H, Migneco F, Lin CY, Hollister SJ. Chemically-Conjugated Bone Morphogenetic Protein-2 on Three-Dimensional Polycaprolactone Scaffolds Stimulates Osteogenic Activity in Bone Marrow Stromal Cells. Tissue Eng Part A. 2010;16(11):3441–8. doi: 10.1089/ten.tea.2010.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schrier JA, DeLuca PP. Porous bone morphogenetic protein-2 microspheres: polymer binding and in vitro release. AAPS PharmSciTech. 2001;2(3):E17. doi: 10.1208/pt020317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee JS, Johnson AJW, Murphy WL. A Modular, Hydroxyapatite-Binding Version of Vascular Endothelial Growth Factor. Adv Mater. 2010;22(48):5494–8. doi: 10.1002/adma.201002970. [DOI] [PubMed] [Google Scholar]
- 62.Suarez-Gonzalez D, Barnhart K, Saito E, Vanderby R, Jr, Hollister SJ, Murphy WL. Controlled nucleation of hydroxyapatite on alginate scaffolds for stem cell-based bone tissue engineering. J Biomed Mater Res A. 2010;95A(1):222–34. doi: 10.1002/jbm.a.32833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee JS, Lee JS, Murphy WL. Modular peptides promote human mesenchymal stem cell differentiation on biomaterial surfaces. Acta Biomater. 2010;6(1):21–8. doi: 10.1016/j.actbio.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee JS, Lu Y, Baer GS, Markel MD, Murphy WL. Controllable protein delivery from coated surgical sutures. J Mater Chem. 2010;20(40):8894–903. [Google Scholar]
- 65.Lu Y, Markel MD, Nemke B, Lee JS, Graf BK, Murphy WL. Influence of Hydroxyapatite-Coated and Growth Factor-Releasing Interference Screws on Tendon-Bone Healing in an Ovine Model. Arthroscopy. 2009;25(12):1427–U92. doi: 10.1016/j.arthro.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jongpoiboonkit L, Franklin-Ford T, Murphy WL. Mineral-Coated Polymer Microspheres for Controlled Protein Binding and Release. Adv Mater. 2009;21(19):1960–3. [Google Scholar]
- 67.McKay WF, Peckham S, Badura JM. Development of a novel compression-resistant carrier for recombinant human bone morphogenetic protein-2 (rhBMP-2) and preliminary clinical results. Bone morphogenetic proteins: from local to systemic therapeutics. 2008:7–23. [Google Scholar]
- 68.Gonzalez-Garcia C, Sousa SR, Moratal D, Rico P, Salmeron-Sanchez M. Effect of nanoscale topography on fibronectin adsorption, focal adhesion size and matrix organisation. Colloids Surf B Biointerfaces. 2010;77(2):181–90. doi: 10.1016/j.colsurfb.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 69.Ehlert N, Hoffmann A, Luessenhop T, Gross G, Mueller PP, Stieve M, Lenarz T, Behrens P. Amino-modified silica surfaces efficiently immobilize bone morphogenetic protein 2 (BMP2) for medical purposes. Acta Biomater. 2011;7(4):1772–9. doi: 10.1016/j.actbio.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 70.Chatzinikolaidou M, Laub M, Rumpf H, Jennissen HP. Biocoating of electropolished and ultra-hydrophilic titanium and cobalt chromium molybdenum alloy surfaces with proteins. Materwiss Werksttech. 2002;33(12):720–7. [Google Scholar]
- 71.Steinmueller-Nethl D, Kloss FR, Najam-U-Haq M, Rainer M, Larsson K, Linsmeier C, Koehler G, Fehrer C, Lepperdinger G, Liu X, Memmel N, Bertel E, Huck CW, Gassner R, Bonn G. Strong binding of bioactive BMP-2 to nanocrystalline diamond by physisorption. Biomaterials. 2006;27(26):4547–56. doi: 10.1016/j.biomaterials.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 72.Hu WW, Elkasabi Y, Chen HY, Zhang Y, Lahann J, Hollister SJ, Krebsbach PH. The use of reactive polymer coatings to facilitate gene delivery from poly (epsilon-caprolactone) scaffolds. Biomaterials. 2009;30(29):5785–92. doi: 10.1016/j.biomaterials.2009.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Han ZJ, Ostrikov K, Tan CM, Tay BK, Peel SAF. Effect of hydrophilicity of carbon nanotube arrays on the release rate and activity of recombinant human bone morphogenetic protein-2. Nanotechnology. 2011;22(29) doi: 10.1088/0957-4484/22/29/295712. [DOI] [PubMed] [Google Scholar]
- 74.Tabata Y, Ikada Y. Protein release from gelatin matrices. Adv Drug Deliv Rev. 1998;31(3):287–301. doi: 10.1016/s0169-409x(97)00125-7. [DOI] [PubMed] [Google Scholar]
- 75.Docherty-Skogh AC, Bergman K, Waern MJ, Ekman S, Hultenby K, Ossipov D, Hilborn J, Bowden T, Engstrand T. Bone Morphogenetic Protein-2 Delivered by Hyaluronan-Based Hydrogel Induces Massive Bone Formation and Healing of Cranial Defects in Minipigs. Plast Reconstr Surg. 2010;125(5):1383–92. doi: 10.1097/PRS.0b013e3181d629dc. [DOI] [PubMed] [Google Scholar]
- 76.Hunt DR, Jovanovic SA, Wikesjo UME, Wozney JM, Bernard GW. Hyaluronan supports recombinant human bone morphogenetic protein-2 induced bone reconstruction of advanced alveolar ridge defects in dogs. A pilot study. J Periodont. 2001;72(5):651–8. doi: 10.1902/jop.2001.72.5.651. [DOI] [PubMed] [Google Scholar]
- 77.Dulbecco R, Vogt M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954;99(2):167–82. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Newman RJ, Francis MJO, Duthie RB. Nuclear magnetic resonance studies of experimentally induced delayed fracture union. Clin Orthop Rel Res. 1987;(216):253–61. [PubMed] [Google Scholar]
- 79.Hsu HP, Zanella JM, Peckham SM, Spector M. Comparing ectopic bone growth induced by rhBMP-2 on an absorbable collagen sponge in rat and rabbit models. J Orthop Res. 2006;24(8):1660–9. doi: 10.1002/jor.20204. [DOI] [PubMed] [Google Scholar]
- 80.Yamamoto M, Tabata Y, Hong L, Miyamoto S, Hashimoto N, Ikada Y. Bone regeneration by transforming growth factor beta 1 released from a biodegradable hydrogel. J Control Release. 2000;64(1-3):133–42. doi: 10.1016/s0168-3659(99)00129-7. [DOI] [PubMed] [Google Scholar]
- 81.He XZ, Ma JY, Jabbari E. Migration of marrow stromal cells in response to sustained release of stromal-derived factor-1 alpha from poly(lactide ethylene oxide fumarate) hydrogels. Int J Pharm. 2010;390(2):107–16. doi: 10.1016/j.ijpharm.2009.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rai B, Teoh SH, Hutmacher DW, Cao T, Ho KH. Novel PCL-based honeycomb scaffolds as drug delivery systems for rhBMP-2. Biomaterials. 2005;26(17):3739–48. doi: 10.1016/j.biomaterials.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 83.Chen FM, Wu ZF, Wang QT, Wu H, Zhang YJ, Nie X, Jin Y. Preparation of recombinant human bone morphogenetic protein-2 loaded dextran-based microspheres and their characteristics. Acta Pharmacol Sin. 2005;26(9):1093–103. doi: 10.1111/j.1745-7254.2005.00180.x. [DOI] [PubMed] [Google Scholar]
- 84.Yamamoto M, Ikada Y, Tabata Y. Controlled release of growth factors based on biodegradation of gelatin hydrogel. J Biomater Sci Polym Ed. 2001;12:77–88. doi: 10.1163/156856201744461. [DOI] [PubMed] [Google Scholar]
- 85.Morgan AW, Chan LL, Sendemir-Urkmez A, Cunningham BT, Jamison RD. Detection of growth factor binding to gelatin and heparin using a photonic crystal optical biosensor. Mater Sci Eng C Mater Biol Appl. 2010;30(5):686–90. [Google Scholar]
- 86.King W, Mohammed J, Murphy W. Modulating growth factor release from hydrogels via a protein conformational change. Soft Matter. 2009;5(12):2399–406. [Google Scholar]
- 87.King WJ, Pytel JN, Ng K, Murphy WL. Triggered drug release from dynamic microspheres via a protein conformational change. Macromol Biosci. 2010;10(6):580–4. doi: 10.1002/mabi.200900382. [DOI] [PubMed] [Google Scholar]
- 88.King WJ, Toepke MW, Murphy WL. A general route for the synthesis of functional, protein-based hydrogel microspheres using tailored protein charge. Chem Commun (Camb) 2011;47(1):526–8. doi: 10.1039/c0cc02716b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murphy WL, Dillmore WS, Modica J, Mrksich M. Dynamic hydrogels: translating a protein conformational change into macroscopic motion. Angew Chem Int Ed Engl. 2007;46(17):3066–9. doi: 10.1002/anie.200604808. [DOI] [PubMed] [Google Scholar]
- 90.Sui ZJ, King WJ, Murphy WL. Dynamic materials based on a protein conformational change. Adv Mater. 2007;19(20):3377–80. [Google Scholar]
- 91.Sui ZJ, King WJ, Murphy WL. Protein-based hydrogels with tunable dynamic responses. Adv Funct Mater. 2008;18(12):1824–31. [Google Scholar]
- 92.Shaikh Mohammed J, Murphy WL. Bioinspired Design of Dynamic Materials. Adv Mater. 2009;21(23):2361–74. [Google Scholar]
- 93.Lee J, Choi WI, Tae G, Kim YH, Kang SS, Kim SE, Kim SH, Jung Y, Kim SH. Enhanced regeneration of the ligament-bone interface using a poly(L-lactide-co-epsilon-caprolactone) scaffold with local delivery of cells/BMP-2 using a heparin-based hydrogel. Acta Biomater. 2011;7(1):244–57. doi: 10.1016/j.actbio.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 94.Chung YI, Ahn KM, Jeon SH, Lee SY, Lee JH, Tae G. Enhanced bone regeneration with BMP-2 loaded functional nanoparticle-hydrogel complex. J Control Release. 2007;121(1-2):91–9. doi: 10.1016/j.jconrel.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 95.Xu X, Jha AK, Duncan RL, Jia X. Heparin-decorated, hyaluronic acid-based hydrogel particles for the controlled release of bone morphogenetic protein 2. Acta Biomater. 2011;7(8):3050–9. doi: 10.1016/j.actbio.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arikava-Hirasawa E, Watanabe H, Takami H, Hassell JR, Yamada Y. Perlecan is essential for cartilage and cephalic development. Nature Genet. 1999;23(3):354–8. doi: 10.1038/15537. [DOI] [PubMed] [Google Scholar]
- 97.Hudalla GA, Kouris NA, Koepsel JT, Ogle BM, Murphy WL. Harnessing endogenous growth factor activity modulates stem cell behavior. Integr Biol. 2011;3(8):832–42. doi: 10.1039/c1ib00021g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koepsel JT, Murphy WL. Patterning Discrete Stem Cell Culture Environments via Localized Self-Assembled Monolayer Replacement. Langmuir. 2009;25(21):12825–34. doi: 10.1021/la901938e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ackerman SJ, Mafilios MS, Polly DW. Economic evaluation of bone morphogenetic protein Versus autogenous iliac crest bone graft in single-level anterior lumbar fusion - An evidence-based modeling approach. Spine. 2002;27(16):S94–S9. doi: 10.1097/00007632-200208151-00017. [DOI] [PubMed] [Google Scholar]
- 100.Hintze V, Moeller S, Schnabelrauch M, Bierbaum S, Viola M, Worch H, Scharnweber D. Modifications of Hyaluronan Influence the Interaction with Human Bone Morphogenetic Protein-4 (hBMP-4) Biomacromolecules. 2009;10(12):3290–7. doi: 10.1021/bm9008827. [DOI] [PubMed] [Google Scholar]
- 101.Capila I, Linhardt RJ. Heparin - Protein interactions. Angew Chem Int Ed Engl. 2002;41(3):391–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 102.Kisiday J, Jin M, Kurz B, Hung H, Semino C, Zhang S, Grodzinsky AJ. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: Implications for cartilage tissue repair. Proc Natl Acad Sci USA. 2002;99(15):9996–10001. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mason JM, Arndt KM. Coiled coil domains: Stability, specificity, and biological implications. Chembiochem. 2004;5(2):170–6. doi: 10.1002/cbic.200300781. [DOI] [PubMed] [Google Scholar]
- 104.Stetefeld J, Jenny M, Schulthess T, Landwehr R, Engel J, Kammerer RA. Crystal structure of a naturally occurring parallel right-handed coiled coil tetramer. Nat Struct Biol. 2000;7(9):772–6. doi: 10.1038/79006. [DOI] [PubMed] [Google Scholar]
- 105.Murota K, Sakamoto S, Kudo K. Reversible immobilization of protein into hydrogel using designed coiled-coil peptides. Chem Lett. 2007;36(11):1320–1. [Google Scholar]
- 106.Zhang S, Gelain F, Zhao X. Designer self-assembling peptide nanofiber scaffolds for 3D tissue cell cultures. Semin Cancer Biol. 2005;15(5):413–20. doi: 10.1016/j.semcancer.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 107.Reves BT, Bumgardner JD, Cole JA, Yang YZ, Haggard WO. Recipient of 2009 Society for Biomaterials Student Award for Outstanding Research, Master's Degree Candidate Category Lyophilization To Improve Drug Delivery For Chitosan-Calcium Phosphate Bone Scaffold Construct: A Preliminary Investigation. J Biomed Mater Res Part B. 2009;90B(1):1–10. doi: 10.1002/jbm.b.31390. [DOI] [PubMed] [Google Scholar]
- 108.Dimar JR, Glassman SD, Burkus KJ, Carreon LY. Clinical outcomes and fusion success at 2 years of single-level instrumented posterolateral fusions with recombinant human bone morphogenetic protein-2/compression resistant matrix versus iliac crest bone graft. Spine. 2006;31(22):2534–9. doi: 10.1097/01.brs.0000240715.78657.81. [DOI] [PubMed] [Google Scholar]
- 109.Fang F, Satulovsky J, Szleifer I. Kinetics of protein adsorption and desorption on surfaces with grafted polymers. Biophys J. 2005;89(3):1516–33. doi: 10.1529/biophysj.104.055079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dee KC, Puleo DA, Bizios R. An introduction to tissue-biomaterial interactions 2002 [Google Scholar]
- 111.King WJ, Murphy WL. Bioinspired Conformational Changes: an Adaptable Mechanism for Bio-responsive Protein Delivery. Polymer Chem. 2011;2(3):476–91. [Google Scholar]
- 112.Mitsak AG, Kemppainen JM, Harris MT, Hollister SJ. Effect of Polycaprolactone Scaffold Permeability on Bone Regeneration In Vivo. Tissue Eng Part A. 2011;17(13-14):1831–9. doi: 10.1089/ten.tea.2010.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huang X, Brazel CS. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J Control Release. 2001;73(2-3):121–36. doi: 10.1016/s0168-3659(01)00248-6. [DOI] [PubMed] [Google Scholar]
- 114.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11(6):471–91. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 115.Toth JM, Boden SD, Burkus JK, Badura JM, Peckham SM, McKay WF. Short-term Osteoclastic Activity Induced by Locally High Concentrations of Recombinant Human Bone Morphogenetic Protein-2 in a Cancellous Bone Environment. Spine. 2009;34(6):539–50. doi: 10.1097/BRS.0b013e3181952695. [DOI] [PubMed] [Google Scholar]
- 116.Boerckel JD, Kolambkar YM, Dupont KM, Uhrig BA, Phelps EA, Stevens HY, Garcia AJ, Guldberg RE. Effects of protein dose and delivery system on BMP-mediated bone regeneration. Biomaterials. 2011;32(22):5241–51. doi: 10.1016/j.biomaterials.2011.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kashiwagi K, Tsuji T, Shiba K. Directional BMP-2 for functionalization of titanium surfaces. Biomaterials. 2009;30(6):1166–75. doi: 10.1016/j.biomaterials.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 118.Hiemstra C, Zhong ZY, van Steenbergen MJ, Hennink WE, Feijen J. Release of model proteins and basic fibroblast growth factor from in situ forming degradable dextran hydrogels. Journal of Controlled Release. 2007;122(1):71–8. doi: 10.1016/j.jconrel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 119.Fujioka K, Maeda M, Hojo T, Sano A. Protein release from collagen matrices. Adv Drug Deliv Rev. 1998;31(3):247–66. doi: 10.1016/s0169-409x(97)00119-1. [DOI] [PubMed] [Google Scholar]
- 120.Kim J, Hefferan TE, Yaszemski MJ, Lu LC. Potential of Hydrogels Based on Poly(Ethylene Glycol) and Sebacic Acid as Orthopedic Tissue Engineering Scaffolds. Tissue Eng Part A. 2009;15(8):2299–307. doi: 10.1089/ten.tea.2008.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Patel ZS, Yamamoto M, Ueda H, Tabata Y, Mikos AG. Biodegradable gelatin microparticles as delivery systems for the controlled release of bone morphogenetic protein-2. Acta Biomater. 2008;4(5):1126–38. doi: 10.1016/j.actbio.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kolambkar YM, Dupont KM, Boerckel JD, Huebsch N, Mooney DJ, Hutmacher DW, Guldberg RE. An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials. 2011;32(1):65–74. doi: 10.1016/j.biomaterials.2010.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jha AK, Yang W, Kirn-Safran CB, Farach-Carson MC, Jia X. Perlecan domain I-conjugated, hyaluronic acid-based hydrogel particles for enhanced chondrogenic differentiation via BMP-2 release. Biomaterials. 2009;30(36):6964–75. doi: 10.1016/j.biomaterials.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brown KV, Li B, Guda T, Perrien DS, Guelcher SA, Wenke JC. Improving Bone Formation in a Rat Femur Segmental Defect by Controlling Bone Morphogenetic Protein-2 Release. Tissue Eng Part A. 2011;17(13-14):1735–46. doi: 10.1089/ten.TEA.2010.0446. [DOI] [PubMed] [Google Scholar]
- 125.Uludag H, Friess W, Williams D, Porter T, Timony G, D'Augusta D, Blake C, Palmer R, Biron B, Wozney J. rhBMP-collagen sponges as osteoinductive devices: Effects of in vitro sponge characteristics and protein pI on in vivo rhBMP pharmacokinetics. Bioartificial Organs II: Technology, Medicine, and Materials. 1999;875:369–78. doi: 10.1111/j.1749-6632.1999.tb08519.x. [DOI] [PubMed] [Google Scholar]
- 126.Jongpaiboonkit L, King WJ, Lyons GE, Paguirigan AL, Warrick JW, Beebe DJ, Murphy WL. An adaptable hydrogel array format for 3-dimensional cell culture and analysis. Biomaterials. 2008;29(23):3346–56. doi: 10.1016/j.biomaterials.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jongpaiboonkit L, King WJ, Murphy WL. Screening for 3D Environments That Support Human Mesenchymal Stem Cell Viability Using Hydrogel Arrays. Tissue Eng Part A. 2009;15(2):343–53. doi: 10.1089/ten.tea.2008.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.King WJ, Jongpaiboonkit L, Murphy WL. Influence of FGF2 and PEG hydrogel matrix properties on hMSC viability and spreading. J Biomed Mater Res A. 2010;93A(3):1110–23. doi: 10.1002/jbm.a.32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Beebe DJ, Mensing GA, Walker GM. Physics and applications of microfluidics in biology. Annu Rev Biomed Eng. 2002;4:261–86. doi: 10.1146/annurev.bioeng.4.112601.125916. [DOI] [PubMed] [Google Scholar]
- 130.Schek RM, Hollister SJ, Krebsbach PH. Delivery and protection of adenoviruses using biocompatible hydrogels for localized gene therapy. Mol Ther. 2004;9(1):130–8. doi: 10.1016/j.ymthe.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 131.Molina I, Li SM, Martinez MB, Vert M. Protein release from physically crosslinked hydrogels of the PLA/PEO/PLA triblock copolymer-type. Biomaterials. 2001;22(4):363–9. doi: 10.1016/s0142-9612(00)00192-7. [DOI] [PubMed] [Google Scholar]
- 132.Gao TJ, Kousinioris NA, Wozney JM, Winn S, Uludag H. Synthetic thermoreversible polymers are compatible with osteoinductive activity of recombinant human bone morphogenetic protein 2. Tissue Eng. 2002;8(3):429–40. doi: 10.1089/107632702760184691. [DOI] [PubMed] [Google Scholar]
- 133.Gil ES, Hudson SM. Stimuli-reponsive polymers and their bioconjugates. Prog Polym Sci. 2004;29(12):1173–222. [Google Scholar]
- 134.Yamamoto M, Tabata Y, Ikada Y. Ectopic bone formation induced by biodegradable hydrogels incorporating bone morphogenetic protein. J Biomat Sci-Polym E. 1998;9(5):439–58. doi: 10.1163/156856298x00550. [DOI] [PubMed] [Google Scholar]
- 135.Einerson NJ, Stevens KR, Kao WYJ. Synthesis and physicochemical analysis of gelatin-based hydrogels for drug carrier matrices. Biomaterials. 2003;24(3):509–23. doi: 10.1016/s0142-9612(02)00369-1. [DOI] [PubMed] [Google Scholar]
- 136.Zhang L, Furst EM, Kiick KL. Manipulation of hydrogel assembly and growth factor delivery via the use of peptide-polysaccharide interactions. J Control Release. 2006;114(2):130–42. doi: 10.1016/j.jconrel.2006.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Holmgren SK, Bretscher LE, Taylor KM, Raines RT. A hyperstable collagen mimic. Chem Biol. 1999;6(2):63–70. doi: 10.1016/S1074-5521(99)80003-9. [DOI] [PubMed] [Google Scholar]
- 138.Li CF, McCarthy JB, Furcht LT, Fields GB. An all-D amino acid peptide model of alpha 1(IV)531-543 from type IV collagen binds the alpha(3)beta(1) integrin and mediates tumor cell adhesion, spreading, and motility. Biochemistry. 1997;36(49):15404–10. doi: 10.1021/bi971817g. [DOI] [PubMed] [Google Scholar]
- 139.Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, Hubbell JA. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics. P Natl Acad Sci USA. 2003;100(9):5413–8. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Segers VFM, Tokunou T, Higgins LJ, MacGillivray C, Gannon J, Lee RT. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation. 2007;116(15):1683–92. doi: 10.1161/CIRCULATIONAHA.107.718718. [DOI] [PubMed] [Google Scholar]
- 141.Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, Rizzi SC, Davies N, Schmokel H, Bezuidenhout D, Djonov V, Zilla P, Hubbell JA. Cell-demanded release of VEGF from synthetic, biointeractive cell-ingrowth matrices for vascularized tissue growth. Faseb Journal. 2003;17(13):2260–+. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 142.Henle P, Zimmermann G, Weiss S. Matrix metalloproteinases and failed fracture healing. Bone. 2005;37(6):791–8. doi: 10.1016/j.bone.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 143.Lee KY, Peters MC, Anderson KW, Mooney DJ. Controlled growth factor release from synthetic extracellular matrices. Nature. 2000;408(6815):998–1000. doi: 10.1038/35050141. [DOI] [PubMed] [Google Scholar]
- 144.Seeherman HJ, Li XJ, Bouxsein ML, Wozney JM. rhBMP-2 induces transient bone resorption followed by bone formation in a nonhuman primate core-defect model. J Bone Joint Surg Am. 2010;92A(2):411–26. doi: 10.2106/JBJS.H.01732. [DOI] [PubMed] [Google Scholar]
- 145.Zhao X, Kim J, Cezar CA, Huebsch N, Lee K, Bouhadir K, Mooney DJ. Active scaffolds for on-demand drug and cell delivery. P Natl Acad Sci USA. 2011;108(1):67–72. doi: 10.1073/pnas.1007862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bromberg LE, Ron ES. Temperature-responsive gels and thermogelling polymer matrices for protein and peptide delivery. Adv Drug Deliv Rev. 1998;31(3):197–221. doi: 10.1016/s0169-409x(97)00121-x. [DOI] [PubMed] [Google Scholar]
- 147.Yamamoto M, Takahashi Y, Tabata Y. Controlled release by biodegradable hydrogyels enhances the ectopic bone formation of bone morphogenetic protein. Biomaterials. 2003;24(24):4375–83. doi: 10.1016/s0142-9612(03)00337-5. [DOI] [PubMed] [Google Scholar]
- 148.Vroman L, Adams AL. Identification of rapid changes at plasma-solid interfaces. J Biomed Mater Res. 1969;3(1):43–67. doi: 10.1002/jbm.820030106. [DOI] [PubMed] [Google Scholar]
- 149.Yeung DKW, Wong SYS, Griffith JF, Lau EMC. Bone marrow diffusion in osteoporosis: Evaluation with quantitative MR diffusion imaging. J Magn Reson Imaging. 2004;19(2):222–8. doi: 10.1002/jmri.10453. [DOI] [PubMed] [Google Scholar]
- 150.Alaoui-Ismaili MH, Falb D. Design of second generation therapeutic recombinant bone morphogenetic proteins. Cytokine Growth Factor Rev. 2009;20(5-6):501–7. doi: 10.1016/j.cytogfr.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 151.Tazaki J, Murata M, Akazawa T, Yamamoto M, Ito K, Arisue M, Shibata T, Tabata Y. BMP-2 release and dose-response studies in hydroxyapatite and beta-tricalcium phosphate. Biomed Mater Eng. 2009;19(2-3):141–6. doi: 10.3233/BME-2009-0573. [DOI] [PubMed] [Google Scholar]
- 152.Seeherman HJ, Archambault JM, Rodeo SA, Turner AS, D'Augusta D, Li XJ, Smith E, Wozney JM. rhBMP-12 Accelerates Healing of Rotator Cuff Repairs in a Sheep Model. J Bone Joint Surg Am. 2008;90A(10):2206–19. doi: 10.2106/JBJS.G.00742. [DOI] [PubMed] [Google Scholar]
- 153.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv Mater. 2006;18(11):1345–60. [Google Scholar]
- 154.Kinsella CR, Bykowski MR, Lin AY, Cray JJ, Durham EL, Smith DM, DeCesare GE, Mooney MP, Cooper GM, Losee JE. BMP-2-Mediated Regeneration of Large-Scale Cranial Defects in the Canine: An Examination of Different Carriers. Plast Reconstr Surg. 2011;127(5):1865–73. doi: 10.1097/PRS.0b013e31820cf2c9. [DOI] [PubMed] [Google Scholar]
- 155.Medtronic Sofamor Danek IU. Premarket Approval Application InFUSE™ Bone Graft/LT-CAGE™ Lumbar Tapered Fusion Device. 2002 Application Number: P000058. [Google Scholar]
- 156.Vihola H, Laukkanen A, Valtola L, Tenhu H, Hirvonen J. Cytotoxicity of thermosensitive polymers poly(N-isopropylacrylamide), poly(N-vinylcaprolactam) and amphiphilically modified poly(N-vinylcaprolactam) Biomaterials. 2005;26(16):3055–64. doi: 10.1016/j.biomaterials.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 157.Yamamoto M, Takahashi Y, Tabata Y. Bone induction by controlled release of BMP-2 from a biodegradable hydrogel in various animal species - from mouse to non-human primate. Asbm6: Advanced Biomaterials Vi. 2005;288-289:253–6. [Google Scholar]
- 158.Hu WW, Ward BB, Wang Z, Krebsbach PH. Bone regeneration in defects compromised by radiotherapy. J Dent Res. 2010;89(1):77–81. doi: 10.1177/0022034509352151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Alanay A, Chen CH, Lee S, Murray SS, Brochmann EJ, Miyazaki M, Napoli A, Wang JC. The adjunctive effect of a binding peptide on bone morphogenetic protein enhanced bone healing in a rodent model of spinal fusion. Spine. 2008;33(16):1709–13. doi: 10.1097/BRS.0b013e31817e9dfd. [DOI] [PubMed] [Google Scholar]
- 160.Behnam K, Phillips ML, Sliva JDP, Brochmann EJ, Duarte MEL, Murray SS. BMP binding peptide: a BMP-2 enhancing factor deduced from the sequence of native bovine bone morphogenetic protein/non-collagenous protein. J Orthop Res. 2005;23(1):175–80. doi: 10.1016/j.orthres.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 161.Lee KB, Murray SS, Taghavi CE, Song KJ, Brochmann EJ, Johnson JS, Keorochana G, Liao JC, Wang JC. Bone morphogenetic protein-binding peptide reduces the inflammatory response to recombinant human bone morphogenetic protein-2 and recombinant human bone morphogenetic protein-7 in a rodent model of soft-tissue inflammation. Spine J. 2011;11(6):568–76. doi: 10.1016/j.spinee.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 162.Taghavi CE, Lee KB, He W, Keorochana G, Murray SS, Brochmann EJ, Uludag H, Behnam K, Wang JC. Bone Morphogenetic Protein Binding Peptide Mechanism and Enhancement of Osteogenic Protein-1 Induced Bone Healing. Spine. 2010;35(23):2049–56. doi: 10.1097/BRS.0b013e3181cc0220. [DOI] [PubMed] [Google Scholar]
- 163.Takahashi Y, Yamamoto M, Yamada K, Kawakami O, Tabata Y. Skull bone regeneration in nonhuman primates by controlled release of bone morphogenetic protein-2 from a biodegradable hydrogel. Tissue Eng. 2007;13(2):293–300. doi: 10.1089/ten.2006.0088. [DOI] [PubMed] [Google Scholar]
- 164.Seeherman H, Kirker-Head C, Li X, Bouxsein ML, Wozney J. rhBMP-2 stimulates bone formation in a femoral head core decompression model in adult sheep. Transactions of the 44th Annual Meeting of the Orthopaedic Research Society 1998 [Google Scholar]
- 165.Dickerman RD, Reynolds AS, Morgan BC, Tompkins J, Cattorini J, Bennett M. rh-BMP-2 can be used safely in the cervical spine: dose and containment are the keys! Spine J. 2007;7(4):508–9. doi: 10.1016/j.spinee.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 166.Seeherman H, Li R, Bouxsein M, Kim H, Li XJ, Smith-Adaline EA, Aiolova M, Wozney JM. rhBMP-2/calcium phosphate matrix accelerates osteotomy-site healing in a nonhuman primate model at multiple treatment times and concentrations. J Bone Joint Surg Am. 2006;88A(1):144–60. doi: 10.2106/JBJS.D.02453. [DOI] [PubMed] [Google Scholar]
- 167.Klimo P, Peelle MW. Use of polyetheretherketone spacer and recombinant human bone morphogenetic protein-2 in the cervical spine: a radiographic analysis. Spine J. 2009;9(12):959–66. doi: 10.1016/j.spinee.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 168.Martin GJ, Boden SD, Morone MA, Moskovitz YA. Posterolateral intertransverse process spinal arthrodesis with rhBMP-2 in a nonhuman primate: Important lessons learned regarding dose, carrier, and safety. J Spinal Disord. 1999;12(3):179–86. [PubMed] [Google Scholar]
- 169.Bouxsein ML, Turek TJ, Blake CA, D'Augusta D, Li X, Stevens M, Seeherman HJ, Wozney JM. Recombinant human bone morphogenetic protein-2 accelerates healing in a rabbit ulnar osteotomy model. J Bone Joint Surg Am. 2001;83A(8):1219–30. doi: 10.2106/00004623-200108000-00012. [DOI] [PubMed] [Google Scholar]
- 170.Akamaru T, Suh D, Boden SD, Kim HS, Minamide A, Louis-Ugbo J. Simple carrier matrix modifications can enhance delivery of recombinant human bone morphogenetic protein-2 for posterolateral spine fusion. Spine. 2003;28(5):429–34. doi: 10.1097/01.BRS.0000048644.91330.14. [DOI] [PubMed] [Google Scholar]
- 171.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19(11):1029–34. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 172.Huang YC, Kaigler D, Rice KG, Krebsbach PH, Mooney DJ. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J Bone Miner Res. 2005;20(5):848–57. doi: 10.1359/JBMR.041226. [DOI] [PubMed] [Google Scholar]
- 173.Kempen DHR, Lu L, Heijink A, Hefferan TE, Creemers LB, Maran A, Yaszemski MJ, Dhert WJA. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials. 2009;30(14):2816–25. doi: 10.1016/j.biomaterials.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 174.Springer ING, Niehoff P, Acil Y, Marget M, Lange A, Warnke PH, Pielenz H, Roldan JC, Wiltfang J. BMP-2 and bFGF in an irradiated bone model. J Cranio Maxill Surg. 2008;36(4):210–7. doi: 10.1016/j.jcms.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 175.Lan J, Wang Z, Wang Y, Wang J, Cheng X. The effect of combination of recombinant human bone morphogenetic and protein-2 and basic fibroblast growth factor or insulin-like growth factor-I on dental implant osseointegration by confocal laser scanning microscopy. J Periodont. 2006;77(3):357–63. doi: 10.1902/jop.2006.050016. [DOI] [PubMed] [Google Scholar]
- 176.Canalis E. Growth Factor Control of Bone Mass. J Cell Biochem. 2009;108(4):769–77. doi: 10.1002/jcb.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shiozawa Y, Jung Y, Ziegler AM, Pedersen EA, Wang J, Wang Z, Song J, Wang J, Lee CH, Sud S, Pienta KJ, Krebsbach PH, Taichman RS. Erythropoietin Couples Hematopoiesis with Bone Formation. PLoS One. 2010;5(5) doi: 10.1371/journal.pone.0010853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Eschbach JW, Egrie JC, Downing MR, Browne JK, Adamson JW. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N Engl J Med. 1987;316(2):73–8. doi: 10.1056/NEJM198701083160203. [DOI] [PubMed] [Google Scholar]
- 179.Kempen DHR, Lu L, Hefferan TE, Creemers LB, Heijink A, Maran A, Dhert WJA, Yaszemski MJ. Enhanced Bone Morphogenetic Protein-2-Induced Ectopic and Orthotopic Bone Formation by Intermittent Parathyroid Hormone (1-34) Administration. Tissue Eng Part A. 2010;16(12):3769–77. doi: 10.1089/ten.tea.2010.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Krebsbach PH, Kuznetsov SA, Bianco P, Robey PG. Bone marrow stromal cells: Characterization and clinical application. Crit Rev Oral Biol Med. 1999;10(2):165–81. doi: 10.1177/10454411990100020401. [DOI] [PubMed] [Google Scholar]
- 181.Krebsbach PH, Zhang KZ, Malik AK, Kurachi K. Bone marrow stromal cells as a genetic platform for systemic delivery of therapeutic proteins in vivo: human factor IX model. J Gene Med. 2003;5(1):11–7. doi: 10.1002/jgm.292. [DOI] [PubMed] [Google Scholar]
- 182.Nussenbaum B, Krebsbach PH. The role of gene therapy for craniofacial and dental tissue engineering. Adv Drug Deliv Rev. 2006;58(4):577–91. doi: 10.1016/j.addr.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 183.Peterson B, Zhang J, Iglesias R, Kabo M, Hedrick M, Benhaim P, Lieberman JR. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Eng. 2005;11(1-2):120–9. doi: 10.1089/ten.2005.11.120. [DOI] [PubMed] [Google Scholar]
- 184.Ekrol I, Hajducka C, Court-Brown C, McQueen MM. A comparison of RhBMP-7 (OP-1) and autogenous graft for metaphyseal defects after osteotomy of the distal radius. Injury-Int J Care Inj. 2008;39:S73–S82. doi: 10.1016/S0020-1383(08)70018-4. [DOI] [PubMed] [Google Scholar]
- 185.Chen XQ, Kidder LS, Lew WD. Osteogenic protein-1 induced bone formation in an infected segmental defect in the rat femur. Journal of Orthopaedic Research. 2002;20(1):142–50. doi: 10.1016/S0736-0266(01)00060-2. [DOI] [PubMed] [Google Scholar]
- 186.Southwood LL, Frisbie DD, Kawcak CE, Ghivizzani SC, Evans CH, McIlwraith CW. Evaluation of Ad-BMP-2 for enhancing fracture healing in an infected defect fracture rabbit model. Journal of Orthopaedic Research. 2004;22(1):66–72. doi: 10.1016/S0736-0266(03)00129-3. [DOI] [PubMed] [Google Scholar]
- 187.Horii A, Wang X, Gelain F, Zhang S. Biological Designer Self-Assembling Peptide Nanofiber Scaffolds Significantly Enhance Osteoblast Proliferation, Differentiation and 3-D Migration. PLoS One. 2007;2(2) doi: 10.1371/journal.pone.0000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gelain F, Unsworth LD, Zhang S. Slow and sustained release of active cytokines from self-assembling peptide scaffolds. Journal of Controlled Release. 2010;145(3):231–9. doi: 10.1016/j.jconrel.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 189.Chau Y, Luo Y, Cheung ACY, Nagai Y, Zhang S, Kobler JB, Zeitels SM, Langer R. Incorporation of a matrix metalloproteinase-sensitive substrate into self-assembling peptides - A model for biofunctional scaffolds. Biomaterials. 2008;29(11):1713–9. doi: 10.1016/j.biomaterials.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 190.Nakahara H, Misawa H, Yoshida A, Hayashi T, Tanaka M, Furumatsu T, Tanaka N, Kobayashi N, Ozaki T. Bone Repair Using a Hybrid Scaffold of Self-Assembling Peptide PuraMatrix and Polyetheretherketone Cage in Rats. Cell Transplantation. 2010;19(6-7):791–7. doi: 10.3727/096368910X508906. [DOI] [PubMed] [Google Scholar]
- 191.Hollister SJ, Murphy WL. Scaffold Translation: Barriers between Concept and Clinic. Tissue Eng B. 2011 doi: 10.1089/ten.teb.2011.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Schuckert KH, Jopp S, Teoh SH. Mandibular Defect Reconstruction Using Three-Dimensional Polycaprolactone Scaffold in Combination with Platelet-Rich Plasma and Recombinant Human Bone Morphogenetic Protein-2: De Novo Synthesis of Bone in a Single Case. Tissue Engineering Part A. 2009;15(3):493–9. doi: 10.1089/ten.tea.2008.0033. [DOI] [PubMed] [Google Scholar]
- 193.Murphy WL, Kohn DH, Mooney DJ. Growth of continuous bonelike mineral within porous poly(lactide-co-glycolide) scaffolds in vitro. Journal of Biomedical Materials Research. 2000;50(1):50–8. doi: 10.1002/(sici)1097-4636(200004)50:1<50::aid-jbm8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 194.Murphy WL, Mooney DJ. Bioinspired growth of crystalline carbonate apatite on biodegradable polymer substrata. Journal of the American Chemical Society. 2002;124(9):1910–7. doi: 10.1021/ja012433n. [DOI] [PubMed] [Google Scholar]
- 195.Suarez-Gonzalez D, Barnhart K, Migneco F, Flanagan C, Hollister SJ, Murphy WL. Controllable mineral coatings on PCL scaffolds as carriers for growth factor release. Biomaterials. 2012;33(2):713–21. doi: 10.1016/j.biomaterials.2011.09.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hu WW, Elkasabi Y, Chen HY, Zhang Y, Lahann J, Hollister SJ, Krebsbach PH. The use of reactive polymer coatings to facilitate gene delivery from poly (epsilon-caprolactone) scaffolds. Biomaterials. 2009;30(29):5785–92. doi: 10.1016/j.biomaterials.2009.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Elkasabi YM, Lahann J, Krebsbach PH. Cellular transduction gradients via vapor-deposited polymer coatings. Biomaterials. 2011;32(7):1809–15. doi: 10.1016/j.biomaterials.2010.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ito Y. Covalently immobilized biosignal molecule materials for tissue engineering. Soft Matter. 2008;4(1):46–56. doi: 10.1039/b708359a. [DOI] [PubMed] [Google Scholar]
- 199.Masters KS. Covalent Growth Factor Immobilization Strategies for Tissue Repair and Regeneration. Macromolecular Bioscience. 2011;11(9):1149–63. doi: 10.1002/mabi.201000505. [DOI] [PubMed] [Google Scholar]
- 200.Tsujigiwa H, Nagatsuka H, Gunduz M, Rodriguez A, Rivera RS, LeGeros RZ, Inoue M, Nagai N. Effects of immobilized recombinant human bone morphogenetic protein-2/succinylated type I atelocollagen on cellular activity of ST2 cells. Journal of Biomedical Materials Research Part A. 2005;75A(1):210–5. doi: 10.1002/jbm.a.30416. [DOI] [PubMed] [Google Scholar]
- 201.Tsujigiwa H, Nagatsuka H, Lee YJ, Han PP, Gunduz M, LeGeros RZ, Inoue M, Yamada M, Nagai N. Immobilized rhBMP-2/succinylated type I atelocollagen gene expression of intracellular signaling molecules on ST2 cells. Journal of Biomedical Materials Research Part A. 2006;77A(3):507–11. doi: 10.1002/jbm.a.30661. [DOI] [PubMed] [Google Scholar]
- 202.Liu HW, Chen CH, Tsai CL, Lin IH, Hsiue GH. Heterobifunctional poly(ethylene glycol)-tethered bone morphogenetic protein-2-stimulated bone marrow mesenchymal stromal cell differentiation and osteogenesis. Tissue Engineering. 2007;13(5):1113–24. doi: 10.1089/ten.2006.0209. [DOI] [PubMed] [Google Scholar]
- 203.Puleo DA, Kissling RA, Sheu MS. A technique to immobilize bioactive proteins, including bone morphogenetic protein-4 (BMP-4), on titanium alloy. Biomaterials. 2002;23(9):2079–87. doi: 10.1016/s0142-9612(01)00339-8. [DOI] [PubMed] [Google Scholar]
- 204.Lorenz C, Hoffmann A, Gross G, Windhagen H, Dellinger P, Mohwald K, Dempwolf W, Menzel H. Coating of Titanium Implant Materials with Thin Polymeric Films for Binding the Signaling Protein BMP2. Macromolecular Bioscience. 2011;11(2):234–44. doi: 10.1002/mabi.201000342. [DOI] [PubMed] [Google Scholar]
- 205.Hu JL, Duppatla V, Harth S, Schmitz W, Sebald W. Site-Specific PEGylation of Bone Morphogenetic Protein-2 Cysteine Analogues. Bioconjugate Chemistry. 2010;21(10):1762–72. doi: 10.1021/bc9005706. [DOI] [PubMed] [Google Scholar]
- 206.Kinstler O, Molineux G, Treuheit M, Ladd D, Gegg C. Mono-N-terminal poly(ethylene glycol)-protein conjugates. Advanced Drug Delivery Reviews. 2002;54(4):477–85. doi: 10.1016/s0169-409x(02)00023-6. [DOI] [PubMed] [Google Scholar]
- 207.Hu JL, Sebald W. N-terminal specificity of PEGylation of human bone morphogenetic protein-2 at acidic pH. Int J Pharm. 2011;413(1-2):140–6. doi: 10.1016/j.ijpharm.2011.04.043. [DOI] [PubMed] [Google Scholar]
- 208.Link AJ, Mock ML, Tirrell DA. Non-canonical amino acids in protein engineering. Current Opinion in Biotechnology. 2003;14(6):603–9. doi: 10.1016/j.copbio.2003.10.011. [DOI] [PubMed] [Google Scholar]







