Abstract
Lymphocytic infiltration of primary cutaneous melanoma has been demonstrated to be of prognostic significance. Tumor infiltrating lymphocytes (TILs) were evaluated on histologic sections of pT4 primary cutaneous melanoma from 293 patients, accrued in protocols 1690 and 1694 of the Eastern Cooperative Oncology Group. Data for the 60-month follow-up were available. Statistical analysis of the pathologic data evaluated the correlation of regional lymph node metastasis and response to interferon therapy, overall survival, and relapse-free survival.
In multivariate analysis, there was significant correlation of the presence of TILs and improved survival. The presence of TILs did not affect the survival of patients treated with interferon alfa-2b. Presence of a localized dominant tumor nodule within the primary tumor had an adverse effect on relapse-free survival (P = .044) that was also marginally present for overall survival (P = .112).
The presence of TILs has prognostic but not predictive value, and the presence of a dominant nodule in the primary lesion represents a new adverse prognostic factor that should be incorporated in the evaluation of primary melanoma. This study confirmed the importance of tumor ulceration and the number of positive lymph nodes on outcome.
Keywords: Primary melanoma, Tumor-infiltrating lymphocytes, Dominant nodule, Relapse-free survival, Overall survival, Histology
The major predictors of survival for localized cutaneous melanoma include primary tumor thickness, ulceration, and the presence or absence of lymph node involvement.1,2 Other factors of potential prognostic importance that have been investigated are tumor-infiltrating lymphocytes (TILs),3-7 mitotic rate,8,9 the presence of tumor regression, and tumor vascularity.10-12 Many of these prognostic factors are inter-related. For metastatic melanoma, the most important prognostic factors are the organ site of metastasis and the presence or absence of elevated blood serum levels of lactic dehydrogenase.13
TILs are a histopathologic marker of host immune response to melanoma. There is ample evidence that melanoma elicits specific humoral and cellular immune responses. The majority of melanomas, especially those less than 1 mm thick, manifest lymphoid host response that is located peripherally to or permeating the tumor.14 Previous studies have correlated the presence of TILs and the intensity of TIL infiltrates with other tumor characteristics and with clinical variables such as overall survival (OS).2 Clinical trials using irradiated autologous melanoma cells genetically engineered to produce granulocyte-macrophage colony-stimulating factor in patients with melanoma have suggested the relevance of TILs to immunotherapy response of human melanoma.15
A number of tumor-associated antigens have been found to stimulate CD8+ and CD4+ TILs after presentation by MHC class I or II molecules, resulting in tumor-specific cytokine production or tumor lysis.16-18 Analysis of the presence of a granule-associated protein of cytotoxic T cells and NK cells (TIA-1) in TIL populations of primary invasive melanoma and nevi revealed significant variability in the TIA-1+ TILs in advanced primary melanoma and suggested that this marker may prove to be an important prognostic parameter in the evaluation of primary melanoma.19 The CD4+/CD8 ratio in tumor and peripheral blood was found to be a statistically significant prognostic parameter.20,21 The appearance of autoantibodies to antigens of the thyroid and other endocrine tissues and the clinical manifestations of autoimmunity some-times directed against melanocytic target antigens developing during treatment with interferon alfa-2b (IFNα2b) has more recently been shown to be associated with statistically significant improvements in relapse-free survival (RFS) and OS of patients with high-risk melanoma.22
This study was undertaken to determine whether a reproducible and relatively simple histologic evaluation of the pattern of lymphocytic infiltrates in thick advanced primary melanoma might predict RFS and OS and to determine if the presence or absence of TILs influenced the response to IFNα2b treatment in cohorts of patients who were uniformly staged and treated in 2 prospective randomized Intergroup trials for which follow-up exceeds 60 months.
Materials and Methods
Eligibility criteria for intergroup trials E1694 and E1690 included patients with primary cutaneous melanoma thicker than 4 mm in the absence of regional node metastases (American Joint Committee on Cancer stage II B disease) or the presence of regional lymph node involvement (American Joint Committee on Cancer stage III disease) at diagnosis or at subsequent recurrence.23,24 All pathologic material received for each of the patients accrued into these Eastern Cooperative Oncology Group (ECOG)-led intergroup protocols was reviewed on entry, and the diagnosis of melanoma was confirmed along with the assessment of 15 characteristics of the primary tumor. Data were recorded on standardized ECOG pathology forms by the ECOG pathologist and study cochair (U.N.M.R.). This information included tumor size, ulceration, levels of invasion as defined by Clark et al,2 Breslow thickness, presence of partial regression, mitoses, and surgical treatment. For each case, the pathology slides were assigned ECOG protocol case sequence numbers at the pathology coordinating office of ECOG located at Northwestern University, Chicago, IL, such that patient information could not be linked to the slides by the reviewing pathologists. The information from the completed pathology forms was entered into the central ECOG database at the Coordinating Center in Boston, MA.
All slides were reexamined by 2 pathologists (U.N.M.R. and M.C.M.) following approval of the study protocol (IRB No. 020746) by the institutional review board at the University of Pittsburgh, Pittsburgh, PA, and the ECOG scientific review committees. Pathology slides of the primary tumor were available from 14 patients in the E1690 trial and 342 patients in the E1694 trial. This study of advanced-stage melanoma included cases at high risk by virtue of primary tumor thickness (≥4 mm) or because they were associated with regional lymph node metastases at diagnosis. The thickness of the primary tumor in this latter group ranged from 0.76 to 1.5 mm (49 cases). Of these 49 cases, 34 patients had tumors that were 1 mm or less in thickness, for which there was evidence of partial regression. In 94 cases, tumors ranged from 1.5 to 4 mm in thickness, and the rest were greater than 4 mm in thickness (188 cases).
The patterns of TILs were reevaluated according to consensus guidelines that were adopted for wider corroboration of the importance of TILs in melanoma. TILs were defined as those that percolated between tumor cells and surrounded individual tumor cells. The infiltrate was assessed quantitatively and reached an approximate tumor/lymphocyte ratio of 1:5.
The intensity of lymphocytic infiltrates and their locations were evaluated. Focal refers to a single collection of few lymphocytes; multifocal refers to multiple collections of few lymphocytes; and segmental is a large confluent collection of lymphocytes in the center or at the periphery of the tumor occupying at least a third but not the entire portion of the vertical growth phase of the tumor or at its circumference.
The intensity of lymphocytic infiltrates, albeit partly subjective, was evaluated. Grade 1 refers to few lymphocytes, no less than 10 per high-power field (×40) (nonbrisk); grade 2 to approximately 10 to 20 lymphocytes per high-power field; and grade 3 to a density of lymphocytes greater than 20 per high-power field throughout the lesion (brisk).
These data with respect to the tumor were assigned specific numbers for statistical computation. In each of the cases, the findings were entered on a standardized form.
Partial regression was defined as the presence of fibrosis of the papillary dermis with ectatic vessels, associated with flattening of overlying epidermal rete and a scant to absent melanoma in situ component in this area. In addition, variable amounts of lymphocytes and melanophages were associated with lesions in which the diagnosis of partial regression was made. The cases with sectioning artifacts, cases of suboptimal quality, or cases in which a small and inadequate portion of the primary tumor was submitted were excluded from the study. Any unusual findings were recorded in the comment section.
Five-year truncated OS and RFS data were used for this analysis because it was deemed most reasonable to use the 5-year OS and RFS data in assessing the TIL data. Patients who died or in whom disease progressed after 5 years were censored at 5 years for OS and RFS, respectively. For simplicity in terminology, OS and RFS truncated at 5 years are referred to as OS and RFS henceforth. The method of Kaplan and Meier was used to evaluate OS and RFS in univariate analysis. The distribution of OS and RFS by patterns of lymphocytic infiltration and their location were compared using the log-rank test. Other prognostic factors such as ECOG performance status, ulceration, Breslow thickness, Clark level of the primary tumor, presence of angiolymphatic and perineural invasion, stage and nodal involvement, and interferon treatment were also evaluated. The stratified log-rank test was also used. Cox proportional hazards regression models were used to evaluate the patterns of infiltration and locations, adjusting for other prognostic factors. Associations of categorical data were assessed by using the Fisher exact test. All P values reported in this article are based on 2-sided testing.
Results
For the study, 293 patients were evaluated (12 from E1690 and 281 from E1694). There were 188 male and 105 female patients; 151 patients were 50 years or younger, and 142 were older than 50 years. Of the patients, 144 received high-dose interferon and 149 patients were assigned observation (E1690) or vaccination (E1694) with GMK (Progenics, Tarrytown, NY).
Among patients with thick tumors, a distinct pathologic variant was identified in which an expansile nodule composed of morphologically homogeneous cells of nevoid or pleomorphic morphologic features was identified Image 1 and Image 2. This pathologic finding may represent a clonal expansion of morphologically similar cells within the vertical growth phase and is referred to as a “dominant nodule” (DN). DN was not a rare finding, occurring in 53 of 293 cases evaluated Table 1, of which only 9 had discernible TILs.
Image 1.
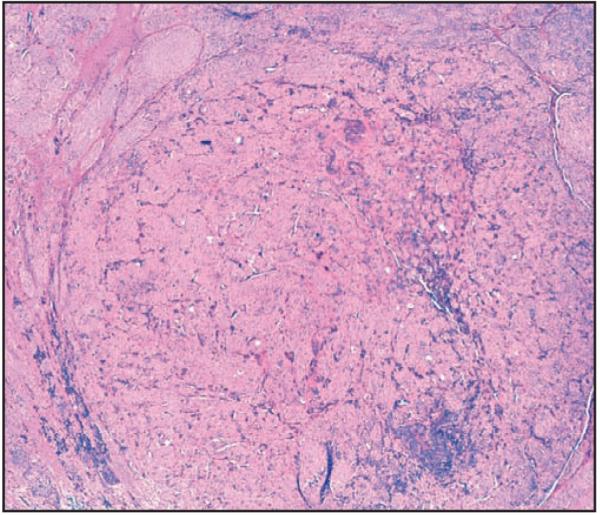
Low magnification of a primary melanoma demonstrating the distinct expansile dominant nodule that contains melanin pigment (H&E, ×4).
Image 2.
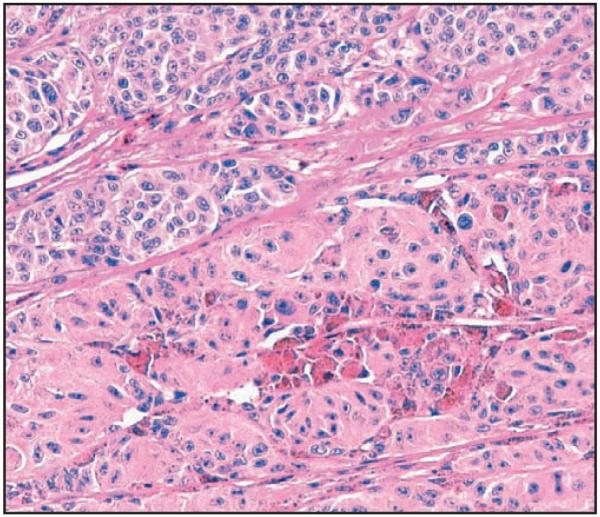
High magnification of a dominant nodule demonstrating the differences in morphologic features in comparison with adjacent tumor (H&E, ×20).
Table 1.
Frequency of Dominant Nodules in 293 Cases of pT4 Primary Cutaneous Melanoma
| Dominant Nodule | Frequency (%) |
|---|---|
| Nodule absent | 240 (81.9) |
| Nodule present | 53 (18.1) |
| Tumor-infiltrating lymphocytes present within nodule |
9/53 (17) |
The presence of a DN was correlated with an adverse prognosis in relation to RFS (P = .044) Figure 1A, but only an adverse trend in relation to OS (P = .112) Figure 1B. The 5-year RFS rate for patients with DN was 0.35 (95% confidence interval [CI], 0.22-0.49), while the RFS for patients without DN was 0.47 (95% CI, 0.40-0.53).
Figure 1.
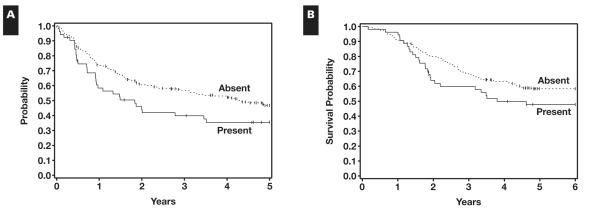
A, Dominant nodule correlated with adverse prognosis in relation to relapse-free survival. Nodule present, n = 53; relapse or death without relapse, 32; censored, 21; median, 1.8 years. Nodule absent, n = 240; relapse or death without relapse, 121; censored, 119; median, 4.3 years. B, Presence of the dominant nodule shows an adverse trend in relation to overall survival. Nodule present, n = 53; died, 27; alive, 26; median, 3.8 years. Nodule absent, n = 240; died, 99; alive, 141; median, not reached.
The TIL status of the tumor adjacent to the DN was evaluated to determine whether this had any effect on prognosis in these 53 patients. The presence of a brisk or nonbrisk TIL infiltrate in the surrounding tumor was a marginally significant factor (P = .09) compared with cases in which there were no TILs observed in any of the tumor. However, there were few cases in which the tumor adjacent to the DN demonstrated brisk TILs. The presence of a DN had a significantly adverse effect on RFS when analysis was stratified by TIL pattern (P =.038). Treatment had no impact on survival in these cases.
The presence of a DN had an adverse effect on OS (P = .089) and RFS (P = .051) when stratified according to lymph node involvement. In the cases in which a DN was present, 21 patients had positive lymph nodes and 32 had negative lymph nodes, but there was no difference in OS or RFS in the lymph node–positive and lymph node–negative groups.
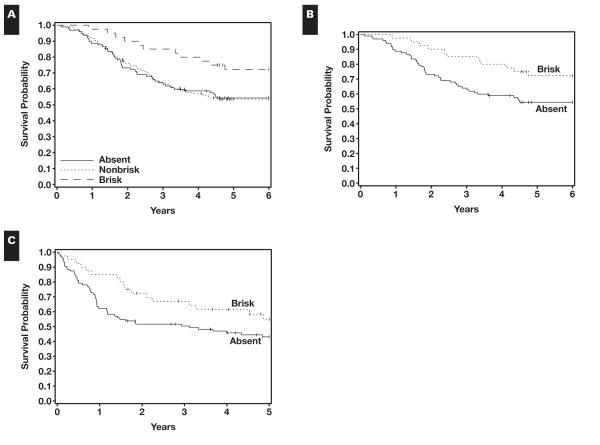
In the study, 156 patients (53.2%) had nonbrisk TILs, 40 (13.7%) had brisk TILs, and 97 (33.1%) had no TILs. Among the 156 patients with nonbrisk TILs, the most common location of TILs was peripheral (55.8%), and the most common distribution was multifocal (58.3%). The most common intensity of TILs was nonbrisk (54.5%). Among the 40 patients with brisk TILs, the most common intensity was brisk (60%) Table 2. When the OS and RFS were evaluated with respect to the location and intensity of lymphocytic infiltrates, there were no significant differences. The 5-year OS rates were 0.72 (95% CI, 0.58-0.86) in patients with brisk TILs, 0.54 (95% CI, 0.46-0.62) in patients with nonbrisk TILs, and 0.54 (95% CI, 0.44-0.64) in patients with no TILs. The difference in OS was marginally significant for these 3 groups divided according to TIL infiltrate (P = .077) Figure 2A. Comparisons of OS for patients with brisk or nonbrisk vs no TILs revealed significant differences (P = .026 and P = .035, respectively) Figure 2B and Figure 2C. The difference in RFS of patients with brisk vs absent TILs was marginally significant (P = .090). The presence of TILs specified as earlier shown by others to have prognostic value, when evaluated using consensus review by one of us3 of earlier works in which this finding achieved prognostic significance, did not achieve statistical significance in relation to RFS or OS among patients with brisk and nonbrisk TIL groups studied herein.
Table 2.
Characteristics of Tumor-Infiltrating Lymphocytes (TILs) in 293 Cases of pT4 Primary Cutaneous Melanoma
| Frequency (%) | |
|---|---|
| Absent | 97 (33.1) |
| Nonbrisk | 156 (53.2) |
| Brisk | 40 (13.7) |
| TIL location (nonbrisk; n = 156) | |
| Peripheral | 87 (55.8) |
| Central | 13 (8.3) |
| Both | 56 (35.9) |
| TIL distribution (nonbrisk; n = 156) | |
| Focal | 31 (19.9) |
| Multifocal | 91 (58.33) |
| Segmental | 34 (21.8) |
Figure 2.
A, Presence of tumor-infiltrating lymphocytes (TILs) and overall survival. TILs absent, n = 97; died, 44; alive, 53. TILS nonbrisk, n = 156; died, 71; alive, 85. TILs brisk, n = 40; died, 11; alive, 29. B, Overall survival by TILs. TILs absent, n = 97; died, 44; alive, 53. TILs brisk, n = 40; died, 11; alive, 29. C, Relapse-free survival by TILs. TILs absent, n = 97; relapse or death without relapse, 53; censored, 44; median, 3.1 years. TILs brisk, n = 40; relapse or death without relapse, 17; censored, 23; median, not reached.
Multivariate analysis for TILs, adjusting for other prognostic factors of tumor thickness (Breslow), ulceration, and nodal status revealed that TILs still are significant or marginally so after adjusting for prognostic factors. Table 3 summarizes the results from multivariate models. In the Cox model, TIL status grouped according to brisk vs absent/nonbrisk was significantly associated with OS (P = .033) and RFS (P = .055), while adjusting for Breslow thickness, node involvement, and ulceration.
Table 3.
Cox Multivariate Models for Survival in 270 Cases of pT4 Primary Cutaneous Melanoma
| Overall Survival |
Relapse-Free Survival |
|||
|---|---|---|---|---|
| Variable | HR (95% CI) | P | HR (95% CI) | P |
| TILs (brisk/absent, nonbrisk) | 0.48 (0.46-0.92) | .033 | 0.57 (0.32-1.01) | .055 |
| TILs (nonbrisk/absent, brisk) | 0.93 (0.63-1.36) | .695 | 0.81 (0.53-1.15) | .235 |
| Breslow thickness (>1 mm/≤1 mm) | 1.81 (1.22-2.67) | .099 | 1.43 (0.76-2.40) | .259 |
| Node involvement (yes/no) | 2.03 (1.32-3.11) | .001 | 1.44 (0.99-2.08) | .053 |
| Ulcer (yes/no) | 1.87 (1.28-2.73) | .001 | 1.89 (1.34-2.67) | <.001 |
CI, confidence interval; HR, hazards ratio; TILs, tumor-infiltrating lymphocytes.
Discussion
Histologically, primary cutaneous melanomas manifest various patterns of lymphocytic infiltration, varying in density and distributed at the advancing margin of the invasive component of the tumor or associated with regressive changes across the lesion. The TIL infiltrate that has been considered most meaningful is the infiltrate that occurs throughout the parenchyma of the tumor. Various factors have been identified that may have a prognostic role in relation to RFS and OS of patients treated with immunotherapy for advanced stage IV melanoma.
The significance and predictive value of lymphocytic infiltration of primary cutaneous melanoma has been examined in multiple prior studies suggesting that this evaluation should be considered as a routine prognostic parameter for patients with clinical stage I melanoma.25-28 Some studies have focused on the reproducibility of methods for evaluation for TILs among pathologists, concluding that categorization of TILs into groups with brisk, nonbrisk, and absent (as formulated by Clark) TILs could be easily taught and adopted to achieve an acceptable level of reproducibility.5 In this study, we tested these methods and further subcategorized the density and localization of lymphocytes among patients with T4 primary cutaneous melanoma and patients with nodal metastasis from any primary lesion in the intergroup adjuvant therapeutic trials E1690 and E1694 in which treatment and evaluation were well defined and consistent. This trial experience has mature follow-up to serve as the basis for an evaluation of whether TIL infiltrates are predictive of recurrence and/or mortality and to evaluate the predictive usefulness of TIL evaluation in relation to therapeutic benefit of IFNα therapy in these clinical trials.
The results of our analysis indicate that in patients with thick primary and/or high-stage melanoma with nodal involvement, the presence of lymphocytic infiltrates is associated with better RFS that is marginally significant (P = .09). The degree and pattern of lymphocyte localization to specific regions of the tumor did not correlate with treatment response or other outcomes. Results of previous studies26,29,30 and our own data reported herein imply that the subsets of lymphocytes that comprise TILs in primary melanomas vary with thickness, and it may be useful to study and compare the role of TILs in larger groups of patients with tumors of various thicknesses evaluated for lymphocyte subsets and functional capacities.
A very interesting and novel component of this study relates to the identification of a DN in 53 cases of deep primary melanoma in which this morphologic finding was documented within the vertical growth phase of the melanoma. This new feature was associated with an adverse outcome in relation to RFS that was statistically significant and a trend toward an adverse effect on OS that was marginally significant. The group of patients with primary tumors containing a DN and absent TILs had the shortest median survival of all groups evaluated in this study. Patients without a DN had a longer median RFS than patients in whom a DN was found. Among the 53 cases in which a DN was present in the vertical growth phase, 21 patients had positive lymph nodes, and 32 had negative lymph nodes. No differences in OS or RFS according to the presence of lymph node involvement were observed, suggesting that the adverse significance of the primary pathologic feature we have termed dominant nodule overwhelmed nodal status. This feature has not previously been documented in the literature, and we suspect that the presence of DNs among more aggressive melanomas may have been interpreted as a component of vertical growth phase. We have previously noted the adverse prognostic significance of microsatellites in the analysis of the pathology of the ECOG trial E1690,23 but in contrast with microsatellites, the DN is contiguous with or located within the invasive component. The DN is best appreciated at low-power magnification and is morphologically distinct from adjacent melanoma cell populations. The finding of a DN has a prognostic significance that appears to be tanta-mount to nodal metastasis.
The presence or absence of TILs and the pattern of infiltration in this series has not revealed a significant impact on survival among patients with DNs, although patients with tumors containing TILs overall had a better RFS, and only a few (9 tumors) contained TILs. Therefore, although the numbers are small to achieve statistical significance, this finding may reflect the fact that DNs may be more frequent in the tumors of lymphocyte-depleted patients in whom TILs are absent or nonbrisk. Future molecular studies should address samples of the DN nodules to determine if they are truly clonal. If clonality can be documented, these findings would be of interest to compare with samples from adjacent tumor, matched metastases in the draining lymph nodes, and distant metastases. The presence of the DN should be identified and systematically documented in patients with melanoma of low and intermediate risk.
A recent study noted the paucity of nodal involvement among patients with thin primary melanomas (3.6%),31 and the prognostic significance of positive sentinel nodes for patients with thin primary melanomas was undetermined.32 We found 34 cases (11.6%) in which the melanoma was less than 1 mm Breslow thickness, but regional lymph nodes were positive, of which 9 cases were microstaged as Clark level II with a Breslow thickness less than 1 mm. These tumors demonstrated partial regression.
The presence of lymph node metastases was a significant adverse prognostic factor for RFS and OS (P = .01 and P = .02, respectively) among patients who entered this trial in relation to the development of metastatic disease after entry to the study (P = .0002). The number of positive nodes was associated with increased risk of relapse compared with node-negative cases. This study has confirmed the previously documented prognostic importance of ulceration and thickness of the primary melanoma in relation to RFS and OS and the importance of the number of lymph nodes involved.33 Our data also support the current concept that in primary cutaneous melanoma, the presence of lymph node metastases at initial diagnosis is more important than tumor thickness and other conventional morphologic parameters. Although the presence of brisk or nonbrisk TILs in the primary tumor was a significant prognostic factor in multivariate analysis (adjusting for other risk factors), TILs did not achieve significance in univariate analysis. We suggest that TILs have a prognostic role in thinner tumors before they enter the high-risk category. The fact that no favorable prognostic association was found in relation to survival, even in tumors with a brisk infiltrate treated with interferon adjuvant therapy, suggests that the importance of this index of the immune system may be diminished in the high-risk patient populations we have studied. This finding will be important to further investigate in relation to other adjuvant therapies as more effective agents are developed in the future.
References
- 1.Balch CM, Soong SJ, Greshenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 2.Clark WH, Elder DE, Guerry D, et al. A model for predicting survival in stage 1 melanoma based upon tumor progression. J Natl Cancer Inst. 1981;81:1893–1904. doi: 10.1093/jnci/81.24.1893. [DOI] [PubMed] [Google Scholar]
- 3.Clemente CG, Mihm MC, Jr, Bufalino R, et al. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–1310. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Taylor RC, Patel A, Panageas KS, et al. Tumor infiltrating lymphocytes predict sentinel lymph node positivity in patients with cutaneous melanoma. J Clin Oncol. 2007;25:869–875. doi: 10.1200/JCO.2006.08.9755. [DOI] [PubMed] [Google Scholar]
- 5.Busam KJ, Antonescu CR, Marghoob AA, et al. Histologic classification of tumor-infiltrating lymphocytes in primary cutaneous malignant melanoma: a study of interobserver agreement. Am J Clin Pathol. 2001;115:856–860. doi: 10.1309/G6EK-Y6EH-0LGY-6D6P. [DOI] [PubMed] [Google Scholar]
- 6.Paek SC, Griffith KA, Johnson TM, et al. The impact of factors beyond Breslow depth on predicting sentinel node positivity in melanoma. Cancer. 2007;109:100–108. doi: 10.1002/cncr.22382. [DOI] [PubMed] [Google Scholar]
- 7.Mihm MC, Jr, Clemente CG, Cascinelli N. Tumor infiltrating lymphocytes in lymph node melanoma metastases: a histopathologic prognostic indicator and an expression of local immune response. Lab Invest. 1996;74:43–47. [PubMed] [Google Scholar]
- 8.Gimotty PA, Van Belle P, Elder DE, et al. Biologic and prognostic significance of dermal Ki67 expressions, mitoses, and tumorigenicity in thin invasive cutaneous melanoma. J Clin Oncol. 2005;23:8048–8056. doi: 10.1200/JCO.2005.02.0735. [DOI] [PubMed] [Google Scholar]
- 9.Sondak VK, Taylor JM, Sabel MS, et al. Mitotic rate and younger age are predictors of sentinel node positivity: lessons learned from a generation of a probabilistic model. Ann Surg Oncol. 2004;11:247–258. doi: 10.1245/aso.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 10.Kashani-Sabet M, Sagebiel RW, Ferreira CM, et al. Tumor vascularity in the prognostic assessment of primary cutaneous melanoma. J Clin Oncol. 2002;20:1826–1831. doi: 10.1200/JCO.2002.07.082. [DOI] [PubMed] [Google Scholar]
- 11.Humphrey S, Walsh NM, Delaney L, et al. Prognostic significance of vascularity in cutaneous melanoma: pilot study using in vivo confocal scanning laser microscopy. J Cutan Med Surg. 2006;10:122–127. doi: 10.2310/7750.2006.00031. [DOI] [PubMed] [Google Scholar]
- 12.Kiss J, Tímár J, Somlai B, et al. Association of microvessel density with infiltrating cells in human cutaneous malignant melanoma. Pathol Oncol Res. 2007;13:21–31. doi: 10.1007/BF02893437. [DOI] [PubMed] [Google Scholar]
- 13.Balch CM, Buzaid AC, Soong SJ, et al. New TNM melanoma staging system: linking biology and natural history to clinical outcome. Semin Surg Oncol. 2003;21:43–52. doi: 10.1002/ssu.10020. [DOI] [PubMed] [Google Scholar]
- 14.King R, Googe PB, Mihm MC., Jr Thin melanomas. Clin Lab Med. 2000;20:713–729. [PubMed] [Google Scholar]
- 15.Soiffer R, Lynch T, Mihm M, et al. Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte-macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 1998;95:13141–13146. doi: 10.1073/pnas.95.22.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng G. MHC class II–restricted tumor antigens recognized by CD4 positive T cells: new strategies for cancer vaccine design. J Immunother. 2001;24:195–204. [PubMed] [Google Scholar]
- 17.Zarour H, De Smet C, Lehmann F, et al. The majority of autologous cytolytic T-lymphocyte clones derived from peripheral blood lymphocytes of a melanoma patient recognize an antigenic peptide derived from gene Pmel17/gp100. J Invest Dermatol. 1996;107:63–67. doi: 10.1111/1523-1747.ep12298177. [DOI] [PubMed] [Google Scholar]
- 18.Kirken A, Dzhandzhugazyan K, Zeuthen J. Melanoma associated antigens recognized by cytotoxic T lymphocytes. APMIS. 1998;106:665–679. doi: 10.1111/j.1699-0463.1998.tb00210.x. [DOI] [PubMed] [Google Scholar]
- 19.Lyle S, Salhany KE, Elder D. T1A-1 positive tumor infiltrating lymphocytes in nevi and melanomas. Mod Pathol. 2000;13:52–55. doi: 10.1038/modpathol.3880009. [DOI] [PubMed] [Google Scholar]
- 20.Kirkwood JM, Bryant J, Schiller JH, et al. Immunomodulatory function of interferon-gamma in patients with metastatic melanoma: results of a phase II-B trial in subjects with metastatic melanoma, ECOG study E 4987. Eastern Cooperative Oncology Group. J Immunother. 1997;20:146–157. doi: 10.1097/00002371-199703000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Hernberg M, Turunen JP, Muhonen T, et al. Tumor-infiltrating lymphocytes in patients with metastatic melanomas receiving chemoimmunotherapy. J Immunother. 1997;20:488–495. doi: 10.1097/00002371-199711000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Gogas H, Ioannovich J, Dafni U, et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med. 2006;354:709–718. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]
- 23.Rao UN, Ibrahim J, Flaherty LE, et al. Implications of microscopic satellites of the primary and extracapsular lymph node spread in patients with high-risk melanoma: pathologic corollary of Eastern Cooperative Oncology Group Trial E1690. J Clin Oncol. 2002;20:2053–2057. doi: 10.1200/JCO.2002.08.024. [DOI] [PubMed] [Google Scholar]
- 24.Kirkwood JM, Ibrahim JG, Sondak VK, et al. High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of Intergroup Trial E1690/S9111/C9190. J Clin Oncol. 2000;18:2444–2458. doi: 10.1200/JCO.2000.18.12.2444. [DOI] [PubMed] [Google Scholar]
- 25.McGovern VJ, Shaw HM, Milton JW. Lymphocytic infiltration and survival in malignant melanoma. In: Ackerman AB, editor. Pathology of Malignant Melanoma. Mason Publishing; New York, NY: 1981. pp. 341–344. [Google Scholar]
- 26.Hanson MG, McCarten AB. Tumor thickness and lymphocytic infiltration in melanoma of head and neck. Am J Surg. 1974;128:557–561. doi: 10.1016/0002-9610(74)90275-x. [DOI] [PubMed] [Google Scholar]
- 27.Larson TF, Grude TH. A retrospective histological study of 669 cases of primary cutaneous malignant melanoma in clinical stage I. 3. The relation between the tumour-associated lymphocyte infiltration and age and sex, tumour cell type, pigmentation, cellular atypia, mitotic count, depth of invasion, ulceration, tumour type and prognosis. Acta Pathol Microbiol Scand A. 1978;86:523–530. [PubMed] [Google Scholar]
- 28.Haanen JB, Baaras A, Gomez R, et al. Melanoma-specific tumor-infiltrating lymphocytes but not circulating melanoma-specific T cells may predict survival in resected advanced-stage melanoma patients. Cancer Immunol Immunother. 2006;55:451–458. doi: 10.1007/s00262-005-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clemente C, Rao S, Lupetti R, et al. Immunohistochemical analysis of the T-cell receptor beta-chain variable regions expressed by T lymphocytes infiltrating primary human melanoma. Lab Invest. 1998;78:619–627. [PubMed] [Google Scholar]
- 30.Yazdi AS, Morstedt K, Puchta U, et al. Heterogeneity of T-cell clones infiltrating primary malignant melanomas. J Invest Dermatol. 2006;126:393–398. doi: 10.1038/sj.jid.5700082. [DOI] [PubMed] [Google Scholar]
- 31.Bedrosian I, Faries MB, Guerry D, IV, et al. Incidence of sentinel node metastasis in patients with thin primary melanoma (≤ 1 mm) with vertical growth phase. Ann Surg Oncol. 2000;7:262–267. doi: 10.1007/s10434-000-0262-z. [DOI] [PubMed] [Google Scholar]
- 32.Wong SL, Brady MS, Busam KJ, et al. Results of sentinel lymph node biopsy in patients with thin melanoma. Ann Surg Oncol. 2006;13:302–309. doi: 10.1245/ASO.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 33.Edge SB, Byrd DR, Compton CC, et al.for the American Joint Committee on Cancer, editors. Cancer Staging Manual. 7th ed Springer; New York, NY: 2010. Melanoma of skin; pp. 325–343. [Google Scholar]