Abstract
Osteoarthritis (OA) affects over 40 million people annually. We evaluated interleukin-1 receptor antagonist (IL-1ra) gene transfer in an equine model based on IL-1ra protein therapy which inhibits inflammation through blocking IL-1. Using the self-complementary adeno-associated virus (scAAV)IL-1ra equine gene as a starting construct, we optimized the transgene cassette by analyzing promoters (cytomegalovirus (CMV) versus chicken β-actin hybrid (CBh)), coding sequences (optimized versus unoptimized), vector capsid (serotype 2 versus chimeric capsid), and biological activity in vitro. AAV serotypes 2 and 2.5 CMV scAAVoptIL-1ra were tested in equine joints. We evaluated two doses of scAAVIL-1ra, scAAVGFP, and saline. We developed a novel endoscopy procedure and confirmed vector-derived transgene expression (GFP) in chondrocytes 6 months post-injection. AAVIL-1ra therapeutic protein levels were 200–800 ng/ml of synovial fluid over 23 and 186 days, respectively. No evidence of intra-articular toxicity was detected and no vector genomes were found in contralateral joints based on GFP fluorescence microscopy and quantitative PCR. Finally, we assayed vector-derived IL-1ra activity based on functional assays which supported anti-inflammatory activity of our protein. These studies represent the first large animal intra-articular gene transfer approach with a therapeutic gene using scAAV and demonstrate high levels of protein production over extended time supporting further clinical investigation using scAAV gene therapy for OA.
Keywords: IL-1ra, intraarticular, joints and arthroscopy, scAAV
Introduction
Osteoarthritis (OA) is a progressive and debilitating joint disease for which there is no cure. It affects over 40 million people annually and is responsible for tremendous financial burdens on health systems especially in societies with growing populations of elderly and obese individuals.1 Furthermore, the disease negatively affects younger, active individuals that participate in high demand sports and suffer from post-traumatic arthritis or overuse syndrome.2 The proinflammatory cytokine primarily responsible for many of the pathological features of OA is interleukin-1β (IL-1β).3 IL-1β has been found to be upregulated in synovial tissues and cartilage and a strong correlation has been consistently found with levels of IL-1β and osteoarthritic changes such as cartilage degeneration and presence of inflammation.1 Initially, treatment for OA consisted of nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors, which reduced symptoms of pain and inflammation, caused by IL-1β but did not deter progression of OA.4,5 In addition, with prolonged use, these drugs can be associated with gastrointestinal disorders and cardiovascular ischemic events that can sometimes even lead to the demise of the patient.6
In the last two decades, researchers have sought to explore pharmaceuticals that can not only control symptoms of OA but also slow the progression and even prevent or stop degeneration of the joint thus bypassing the need of inevitable joint replacement. Although drugs such as hyaluronan and glucosamine/chondroitin sulfate have been somewhat disease modifying, they have not alleviated symptoms as effectively as originally forecasted.7,8,9,10,11 A logical target for blocking effects of IL-1β is the molecule IL-1 receptor antagonist (IL-1ra). This molecule is the natural inhibitor of IL-1β and competes with IL-1β for occupancy of the IL-1 cell surface receptors but cannot initiate cellular signals when bound to these receptors.3 Studies have revealed that the IL-1ra concentration is low in inflamed joints and a level of tenfold to 1,000-fold excess of IL-1ra over IL-1β is required to effectively block all of the available IL-1 receptors enough to inhibit joint degeneration.12,13,14 Autologous-conditioned serum, harvested from patient's blood, is a biological treatment that has high levels of constitutive IL-1ra concentrations and has been met with varying degrees of success in both humans and horses suffering from OA.15,16 One reason may be that levels of IL-1ra protein are not high enough and not sustained for periods required to inhibit ongoing inflammation.16
Gene transfer using viral vectors to initiate therapeutic levels of IL-1ra or growth factors in joints is a promising approach and “proof of concept” in delivering therapeutic genes to joints by direct in vivo injection.17,18,19,20,21 Various viral vectors have been utilized for intra-articular gene therapy including adenovirus, retrovirus, lentivirus and adeno-associated virus (AAV).17,19,22,23 Adenoviral vectors have resulted in significant elevations of protein (IL-1ra, insulin-like growth factor-I) when injected intra-articularly; however, levels only remain elevated for 14–21 days and adenoviral vectors themselves can cause significant inflammation due to their immunogenic stimulation.17,18,24 Retroviral vectors have also significantly increased protein levels, however; these vectors do not efficiently transduce nondividing cells and therefore are less suited for joint tissues where cell turnover is low.22 Lentiviral vectors based on integration into the chromosome, offers the potential of long-term expression for OA; however, when injected into the knee joints of immunocompetent rats, the results demonstrate a sharp decrease in protein expression at ~20 days and therefore may not be the vector of choice for long-term protein production for direct intra-articular injections.23 AAV has been studied and validated to result in significant elevations in protein expression.19,25 Kay et al. compared single-stranded AAV to self-complementary AAV (scAAV) and reported a 25-fold greater transgene expression level for scAAV confirming that second strand DNA synthesis can be a major impediment to transduction efficiency in the joint.19 Furthermore, in Kay et al. scAAV not only resulted in dramatically increased transduction efficiency when compared with the single-stranded AAV but also did not reveal a difference in transduction between normal and inflamed articular environments suggesting that scAAV may be an appropriate vector in OA. Suitability of scAAV vectors intra-articularly was further confirmed in equine joints when transduction efficiency of AdGFP, rAAV, and scAAV were compared over a period of 8 weeks.26 Goodrich et al. compared scAAV serotypes in synoviocytes and chondrocytes in vitro and revealed the importance of serotype affinity in that significant differences existed in transduction efficiencies in both synoviocytes and chondrocytes which are the main cell types in joints.27 Serotype 6 was better in chondrocytes and serotype 3 in synoviocytes whereas serotype 2 was best in both chondrocytes and synoviocytes.27 This was also revealed by Sun et al. where various serotypes of scAAVFIX resulted in differing factor IX levels in the joints of mice.28
In addition to varying serotype efficiencies intra-articularly, various promoters may also change protein production.29 Few studies have been performed to investigate optimal use of promoters to enhance transgene expression intra-articularly but existing data suggest different promoters may play an important role in maximizing protein expression and variability exists with different promoters.30,31 Furthermore, optimization of transgene cassette often enhances therapeutic output of protein through favoring codon usage to exploit amino acyl-transfer RNA molecules that are most abundant in mammalian cells.29
Regardless of promising results seen in vitro, protein expression in cell culture may not always represent protein expression in vivo due to unforeseen immunity, transduction efficiency, and species differences. Since gene therapeutic vectors targeted for OA should produce protein levels for long periods of time (over 4 months), the objective of this study was to optimize variables of our scAAV vector such as promoter and transgene, first in vitro and then, using our optimized promoter and transgene, compare our two best scAAV serotypes in vivo to explore the application of scAAV gene therapy in an equine model.11,18,32 We hypothesized that we could optimize promoter and transgene in vitro and test this vector in vivo to determine efficacy of transgene production in the equine joint. This is the first study to report significant protein elevations for up to 6 months using scAAV in large animal (equine) joints.
Results
Optimization of IL-1ra cassette and CMV and CBh promoters
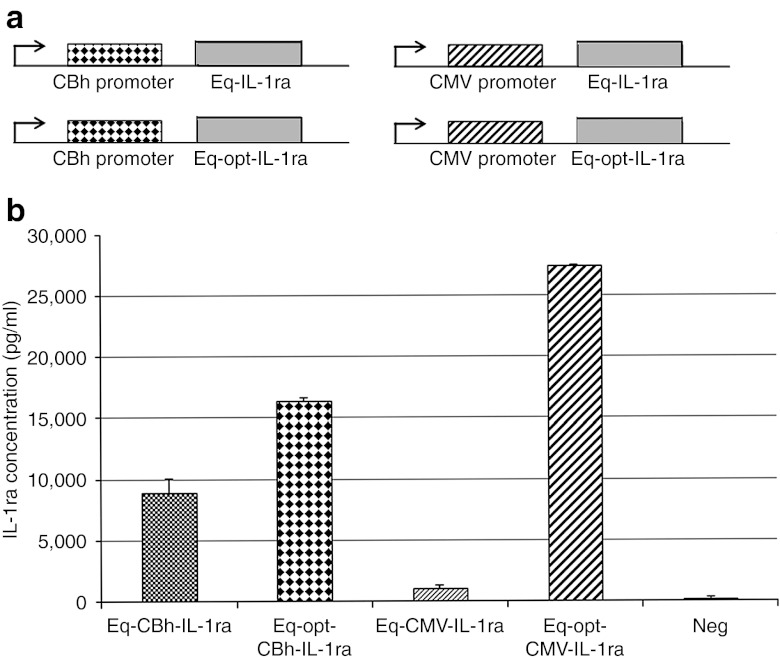
An optimized equine IL-1ra (OptEqIL-1ra) gene sequence was designed and produced by GeneArt to produce greater amounts of protein due to more efficient transcription and translation. Essentially, specific nucleotide sequences were altered so they produced the same protein, but minimized the expression of sequence repeats, splice sites, and secondary structures as this technology has been shown to be successful in clinical trials.33 OptEqIL-1ra and non-optimized EqIL-1ra genes were cloned into mammalian expression vectors (pTRs-ks) containing a cytomegalovirus (CMV) or chicken β-actin hybrid (CBh) promoter (Figure 1a), and evaluated for protein production in transfected 293 cells. The optimized constructs produced higher levels of IL-1ra than their unoptimized (wild-type) counterparts, which had threefold higher (Eq-opt-CBhIL-1ra) and 27-fold higher (Eq-opt-CMVIL-1ra) IL-1ra protein levels, respectively (Figure 1b).
Figure 1.
Plasmid design and protein production. (a) Vector constructs of the non-optimized (top) and optimized (bottom) CBhEqIL-1ra-pTRs-ks plasmids (left) and the CMVEqIL-1ra-pTRs-ks plasmids (right). Specific base pair sequence changes were made in the optimized IL-1ra gene (without affecting the protein sequence) so that the protein would be produced more efficiently by the scAAV construct. (b) IL-1ra production in transduced 293 cells. IL-1ra production was assessed in 293 cells transduced with 2 µg/cm of optimized and non-optimized equine IL-1ra plasmid constructs. CMV, cytomegalovirus; IL, interleukin; scAAV, self-complementary adeno-associated virus.
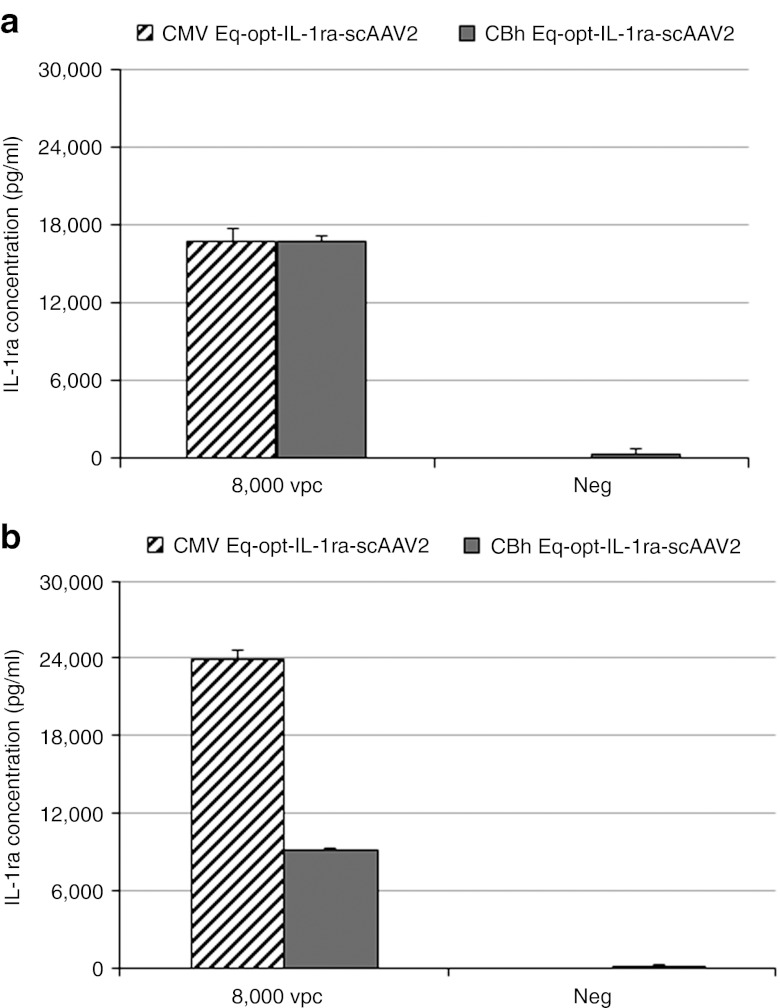
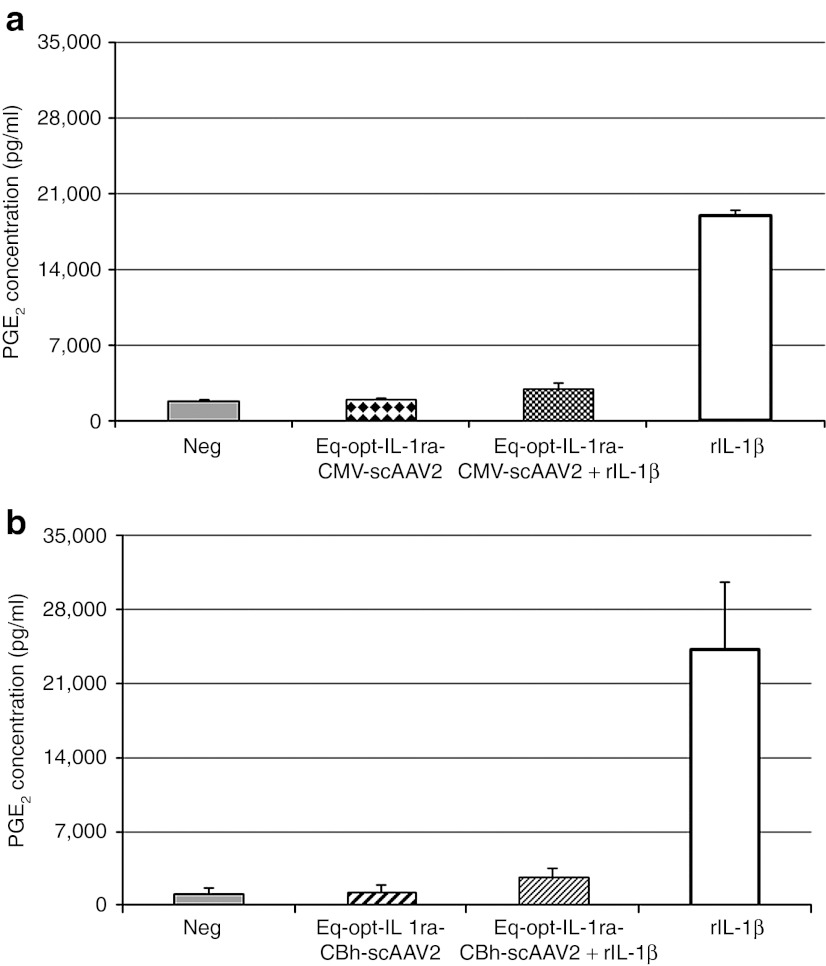
CMV and CBh-Eq-optIL-1ra-pTRs-ks plasmids were packaged into a scAAV2 construct, and evaluated for protein production and functionality in equine chondrocytes and synoviocytes. Transductions were carried out with 8,000 viral genomes per cell based on a previous in vitro study comparing dosages of viral vector per cell.27 CMVEq-opt-IL-1ra-scAAV2 and CBhEq-opt-IL-ra-scAAV2 produced similar levels of IL-1ra protein in chondrocytes (17 ng/ml) D10 post-transduction (Figure 2a), while CMVEq-opt-IL-1ra-scAAV2 produced threefold higher (24 versus 8 ng) levels of CBhEq-opt-IL-1ra-scAAV2 in synoviocytes (Figure 2b). Because both the synovium and cartilage are targets for gene therapy within the joint, the CMV promoter was chosen for use in vivo as it efficiently enhanced protein production in both tissues comparably whereas the CBh promoter had lower protein expression in synoviocytes. Functionally, there was no significant difference between constructs with the two different promoters in their ability to minimize the inflammatory response of cultured cells to rIL-1β stimulation. CMV-Eq-opt-IL-1ra-scAAV2 reduced the inflammatory response of synoviocytes by 88% whereas the CBh-Eq-opt-IL-1ra-scAAV2 construct reduced the response by 82% (Figure 3) suggesting we reached saturation.
Figure 2.
Promoter effects on IL-1ra production in transduced chondrocytes or synoviocytes. (a,b) IL-1ra production in (a) equine chondrocytes transduced with Eq-opt-IL-1ra-scAAV2 and (b) equine synoviocytes transduced with Eq-opt-IL-1ra-scAAV2 containing a CMV (diagonal lines) or CBh (solid bar) promoter. CMV, cytomegalovirus; IL, interleukin; vpc, viral particles per cell.
Figure 3.
Promoter effects on inhibition of PGE2. (a,b) PGE2 inhibition in (a) Eq-opt-IL-1ra-scAAV–transduced chondrocytes and PGE2 inhibition in (b) Eq-opt-IL-1ra-scAAV–transduced synoviocytes. rIL-1β was used to stimulate cells 2 days post-transduction. Assessments were made 6 days post-transduction using a PGE2 ELISA kit. CMV, cytomegalovirus; IL, interleukin; PGE2, prostaglandin E2; scAAV, self-complementary adeno-associated virus.
Optimization of serotype and dose in vivo
In a previous study, we evaluated serotypes 1–6, and 8 using an scAAVeGFP cassette and the results indicated that serotype 2 scAAVGFP was the most efficient for transduction of equine chondrocytes and synoviocytes.27 Comparisons of scAAV2 and 2.5 in this study revealed similar transduction efficiencies (data not shown). For the in vivo pilot study, saline, serotype 2 or S2.5scAAVCMVGFP was administered intra-articularly and other joints received serotype 2 or S2.5scAAVeq-opt-IL-1ra constructs administered at 5 × 1012 or 5 × 1013 viral genomes per joint (Figure 4a). Following injections, horses were measured for lameness, joint effusion or pain on flexion/manipulation of joints until termination. None of these clinical parameters indicated adverse events.
Figure 4.
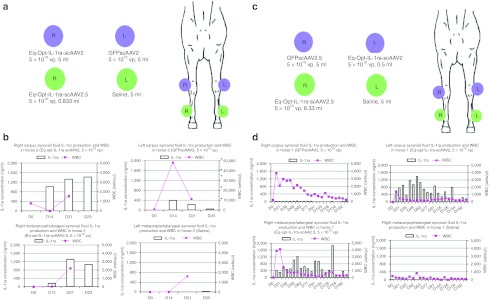
Effects of serotype and dose on in vivo testing of Eq-Opt-IL-1ra-scAAV and GFPscAAV. (a) Clinical design for the dosing of Horse 2 (horse used for short-term administration). The blue circles correspond to the carpal joints, and the green circles correspond to the metacarpophalangeal joints. scAAV constructs were given at specific concentrations per joint, whereas 5 ml of saline was administered into the control joint. (b) IL-1ra expression (left y-axis) in bars and WBC counts (right y-axis) in line graph (cells/µl) in the synovial fluid of the carpus and metacarpophalangeal joints of Horse 2. Each joint is graphed separately over time. IL-1ra levels were assessed with an equine IL-1ra ELISA kit. Note: the WBC numbers on the top right graph are higher due to a higher WBC count in that joint. WBC counts were not determined at D23. (c) Clinical design for the dosing of Horse 1 (horse used for long-term study). The blue circles correspond to the carpal joints, and the green circles correspond to the metacarpal phalangeal joints. scAAV constructs were given at specific concentrations per joint, whereas 5 ml of saline was administered into the control joint. (d) IL-1ra expression (left y-axis) and WBC counts (cells/µl) (right y-axis) in the synovial fluid of the carpus and metacarpophalangeal joints of Horse 1. Each joint is graphed separately over time. GFP, green fluorescent protein; IL, interleukin; scAAV, self-complementary adeno-associated virus; vp, viral particles.
Circulating levels of IL-1ra in the serum from both horses was minimal (<1 ng/ml) and primarily detected in samples taken between D14 and D56 (data not shown). Analysis of the synovial fluid samples in Horse 2 (horse used for short-term study) revealed high levels (200–1,600 ng/ml) of IL-1ra in the S2- and S2.5scAAVIL-1ra–dosed joints for all time points examined beyond D0 (Figure 4a,b), suggesting equal transduction efficiencies of S2 and S2.5 in vivo for the dose tested. Fluid cytology analysis revealed that there was an initial spike of 40,000 WBC (cells/µl) in the scAAVGFP-injected joint (left carpus) that quickly returned to baseline levels by D21. Differential analysis revealed that the majority of WBC's consisted of lymphocytes and less commonly neutrophils. Interestingly, the joints injected with serotype 2, serotype 2.5 scAAVeq-opt-IL-1ra or saline had only a brief and minor rise (<2,000 cells/µl) in WBC.
The left carpus (saline injected) had a brief rise in IL-1ra protein most likely due to a well-published “contralateral effect” in which therapeutic protein in one joint rises and causes a rise in the contralateral joint. This effect was not observed in the metacarpophalangeal joints of Horse 2. Horse 1 (horse used for long-term study) also had high levels (200–1,800 ng/ml) of IL-1ra detected beginning at D14 post-induction and continuing to D182 (Figure 4c,d). More specifically, levels of IL-1ra varied between 400–1,200 ng/ml from D14 to D77 and then had an overall downward trend from D91 to D126 but continued to be above 200 ng/ml at D182. WBC count in all joints except saline had a mild and brief rise (<4,000 cells) but quickly returned to baseline by D21.
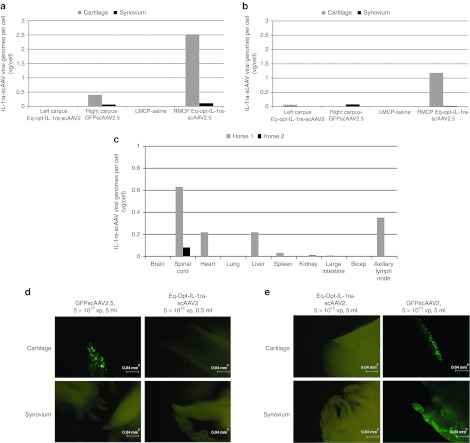
The carpal joints of Horse 1 were arthroscopically examined following euthanasia (Supplementary Video S1a,b). The arthroscope has a fluorescent filter in which fluorescing cells can be imaged within the joint. Several areas of cartilage surface had fluorescent cells (chondrocytes) in the scAAVGFP-injected joint whereas no cartilage surface in the scAAV-Eq-opt-IL-1ra–injected joint exhibited fluorescent cells (Supplementary Video S1). Cartilage biopsies were performed from fluorescing areas of cartilage (viewed arthroscopically) and subsequently viewed with the fluorescent microscope which confirmed intense fluorescing chondrocytes (Figure 5d,e). Synovial tissue from both scAAVGFP- and scAAV-Opt-eq-IL-1ra–injected joints did not fluoresce in the horse that was arthroscopically examined. Based on fluorescent microscopy of the synovium (Horse 2) or arthroscopy (Horse 1) it appears that synovial tissue fluorescence diminishes with the turnover of synovial cells over time.
Figure 5.
Analysis of vector genomes and detection of GFP fluorescent cells. (a) Distribution of IL-1ra viral genomes per cell for Horse 1 and (b) distribution of viral genomes per cell for Horse 2 carpus and metacarpophalangeal tissue samples. (c) Equine-optimized biodistribution of IL-1ra viral genomes per cell for tissues collected at the termination of the study. (d) Horse 1 carpal joint and (e) Horse 2 carpal joint. GFP expression in S2- and S2.5scAAVGFP-injected carpal joints. Metacarpophalangeal joints were not shown because they were not expressive of GFP (as they were injected with scAAVIL-1ra or saline). Images were captured using ×100 magnification, with a FITC fluorescent filter cube (excitation of 495 nm and emission of 521 nm). FITC, fluorescein isothiocyanate; GFP, green fluorescent protein; IL, interleukin; LMCP, left metacarpal phalangeal joint; RMCP, right metacarpal phalangeal joint; scAAV, self-complementary adeno-associated virus; vp, viral particles.
Testing functionality of therapeutic protein from synovial fluid samples
Synovial fluid samples from injected joints were assessed for their ability to minimize the inflammatory response to cultured synoviocytes stimulated with 10 ng/ml of equine rIL-1β (Figure 6). Four time points were randomly selected from Horse 1 and added to synoviocytes at equal volumes simultaneously with equine rIL-1β–stimulated media. Synovial fluid samples collected from the IL-1ra–dosed joints consistently minimized the inflammatory response to the equine rIL-1β suggesting that the level of IL-1ra within the joint fluid decreased IL1-β–induced inflammation. As expected, the synovial fluid from the saline-injected joints had minimal decrease or an elevation in IL-1β.
Figure 6.
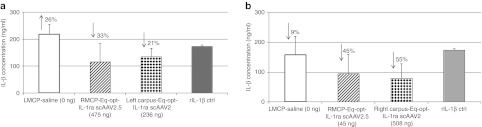
IL-1β inhibition using synovial fluid of transduced joints. (a) IL-1β expression in stimulated synoviocytes treated with Horse 1 synovial fluid and (b) IL-1β expression in stimulated synoviocytes treated with Horse 2 synovial fluid. Functional assessment of rIL-1β–stimulated synoviocytes treated with D14 synovial fluid from Horse 2 and D98 synovial fluid for Horse 1. The amount of IL-1ra present in the synovial fluid for each joint is indicated in parentheses. The amount of inhibition or increase in IL-1β levels are indicated with arrows and percentages (of decrease). Samples were assessed with an equine IL-1β ELISA kit. ctrl, control; IL, interleukin; LMCP, left metacarpal phalangeal joint; RMCP, right metacarpal phalangeal joint; scAAV, self-complementary adeno-associated virus.
Distribution of viral genomes in joints and body tissues and histology of joint tissues
Tissue samples were collected at necropsy from both animals to assess the biodistribution of scAAV-Eq-opt-IL-1ra viral genomes. Quantitative PCR analysis revealed a higher number of viral genomes (specifically detecting the optimized IL-1ra transgene) detected in cartilage samples than synovial samples for both animals (Figure 5a–c). No vector genomes were found in the saline joints and minimal to no scAAV-Eq-opt-IL-1ra vector genomes were found in the scAAVGFP-injected joints.
Fluorescent microscopy of Horse 1 revealed a similar trend to the fluorescent arthroscopic assessment in that the scAAVGFP-dosed joint revealed intensely fluorescing chondrocytes within the cartilage and no fluorescing synoviocytes within synovium (183 days following injection) (Figure 5d). For Horse 2 both cartilage and synovium taken from the scAAVGFP-dosed joint (at day 23) were fluorescent (Figure 5e) again supporting published data that chondrocytes have an extended half-life compared with synoviocytes.34
No evidence of intra-articular toxicity was determined in observation and scoring of synovium and cartilage. Finally, no significant differences were found between joints injected with scAAVGFP, scAAV-Eq-opt-IL-1β or saline in terms of synovial thickening and hyperplasia and cartilage matrix loss.
Discussion
The results of this study reveal the first successful demonstration of dramatic increases in the therapeutic protein, IL-1ra, for extended periods of time in large animal (equine) joints. These data are important as they suggest that scAAV gene therapy will be efficient in transducing joint tissues for extended periods of time without causing intra-articular toxicity in the equine model, a model that is commonly used to mimic OA in people.18,35 Others have demonstrated efficient transduction of scAAV in rats,36 rabbits,19 and horses26 although in those studies extended long-term production of protein was not longer than 8 weeks. Gouze et al. elegantly demonstrated that synovial tissue is efficiently transduced by viral vectors but suggested that synoviocytes may not be ideal gene therapy targets for long-term protein production due to turnover of synoviocytes.34 In that study, synoviocytes efficiently transduced with lentiviral vectors were all but absent by day 162 and authors suggested that neighboring ligaments, tendons, and capsular tissues may be more appropriate targets due to reduced cellular turnover.34 Our study suggests that the stable and low-dividing chondrocytes are ideal targets and will efficiently produce therapeutic protein for up to 183 days (final test day measured, Figure 4d). Although the protein production had a downward trend from day 0 to day 182, the measured protein is consistent with therapeutic amounts in the joints through the final time point of synovial fluid harvest. Fluorescent arthroscopic video of the scAAV injected joint at 6 months had stably transduced cartilage and when biopsies of this tissue were viewed with the fluorescent microscope, large numbers of chondrocytes were fluorescing, confirming this observation. From our analysis, we would conclude that both chondrocytes and synoviocytes are targets for AAV gene transfer, but long-term transgene expression will predominantly utilize chondrocytes as target tissue.
Many studies highlight the importance of immune compatibility between vector, transgene, and host.19,23,34,37 Consistently, expression of a gene of cross-species origin is quickly extinguished regardless of gene delivery with a vector that expresses viral proteins at low levels.34 We sought to not only use the equine transgene in our equine model but to optimize the transgene to more efficiently produce protein potentially allowing for a lower effective dose of vector intra-articularly. In past studies, transgene optimization has resulted in much greater protein production and has shown promise in gene therapy strategies in which more efficient translation of protein is required.29,33 The process of optimizing cDNA results in more efficient transcription due to the elimination of RNA secondary structures and enhanced translation commensurate with the abundance of the transfer RNA within that species. In addition, promoter optimization is another approach to maximizing protein production and reducing the amount of vector administered. Previous studies have compared the CMV and CBA promoters in mammalian cells.38,39 A hybrid of the CBA promoter (CBh) was developed which provided a smaller base pair segment and resulted in a more robust protein expression than CMV in neuronal cells.40 It was our goal to determine whether this was also the case in the cells of joint tissues. Our results revealed that CMV still appears to be the better promoter in joint tissues. Furthermore, the CMV promoter combined with the optimized transgene resulted in optimal protein production of equine IL-1ra, respectively (Figures 1b,2).
Our group previously evaluated serotype affinity and vector dose in vitro using scAAVGFP and, in that study, AAV2 was consistently the most efficient at transducing both synoviocytes and chondrocytes.27 When we began testing the optimized transgene and various promoters in the present study we did not have access to serotype 2.5, an enhanced chimeric vector recently tested in a clinical trial for Duchenne muscular dystrophy, however, following optimization of transgene and promoter, we compared scAAVGFP serotypes 2 and 2.5 in vitro in both synoviocytes and chondrocytes. Since they were comparable in transduction efficiency, we sought to compare them in equine joints using our optimized transgene and promoter. No differences existed in IL-1ra levels between joints injected with serotype 2 and 2.5 chimeric capsid (Figure 4). Both serotypes efficiently transduced the joint tissues and resulted in high levels of protein production of over 23 days and 6 months, respectively. The dosages used in this study were chosen based on a previous study in which rAAV2-TNFR:Fc was administered to human joints in a phase 1/2 study to determine the safety and tolerability of repeat injections intra-articularly.41 In that study, patients had minimal complications and demonstrated good response to the intra-articular gene therapy.41 In the current study, horses did not demonstrate lameness, joint swelling or pain on joint manipulation for the duration of the study. WBC rose in one joint of Horse 2 receiving S2scAAVGFP but quickly returned to baseline by day 21 post-injection. Three joints in Horse 1 had very mild elevations of WBC and those joints received scAAVGFP or scAAV-Eq-opt-IL-1ra. Differential analysis of white cell elevations consisted of mononuclear cells, specifically lymphocytes. Neutrophils may have also increased at earlier time points (before 14 days), however, we did not harvest synovial fluid earlier than this time point so it is not known whether a different type of white cell increased as well. No differences were detected in IL-1ra protein levels between dosages of 5 × 1013 and 5 × 1012 viral particle. This suggests that a saturation effect of the scAAV vector constructs was attained at both doses and that lower doses may be just as efficacious (studies ongoing). Further studies are ongoing to determine how lower dosages may affect protein production.
Consistent with other studies utilizing gene therapy vectors, we observed an interesting rise in IL-1ra in the carpal joint contralateral to the carpal joint injected with scAAV-Eq-opt-IL-1ra.42,43,44,45,46 As in previous publications reporting the “contralateral effect” we believe the protein in the contralateral joint to be a product of the transgene of the joint injected with scEq-opt-IL-1ra and not a product of vector migration to the contralateral joint since no vector genomes were found in either the synovium or cartilage of the joint not receiving scAAV vector, but positive for low level IL-1ra protein (Figure 5a). Furthermore, when synovium and cartilage were examined from all joints injected with either scAAVGFP or scAAVEq-opt-IL-1ra, only the joints injected with scAAVGFP had joint tissues that fluoresced (Figure 5) confirming that vector genomes probably do not migrate to the contralateral joints. Synovial elevations of IL-1ra in the scAAVGFP-injected joint were also most likely not from a rise in the serum levels due to a negligible increase of IL-1ra protein of <1 ng/ml measured at all time points. In a study by Ghivizzani et al.,where adenoviral vectors were tracked following joint injection, authors found that when knee joints of rabbits were injected with an adenoviral vector encoding luciferase (AdLuc), a percentage of the leukocytes present in the joint at the time of AdLuc injection were transduced and then migrated to the lymph node of the contralateral limb and subsequently were found in the contralateral joint.42 This contralateral effect was also seen in Lechman et al. where in addition to transduced leukocytes, transduced immature dendritic cells and macrophages were also suspected of migrating from the injected joint to the contralateral joint by way of the lymph node circulation.46 Leucocytes, macrophages, and immature dendritic cells were not tracked in the current study, however; this method of elevated protein could be responsible for a contralateral effect seen in Horse 2.
It was interesting that we were able to detect vector genomes in some of the tissues (cartilage) of joints injected with scAAVEq-opt-IL-1ra but not others (Figure 5a,b). We believe this may be due to variability where transduction occurs in the joint and whether these areas were harvested at tissue collection. As seen in the arthroscopy videos of the joint injected with scAAVGFP, area of cartilage transduction varied (Supplementary Video S1a). Although the harvest sites of cartilage and synovium were consistent at necropsy, areas of the joint transduction could have varied enough to result in differing amounts of vector genomes measured.
This is the first intra-articular gene therapy study to demonstrate arthroscopic confirmation of transduced joint tissues. The technique is straightforward and easy to use although one must keep in mind that some amount of autofluorescence of the cartilage and synovial tissues exist. We believe this may be an extremely valuable tool to document tissue transduction intra-articularly without disturbing joint tissues (performing a biopsy) throughout gene therapy trials.
Functionality of the IL-1ra protein found in the synovial fluids was proven by stimulating synoviocytes with IL-1β and then measuring the ability of the synovial fluid to reduce inflammation. We chose timepoints of when the synovial fluid was collected (day 14 and day 98) based on whether we had enough fluid to perform the experiment. Reductions in IL-1β resulted whenever synovial fluid containing IL-1ra was placed on IL-1β–stimulated synoviocytes although percent reductions were not proportional. This may be due to the fact that synovial fluid itself has anti-inflammatory properties and therefore proportional decreases in IL-1β may be difficult to measure.
These data are the first to reveal that scAAV gene therapy may be a realistic option to cause high levels of therapeutic protein in the joint to ultimately treat OA in a large animal model that is commonly used to mimic OA in humans. The information gained from this study has wide impact on gene therapy for OA as it reveals that cartilage is stably transduced in situ and can produce significant amounts of therapeutic proteins for periods of at least 6 months adding to and extending information gained from short-term studies in rat, rabbit, dogs, and horse.19,21,26,28 Ishihara et al., attempted to induce high therapeutic levels of BMP-2 in equine joints using scAAVBMP-2 but were not able to detect appreciable amounts of protein.47 Investigators of that study hypothesized that synovial fluid may have had an inhibitory effect, however; this issue was not borne out in the current study. Regardless, our results demonstrate that (i) optimized promoter, (ii) transgene, and (iii) capsid tested first in vitro conveyed to long-term and high level efficacy of transgene production in the equine joint. This study strongly suggests that further investigation of scAAVIL1-ra gene therapy to treat OA in large animal models is warranted.
Materials and Methods
Gene cloning. An optimized sequence for the equine IL-1ra gene in a non-expression vector was purchased from GeneArt (Invitrogen, Carlsbad, CA). The optimized sequence was generated from software, GeneOptimizer (Life Technologies, Grand Island, NY), which provides algorithms which optimize codon usage in a particular species to enhance protein production. The optimized or unoptimized (wild-type) equine IL-1ra gene was removed from the non-expression vector using NotI and AgeI restriction enzyme sites (NEB, Ipswich, MA), and ligated into a pTRs-ks mammalian expression vector obtained from the UNC Vector Core (Chapel Hill, NC) containing either a CMV or CBh promoter.40,48 Ligations were performed using T4 Ligase as per manufacturer's instructions (NEB). Constructs were validated by DNA sequencing and absence of mismatches were confirmed.
Plasmid and virus evaluation. pTRs-ks-IL-1ra plasmids were evaluated in 293 cells to ensure that the vector would produce detectable amounts of IL-1ra protein; 293 cells were plated to 60% confluency and equilibrated for 24 hours in Dulbecco's modified Eagle's medium (Invitrogen) containing 10% fetal bovine serum (Sigma-Aldrich, St Louis, MO), 1X penicillin/streptomycin, and 1 N HEPES (Invitrogen). Cells were transfected with 2 µg/cm2 of plasmid DNA for 24 hours using JetPei transfection reagent (Polyplus, New York, NY). Medium was collected and evaluated with a mouse IL-1ra ELISA (R&D Systems, Minneapolis, MN).
scAAV expressing GFP and serotypes 2 and 2.5 were evaluated to determine which serotype had the highest transduction efficiency in equine chondrocytes and synoviocytes.
Plasmids were transferred into scAAV2 and scAAV2.5 constructs (UNC Vector Core). Recombinant AAV vectors were generated using HEK293 cells grown in serum-free suspension conditions in WAVE Bioreactors (GE Healthsciences, Pittsburgh, PA) as described in Grieger et al. (J.C. Grieger and R.J. Samulski, manuscript in preparation).
In brief, the suspension of HEK293 cells were transfected using polythyleneimine (Polysciences, Warrington, PA) and the following plasmids XX680,49 pXR2,50 pXR2.5,51 with scAAV vector plasmids ptrsKS CMV-eGFP,52 and ptrsKS CMV-IL1-ra to generate scAAV2 CMV-eGFP, scAAV2 IL-1ra, scAAV2.5eGFP, and scAAV2.5 CMV-IL1-ra. Forty-eight hours post-transfection, WAVE bioreactor cell cultures were centrifuged and supernatant was discarded. The cells were re-suspended and lysed through sonication as described by Grieger et al.53 Five hundred and fifty units of DNase was added to the lysate and incubated at 37 °C for 45 minutes, followed by centrifugation at 9,400g to pellet the cell debris and the clarified lysate was loaded onto a modified discontinuous iodixanol gradient followed by ion exchange chromatography.
scAAV2CMVIL-1ra and scAAV2CBhIL-1ra were evaluated for protein production and functionality in chondrocytes and synoviocytes. Mature equine chondrocytes and synoviocytes were harvested from cadaveric stifle joints as previously described.54 Chondrocytes were used immediately; synoviocytes were used after three subsequent passages. Cells were plated to 60% confluency and equilibrated for 48 hours in Ham's F12 media (Invitrogen) supplemented as described above. Cells were transduced with 8,000 viral particles per cell in nonsupplemented F12. Supplemented medium was replaced after 3 hours and cells were cultured for an additional 4 days. Once equilibrated, cells were stimulated with 10 ng/ml of recombinant equine IL-1β (R&D Systems) for 48 hours. Media was collected and evaluated with a mouse IL1-ra kit (R&D Systems) and PGE2 kit (Enzo Life Sciences, Farmingdale, NY).
Clinical trial. All animal work was approved by Colorado State University Institutional Animal Care and Use Committee. An initial pilot study consisting of two skeletally mature horses was performed over a 6-month period to assess dosing and serotype administration. Both fore metacarpophalangeal and mid-carpal joints were dosed with either saline, scAAVGFP or scAAVIL-1ra viral constructs. The scAAV constructs were either serotype 2 or 2.5, and were given at two different dose concentrations (5 × 1013 and 5 × 1012 viral particles). Lameness and physical examinations, serum and synovial fluid samples were collected at day 0 to establish baseline levels. Serum and synovial fluid samples were collected 2 weeks post-injection and every other week thereafter for 3 or 28 weeks. Samples were then collected every other week until day 23 for Horse 2 (horse used for short-term study) and week 28 for Horse 1 (horse used for long-term study), at which time the animals were euthanized and tissue samples were collected for histology, biodistribution, and functional assays.
Arthroscopic examination. Arthroscopic assessment was performed on the carpal joints of Horse 1 at 28 weeks following euthanasia. Fluorescent filters on the arthroscope were used to evaluate synovial joint capsule and cartilage for fluorescing cells. Since one carpal joint had scAAVGFP injected and the contralateral joint had scAAVEq-opt-IL-1ra injected, the joints acted as a positive and negative control, respectively. Fluorescence was imaged and video recorded.
Serum and synovial fluid evaluation and viral biodistribution. Serum samples were evaluated for circulating levels of IL1-ra with an equine IL1-ra ELISA (R&D Systems). Synovial fluid samples were evaluated for total protein and fluid cytology, as well as IL1-ra with the same previously mentioned ELISA kit. Serum samples were diluted 1:20 and synovial fluid samples were diluted 1:40 in reagent diluent (1% bovine serum albumin in phosphate-buffered saline) before IL-1ra evaluation. Once diluted, samples were evaluated per manufacturer instructions.
Synovial fluid samples were also assessed for their functional ability against IL-1β stimulation in synovial monolayer cultures. We first established that the synovial fluid alone would have minimal effects on the IL-1β response in stimulated monolayer culture samples. Non-osteoarthritic equine synovial fluid samples (from nonstudy horses), with non-detectable levels of IL-1ra, were added to synoviocytes at vol/vol concentrations of 10, 25, and 50% synovial fluid simultaneously with equine rIL-1β supplemented media (10 ng rIL1β per ml; R&D Systems). Media was collected after 48 hours and evaluated for IL-1β levels with a commercially available equine IL-1β ELISA kit (King Biofisher, St Paul, MN). To assess the harvested synovial fluids from the study animals, synovial fluids from injected joints were added to synoviocytes at 50% vol/vol concentrations of synovial fluid simultaneously with equine rIL-1β supplemented media. The amount of IL-1ra present in each synovial fluid sample ranged from non-detectable to ~1 µg per ml. Media was collected after 48 hours and evaluated as described above.
Tissue samples were collected from each animal at necropsy to assess the biodistribution of IL-1ra viral particles. Quantitative PCR was used for vector genome biodistribution studies. Tissue DNA was purified and quantified using SyBR Green as described.55 Data is reported as the number of double-stranded IL1-ra vector DNA molecules per two copies of the equine GAPDH locus, or in other words the number of vector DNA copies per diploid equine genome. The equine GAPDH primers are as follows: forward 5′-GATTGTCAGCAATGCCTCCT; reverse 5′-TGCCAAAGTGGTCATGGAT. The IL-1ra primers are as follows: forward 5′-AGCTTCCCGGCAACAATTA; reverse 5′-GCAGAAGTGGTCCTGCAACT.
Histology and explant evaluation. Tissue samples taken from the forelimb carpal and metacarpal phalangeal joints from each horse were visualized with a fluorescent microscope. Images were captured under ×100 magnification, with a FITC fluorescent filter cube with an excitation of 495 nm and emission of 521 nm. Synovium and osteochondral sections were placed into formalin. Osteochondral samples were decalcified using an EDTA-based decalcification solution. All samples were paraffin embedded and mounted onto charged slides. All tissues were deparaffinized and stained with hematoxylin and eosin. Samples were rehydrated with a series of ethanol incubations, followed by hematoxylin stain, blue buffer solution and eosin counterstaining. Slides were rinsed with dH2O between each stain. Subchondral bone fragments were also stained with Alcian blue. Samples were deparaffinized and rehydrated as described, and were incubated in 3% acetic acid, Alcian blue, and nuclear fast red counterstain. Slides were rinsed between stains as described. Slides were dehydrated and cover slipped for analysis. Histological sections were observed and scored for inflammation, sclerosis (synovium), matrix degeneration, and surface defects (cartilage).
SUPPLEMENTARY MATERIAL Video S1.
Acknowledgments
The authors would like to acknowledge our funding sources: NIH1K08AR054903-01A2 and Grayson Jockey Club Foundation Award (to L.R.G. and C.W.M.); and 5U54AR056953 (to R.J.S.) and R01 AI072176 (to R.J.S.) and J.C.G. was supported in part by funding from AFM, Swati Yadav and Sophia Shih for their help with the vector genome analysis and Matthew Hirsch for his editorial assistance. The authors would like to acknowledge that while this paper was under review, Watson et al., Gene Therapy, 2012 (advanced online publication) published a similar study affirming the observations of this approach in the equine model. The authors declared no conflict of interest.
Supplementary Material
References
- Daheshia M., and, Yao JQ. The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J Rheumatol. 2008;35:2306–2312. doi: 10.3899/jrheum.080346. [DOI] [PubMed] [Google Scholar]
- Buckwalter JA., and, Brown TD. Joint injury, repair, and remodeling: roles in post-traumatic osteoarthritis. Clin Orthop Relat Res. 2004. pp. 7–16. [PubMed]
- Dinarello CA., and, Thompson RC. Blocking IL-1: interleukin 1 receptor antagonist in vivo and in vitro. Immunol Today. 1991;12:404–410. doi: 10.1016/0167-5699(91)90142-G. [DOI] [PubMed] [Google Scholar]
- Kivitz A, Eisen G, Zhao WW, Bevirt T., and, Recker DP. Randomized placebo-controlled trial comparing efficacy and safety of valdecoxib with naproxen in patients with osteoarthritis. J Fam Pract. 2002;51:530–537. [PubMed] [Google Scholar]
- Simon LS, Lanza FL, Lipsky PE, Hubbard RC, Talwalker S, Schwartz BD.et al. (1998Preliminary study of the safety and efficacy of SC-58635, a novel cyclooxygenase 2 inhibitor: efficacy and safety in two placebo-controlled trials in osteoarthritis and rheumatoid arthritis, and studies of gastrointestinal and platelet effects Arthritis Rheum 411591–1602. [DOI] [PubMed] [Google Scholar]
- McGettigan P., and, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA. 2006;296:1633–1644. doi: 10.1001/jama.296.13.jrv60011. [DOI] [PubMed] [Google Scholar]
- Barnhill JG, Fye CL, Williams DW, Reda DJ, Harris CL., and, Clegg DO. Chondroitin product selection for the glucosamine/chondroitin arthritis intervention trial. J Am Pharm Assoc (2003) 2006;46:14–24. doi: 10.1331/154434506775268616. [DOI] [PubMed] [Google Scholar]
- Clegg DO, Reda DJ, Harris CL, Klein MA, O'Dell JR, Hooper MM.et al. (2006Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis N Engl J Med 354795–808. [DOI] [PubMed] [Google Scholar]
- Michel BA, Stucki G, Frey D, De Vathaire F, Vignon E, Bruehlmann P.et al. (2005Chondroitins 4 and 6 sulfate in osteoarthritis of the knee: a randomized, controlled trial Arthritis Rheum 52779–786. [DOI] [PubMed] [Google Scholar]
- Uebelhart D, Malaise M, Marcolongo R, de Vathaire F, DeVathaire F, Piperno M.et al. (2004Intermittent treatment of knee osteoarthritis with oral chondroitin sulfate: a one-year, randomized, double-blind, multicenter study versus placebo Osteoarthr Cartil 12269–276. [DOI] [PubMed] [Google Scholar]
- Frisbie DD, Kawcak CE, McIlwraith CW., and, Werpy NM. Evaluation of polysulfated glycosaminoglycan or sodium hyaluronan administered intra-articularly for treatment of horses with experimentally induced osteoarthritis. Am J Vet Res. 2009;70:203–209. doi: 10.2460/ajvr.70.2.203. [DOI] [PubMed] [Google Scholar]
- Arend WP. Interleukin-1 receptor antagonist. Adv Immunol. 1993;54:167–227. doi: 10.1016/s0065-2776(08)60535-0. [DOI] [PubMed] [Google Scholar]
- Arend WP, Welgus HG, Thompson RC., and, Eisenberg SP. Biological properties of recombinant human monocyte-derived interleukin 1 receptor antagonist. J Clin Invest. 1990;85:1694–1697. doi: 10.1172/JCI114622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein GS, Berger AE, Tracey DE, Chosay JG, Chapman DL, Paine MM.et al. (1992IL-1 receptor antagonist protein production and gene expression in rheumatoid arthritis and osteoarthritis synovium J Immunol 1491054–1062. [PubMed] [Google Scholar]
- Yang KG, Raijmakers NJ, van Arkel ER, Caron JJ, Rijk PC, Willems WJ.et al. (2008Autologous interleukin-1 receptor antagonist improves function and symptoms in osteoarthritis when compared to placebo in a prospective randomized controlled trial Osteoarthr Cartil 16498–505. [DOI] [PubMed] [Google Scholar]
- Frisbie DD, Kawcak CE, Werpy NM, Park RD., and, McIlwraith CW. Clinical, biochemical, and histologic effects of intra-articular administration of autologous conditioned serum in horses with experimentally induced osteoarthritis. Am J Vet Res. 2007;68:290–296. doi: 10.2460/ajvr.68.3.290. [DOI] [PubMed] [Google Scholar]
- Goodrich LR, Brower-Toland BD, Warnick L, Robbins PD, Evans CH., and, Nixon AJ. Direct adenovirus-mediated IGF-I gene transduction of synovium induces persisting synovial fluid IGF-I ligand elevations. Gene Ther. 2006;13:1253–1262. doi: 10.1038/sj.gt.3302757. [DOI] [PubMed] [Google Scholar]
- Frisbie DD, Ghivizzani SC, Robbins PD, Evans CH., and, McIlwraith CW. Treatment of experimental equine osteoarthritis by in vivo delivery of the equine interleukin-1 receptor antagonist gene. Gene Ther. 2002;9:12–20. doi: 10.1038/sj.gt.3301608. [DOI] [PubMed] [Google Scholar]
- Kay JD, Gouze E, Oligino TJ, Gouze JN, Watson RS, Levings PP.et al. (2009Intra-articular gene delivery and expression of interleukin-1Ra mediated by self-complementary adeno-associated virus J Gene Med 11605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CH, Robbins PD, Ghivizzani SC, Wasko MC, Tomaino MM, Kang R.et al. (2005Gene transfer to human joints: progress toward a gene therapy of arthritis Proc Natl Acad Sci USA 1028698–8703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne KA, Lee HH, Haleem AM, Martins C, Yuan Z, Qiao C.et al. (2011Single intra-articular injection of adeno-associated virus results in stable and controllable in vivo transgene expression in normal rat knees Osteoarthr Cartil 191058–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandara G, Mueller GM, Galea-Lauri J, Tindal MH, Georgescu HI, Suchanek MK.et al. (1993Intraarticular expression of biologically active interleukin 1-receptor-antagonist protein by ex vivo gene transfer Proc Natl Acad Sci USA 9010764–10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouze E, Pawliuk R, Pilapil C, Gouze JN, Fleet C, Palmer GD.et al. (2002In vivo gene delivery to synovium by lentiviral vectors Mol Ther 5397–404. [DOI] [PubMed] [Google Scholar]
- Nixon AJ, Goodrich LR, Scimeca MS, Witte TH, Schnabel LV, Watts AE.et al. (2007Gene therapy in musculoskeletal repair Ann N Y Acad Sci 1117310–327. [DOI] [PubMed] [Google Scholar]
- Pan RY, Chen SL, Xiao X, Liu DW, Peng HJ., and, Tsao YP. Therapy and prevention of arthritis by recombinant adeno-associated virus vector with delivery of interleukin-1 receptor antagonist. Arthritis Rheum. 2000;43:289–297. doi: 10.1002/1529-0131(200002)43:2<289::AID-ANR8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Ishihara A, Bartlett JS., and, Bertone AL. Inflammation and immune response of intra-articular serotype 2 adeno-associated virus or adenovirus vectors in a large animal model. Arthritis. 2012;2012:735472. doi: 10.1155/2012/735472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich LR, Choi VW, Carbone BA, McIlwraith CW., and, Samulski RJ. Ex vivo serotype-specific transduction of equine joint tissue by self-complementary adeno-associated viral vectors. Hum Gene Ther. 2009;20:1697–1702. doi: 10.1089/hum.2009.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Hakobyan N, Valentino LA, Feldman BL, Samulski RJ., and, Monahan PE. Intraarticular factor IX protein or gene replacement protects against development of hemophilic synovitis in the absence of circulating factor IX. Blood. 2008;112:4532–4541. doi: 10.1182/blood-2008-01-131417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AM, Nicolson SC, Warischalk JK., and, Samulski RJ. AAV's anatomy: roadmap for optimizing vectors for translational success. Curr Gene Ther. 2010;10:319–340. doi: 10.2174/156652310793180706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortolano S, Spuch C., and, Navarro C. Present and future of adeno associated virus based gene therapy approaches. Recent Pat Endocr Metab Immune Drug Discov. 2012;6:47–66. doi: 10.2174/187221412799015245. [DOI] [PubMed] [Google Scholar]
- Byers S, Rothe M, Lalic J, Koldej R., and, Anson DS. Lentiviral-mediated correction of MPS VI cells and gene transfer to joint tissues. Mol Genet Metab. 2009;97:102–108. doi: 10.1016/j.ymgme.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Frisbie DD, Kawcak CE, Werpy NM, Park RD., and, McIlwraith CW. Clinical, biochemical, and histologic effects of intra-articular administration of autologous conditioned serum in horses with experimentally induced osteoarthritis. Am J Vet Res. 2007;68:290–296. doi: 10.2460/ajvr.68.3.290. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC.et al. (2011Adenovirus-associated virus vector-mediated gene transfer in hemophilia B N Engl J Med 3652357–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouze E, Gouze JN, Palmer GD, Pilapil C, Evans CH., and, Ghivizzani SC. Transgene persistence and cell turnover in the diarthrodial joint: implications for gene therapy of chronic joint diseases. Mol Ther. 2007;15:1114–1120. doi: 10.1038/sj.mt.6300151. [DOI] [PubMed] [Google Scholar]
- Frisbie DD., and, McIlwraith CW. Evaluation of gene therapy as a treatment for equine traumatic arthritis and osteoarthritis. Clin Orthop Relat Res. 2000. pp. S273–S287. [DOI] [PubMed]
- Izal I, Acosta CA, Ripalda P, Zaratiegui M, Ruiz J., and, Forriol F. IGF-1 gene therapy to protect articular cartilage in a rat model of joint damage. Arch Orthop Trauma Surg. 2008;128:239–247. doi: 10.1007/s00402-007-0407-7. [DOI] [PubMed] [Google Scholar]
- Boissier MC, Lemeiter D, Clavel C, Valvason C, Laroche L, Begue T.et al. (2007Synoviocyte infection with adeno-associated virus (AAV) is neutralized by human synovial fluid from arthritis patients and depends on AAV serotype Hum Gene Ther 18525–535. [DOI] [PubMed] [Google Scholar]
- Hu C., and, Lipshutz GS. AAV-based neonatal gene therapy for hemophilia A: long-term correction and avoidance of immune responses in mice. Gene Ther. 2012;19:1166–1176. doi: 10.1038/gt.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell P, Wang L, Gao G, Haskins ME, Tarantal AF, McCarter RJ.et al. (2011Inverse zonation of hepatocyte transduction with AAV vectors between mice and non-human primates Mol Genet Metab 104395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SJ, Foti SB, Schwartz JW, Bachaboina L, Taylor-Blake B, Coleman J.et al. (2011Optimizing promoters for recombinant adeno-associated virus-mediated gene expression in the peripheral and central nervous system using self-complementary vectors Hum Gene Ther 221143–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mease PJ, Wei N, Fudman EJ, Kivitz AJ, Schechtman J, Trapp RG.et al. (2010Safety, tolerability, and clinical outcomes after intraarticular injection of a recombinant adeno-associated vector containing a tumor necrosis factor antagonist gene: results of a phase ½ Study J Rheumatol 37692–703. [DOI] [PubMed] [Google Scholar]
- Ghivizzani SC, Lechman ER, Kang R, Tio C, Kolls J, Evans CH.et al. (1998Direct adenovirus-mediated gene transfer of interleukin 1 and tumor necrosis factor alpha soluble receptors to rabbit knees with experimental arthritis has local and distal anti-arthritic effects Proc Natl Acad Sci USA 954613–4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen JD, Lechman EL, Carlos CA, Weiss K, Kovesdi I, Glorioso JC.et al. (1999Adenoviral transfer of the viral IL-10 gene periarticularly to mouse paws suppresses development of collagen-induced arthritis in both injected and uninjected paws J Immunol 1623625–3632. [PubMed] [Google Scholar]
- Miagkov AV, Kovalenko DV, Brown CE, Didsbury JR, Cogswell JP, Stimpson SA.et al. (1998NF-kappaB activation provides the potential link between inflammation and hyperplasia in the arthritic joint Proc Natl Acad Sci USA 9513859–13864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Lechman ER, Kim S, Nash J, Oligino TJ., and, Robbins PD. Ex vivo gene delivery of IL-1Ra and soluble TNF receptor confers a distal synergistic therapeutic effect in antigen-induced arthritis. Mol Ther. 2002;6:591–600. [PubMed] [Google Scholar]
- Lechman ER, Jaffurs D, Ghivizzani SC, Gambotto A, Kovesdi I, Mi Z.et al. (1999Direct adenoviral gene transfer of viral IL-10 to rabbit knees with experimental arthritis ameliorates disease in both injected and contralateral control knees J Immunol 1632202–2208. [PubMed] [Google Scholar]
- Ishihara AB., and, Bertone AL.2010Biologic response to intra-articular Ad/AAV BMP2 vectors56th Annual Meeing of the Orthopaedic Research Society, New Orleans.
- McCarty DM, Monahan PE., and, Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8:1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- Xiao X, Li J., and, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X.et al. (2002Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity J Virol 76791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles DE, McPhee SW, Li C, Gray SJ, Samulski JJ, Camp AS.et al. (2012Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector Mol Ther 20443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P., and, Samulski RJ. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003;10:2112–2118. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- Grieger JC, Choi VW., and, Samulski RJ. Production and characterization of adeno-associated viral vectors. Nat Protoc. 2006;1:1412–1428. doi: 10.1038/nprot.2006.207. [DOI] [PubMed] [Google Scholar]
- Nixon AJ, Lust G., and, Vernier-Singer M. Isolation, propagation, and cryopreservation of equine articular chondrocytes. Am J Vet Res. 1992;53:2364–2370. [PubMed] [Google Scholar]
- Gray SJ, Matagne V, Bachaboina L, Yadav S, Ojeda SR., and, Samulski RJ. Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol Ther. 2011;19:1058–1069. doi: 10.1038/mt.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.