Abstract
Background and Purpose
The sphingosine 1-phosphate receptor agonist fingolimod reduces infarct size in rodent models of stroke and enhances Blood Brain Barrier (BBB) integrity. Based on these observations, we hypothesized that combination of fingolimod with tissue Plasminogen Activator (tPA) would reduce the risk of hemorrhagic transformation associated with delayed administration of tPA.
Methods
We evaluated the effects of fingolimod in a mouse model of thromboembolic stroke, in which both the beneficial effect of reperfusion associated with early tPA treatment and hemorrhagic transformation associated with delayed administration mimic clinical observations in humans.
Results
Our results demonstrate that fingolimod treatment attenuates the neurological deficit and reduces infarct volume after in situ thromboembolic occlusion of the middle cerebral artery. Combination of fingolimod and tPA improves the neurological outcome of the thrombolytic therapy and reduces the risk of hemorrhagic transformation associated to delayed administration to tPA.
Conclusion
This study confirms the protective efficacy of fingolimod as a treatment against ischemic stroke in another rodent model of stroke (thromboembolic occlusion), and suggests that fingolimod could potentially be used in combination with tPA to reduce the risk of brain hemorrhage.
Keywords: fingolimod, hemorrhage, stroke, tPA, thromboembolic model
INTRODUCTION
Acute cerebral ischemia is a major cause of mortality and disability worldwide, but no successful pharmacotherapy has been established that can benefit patients beyond the time window of thrombolysis with recombinant tissue type plasminogen activator (tPA) 1. Because of risks related to hemorrhage, this window is within 3 to 4.5 hours after the onset of symptoms 2. tPA has also shown neurotoxicity in experimental models of cerebral ischemia 3. Therefore a combination treatment that would reduce the deleterious effects of tPA while maintaining the benefits of recanalization might extend the usability and/or efficacy of tPA.
Fingolimod (FTY720, Gilenya®) is a sphingosine analog that, when phosphorylated, acts on sphingosine-1-phosphate receptors, regulating cellular and physiological mechanisms including proliferation, apoptosis, adhesion, migration, differentiation/morphogenesis, inflammation or blood–brain barrier (BBB) integrity 4–6. Fingolimod has emerged as a new treatment for multiple sclerosis 7–8. The effectiveness of fingolimod has also been shown by several groups in rodent models of ischemic brain injury9–12. These studies suggest that anti-inflammatory mechanisms and vasculo-protection possibly underlie the beneficial effects of fingolimod after stroke 12. We therefore hypothesized that combining fingolimod with tPA might reduce hemorrhagic transformation associated with delayed administration of tPA.
In this study, we evaluated the effect of fingolimod in a mouse model of thromboembolic stroke, in which the beneficial effect tPA-induced reperfusion and hemorrhagic transformation associated with delayed administration are similar to those occurring in humans.
MATERIAL AND METHODS
Animals
C57BL/6 male mice (25 to 30 g, Charles River Laboratory) were maintained on a 12/12 hours light/dark cycle and fed ad libitum. Experiments were conducted according to protocols approved by the Animal Research Committee of Massachusetts General Hospital and NIH guide for the Care and Use of Laboratory Animals.
Experimental groups
Three cohorts were studied (see online supplement, http://stroke.ahajournals.org, figure S1): 1) MCAO not treated with tPA (permanent occlusion); in this group, animals were treated (i.p.) with 0.5mg/kg fingolimod or saline 45 min, 24 and 48 hours after occlusion. 2) MCAO + early tPA, in which tPA was administered intravenously 30 min after thrombin injection (transient occlusion); in this group, animals received fingolimod or saline 30 min (together with tPA), 24 and 48 hours after occlusion. 3) MCAO + delayed tPA, in which tPA was administered intravenously 3 hours after thrombin injection (transient occlusion); fingolimod or saline was administered 3 hours (together with tPA), 24 and 48 hours after occlusion.
Fingolimod and Phospho-fingolimod (generous gifts from Dr. Volker Brinkmann, Novartis Institutes for Biomedical Research, Basel) were dissolved in saline and stored at 4°C for less than 48 hours.
Middle cerebral artery (MCA) occlusion
Experimental ischemia was carried out as described in 13–14 (see Supplemental Data for details). Isoflurane-anesthetized mice were placed in a stereotaxic frame, the skin between the right ear and eye was cut, the temporal muscle retracted, a craniotomy was performed over the artery bifurcation, the meninges were cut and the MCA, with its parietal and frontal branches, was exposed.
A micropipette (tip size: 30–50 μm), filled with 2 UI/μl mouse α-thrombin (Haematologic Technologies Inc., USA) dissolved in 18% glycerol/saline, was placed in a micromanipulator and 0.5 μl of thrombin solution or vehicle was injected into the lumen of the artery bifurcation to induce the formation of a clot. The micropipette was removed 15 minutes later, when the clot had stabilized. Artery occlusion was considered successful when Laser Speckle Flowmetry showed a rapid and drastic fall of brain perfusion that remained stable during 80 min (mean reduction of 70–80%) (Figure 2SA). To induce reperfusion, tPA (10 mg/kg; Alteplase, Activase®) was administered i.v. (200 μL, 10% bolus, 90% perfusion during 40 min), 30 min (early recanalization) or 3 hours (delayed recanalization) after the injection of thrombin. In mice with early recanalization, we defined effective reperfusion when blood flow recovered to at least 75% of basal values (Figure S2B).
Assessment of lesion volume and histology
Assessment of lesion volume and histology is described in Supplemental Data.
Neurological deficit evaluation
Neurological deficit was evaluated after ischemia using the grid and cylinder tests, described in Supplemental Data.
Quantitative evaluation of Evans Blue extravasation
To evaluate the effect of fingolimod on blood-barrier damage Evans Blue extravasation was determined as described in Supplemental Data.
Absolute CBF measurement
Absolute CBF measurement details are provided in Supplemental Data.
In vitro tPA activity analysis
In vitro interaction assay between tPA activity and fingolimod or P-fingolimod is described in Supplemental Data.
Statistical analysis
Mice were randomly allocated; treatment groups were coded with tail marks to assess infarct, hemorrhage area and neurological deficit in a blinded fashion. The number of mice in each group was based on power analysis assuming a treatment effect of 30% and an SD of 25%. Total number of mice included and mortality during and after surgery are summarized in Table S1. Data are expressed as mean ± SD. For infarct, hemorrhage volumes and Evans Blue extravasations, statistical difference between groups was calculated by analysis of variance. Statistical significance was evaluated for the grid test using a two-way analysis of variance (ANOVA). Statistical significance was evaluated in the cylinder test using one-way ANOVA. P<0.05 was considered significant.
RESULTS
Effect of thrombin and tPA administration on cerebral blood flow
Blood pressure and blood gases were stable in all groups under both basal and ischemic conditions (Table 1). No significant differences in these parameters were observed between treated and non treated mice.
Table 1.
Physiological parameters: blood pressure was monitored during the whole surgical procedure. pH, pCO2 and pO2, values were determined before occlusion (pre-ischemia) and 80 min after occlusion (post-ischemia).
| Thrombin | Thrombin + tPA 30 min | Thrombin + tPA 3 h | ||||
|---|---|---|---|---|---|---|
| Saline | FTY720 | Saline | FTY720 | Saline | FTY720 | |
| Blood pressure (mm Hg) | 84.3 ± 6.0 | 86.1 ± 7.3 | 86.0 ± 7.3 | 86.9±7.0 | 85.7 ± 7.7 | 82.7± 5.0 |
|
| ||||||
| pH (pre-ischemia) | 7.38 ± 0.07 | 7.39 ± 0.06 | 7.37 ± 0.08 | 7.38 ± 0.07 | 7.36 ± 0.06 | 7.35 ± 0.08 |
|
| ||||||
| pCO2 (pre-ischemia, mmHg) | 30.8 ± 4.8 | 31.0 ± 7.6 | 35.4 ± 7.7 | 34.5 ± 6.0 | 36.0 ± 7.2 | 37.2 ± 8.1 |
|
| ||||||
| pO2 (pre-ischemia, mmHg) | 142.7 ± 35.5 | 146.2 ± 23.3 | 160.3 ± 34.5 | 131.7 ± 31.5 | 133.9 ± 26.3 | 173.7 ± 25.9 |
|
| ||||||
| pH (post-ischemia) | 7.39 ± 0.08 | 7.33 ± 0.05 | 7.35 ± 0.09 | 7.32 ± 0.09 | 7.25 ± 0.12 | 7.25 ± 0.11 |
|
| ||||||
| pCO2 (post-ischemia, mmHg) | 43.7 ± 12.0 | 45.8 ± 12.2 | 51.1 ± 14.5 | 49.4 ± 11.6 | 48.4 ± 12.9 | 52.2 ± 15.5 |
|
| ||||||
| pO2 (post-ischemia, mmHg) | 132.2 ± 38.6 | 119.3 ± 38.4 | 142.4 ± 37.1 | 109.8 ± 34.4 | 115.7 ± 30.4 | 145.8 ± 14.6 |
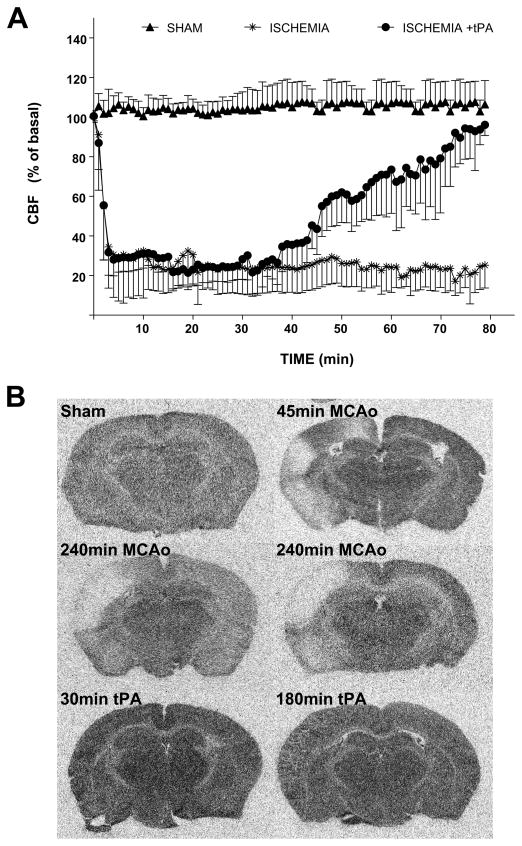
In all groups, local injection of thrombin into the MCA caused an immediate drop (to ~10% of baseline) of cerebral blood flow. In animals not treated with tPA (permanent occlusion), the occlusion was stable during 80 min (as assessed by LSF; Figure 1A) and for at least 4 hours (assessed by [14C]iodoantipyrine autoradiography in a separate cohort of 4 mice, Figure 1B).
Figure 1.
A) Analysis of cerebral blood flow by laser speckle flowmetry (LSF) in the sham group (18% glycerol injection), in animals receiving thrombin injection (ischemia) and animals with early tPA-induced recanalization (ischemia + tPA). Cerebral blood flow is expressed as percent of basal level. B) Regional cerebral blood flood using [14C]iodoantipyrine autoradiography was performed 45 min after 18% glycerol injection (sham, n=2); 45 min (n=2) or 240 min (n=4) after MCA occlusion (note that the ischemic area extends beyond the territory of the occluded MCA branch, probably due to the occurrence of CSD/peri-infarct depolarization); 4 hrs after tPA injection in mice undergoing early (30 min, n=2) or late (180 min, n=2) tPA recanalization.
Treatment with tPA 30 min after thrombin injection induced a gradual reperfusion starting around 30 min after its administration (Figure 1A). No significant differences on reperfusion time were observed between animals treated and non-treated with fingolimod. In mice receiving tPA after 3 hours, the cerebral flow was only measured during the first 80 min of the procedure. The occlusion was stable during this period.
The effectiveness of reperfusion in mice with transient occlusion (see material and methods) was confirmed by [14C]iodoantipyrine autoradiography. [14C]iodoantipyrine autoradiography of brains obtained 4 hours after tPA administration confirmed that the clot was completely dissolved in both transient ischemic groups.
In the sham group, local administration of thrombin vehicle (18% glycerol) did not alter cerebral blood flow. Mice in which baseline cerebral flow was altered as result of the craniotomy were not included in the study (Table S1). No persistent CSD events were observed after thrombin injection in any of the three groups studied.
Effect of fingolimod on infarct volume and neuronal deficit
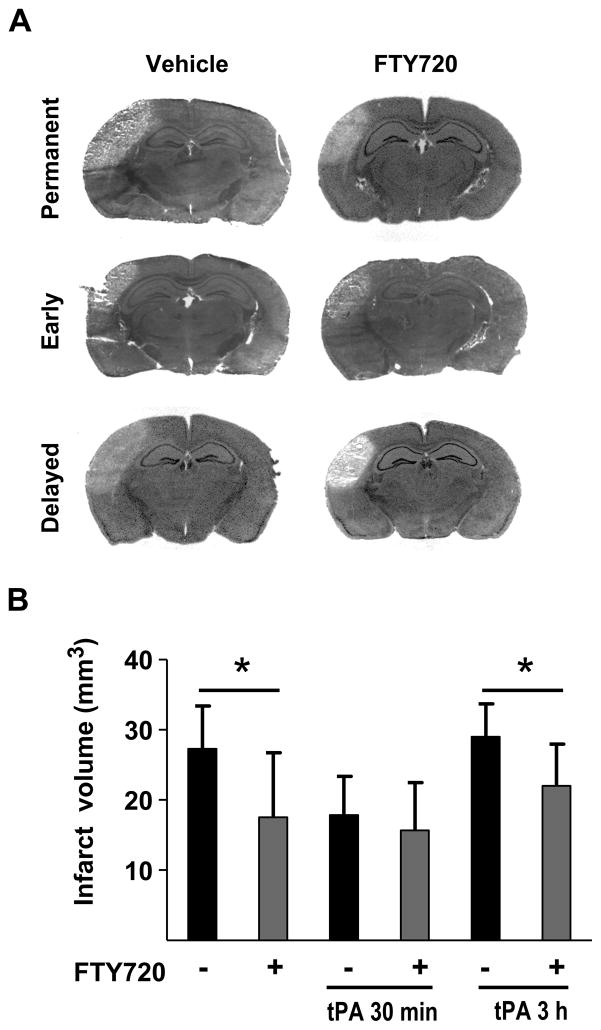
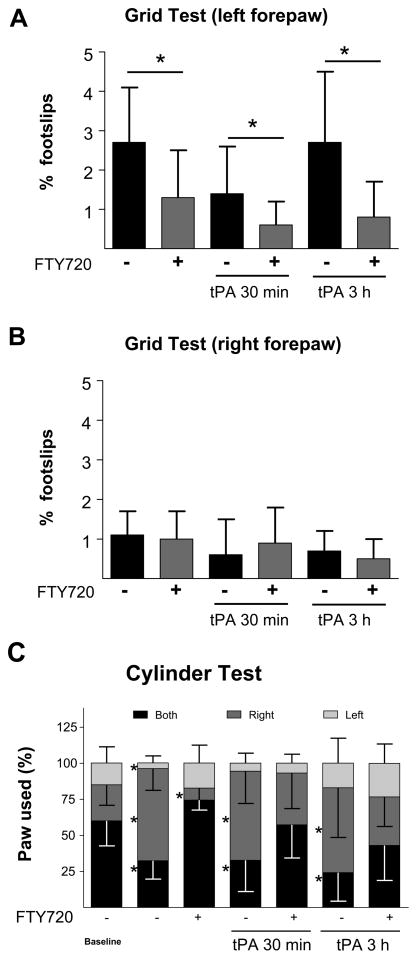
Permanent occlusion caused a cortical infarct of 27.3 ± 6.1 mm3 after 3 days. Ischemia was associated with neurological deficits evaluated using the cylinder and grid walking tests on day 3. In the absence of tPA treatment, infarct volume was significantly reduced (17.5 ± 9.1 mm3, p < 0.05) when animals received three injections of fingolimod, 45 min, 24 h and 48 h after thrombin (Figure 2). Reduction of infarct volume by fingolimod was paralleled by better functional outcome (Figure 3).
Figure 2.
A) Representative H&E-stained sections illustrating the effect of vehicle or fingolimod (0.5 mg/kg FTY720) on infarct volume in animals with permanent occlusion, early recanalization (tPA 30 min after occlusion) and delayed recanalization (tPA 3 hours after occlusion). B) Infarct volumes were measured 3 days after occlusion. Data are means ± SD; n=9 to 10; *P<0.05.
Figure 3.
Effect of vehicle and fingolimod (FTY720, 0.5 mg/kg) on functional deficit, evaluated by the grid walking test in the three experimental groups tested. Panels A and B show the percentage of footslips for the left and right forepaws. The grid walking test was performed 3 days after occlusion. C shows the effect of vehicle and fingolimod (FTY720, 0.5 mg/kg) on functional deficit, evaluated by the cylinder test in the three experimental groups tested. This test was performed before surgery (baseline) and 3 days after occlusion. Animals treated with FTY720 show a behavior close to baseline. Data are means ± SD; n=9 to 10; *P<0.05.
Early vessel recanalization with tPA reduced infarct volume (18.0 ± 5.5 mm3) and improved functional outcome, compared with the permanent ischemic group. Fingolimod administered in combination with tPA, and 24 and 48 h after occlusion further improved the neuronal deficit in both behavioral tests, but did not decrease lesion volume beyond the effect of tPA alone (15.7 ± 6.7 mm3).
Delayed administration of tPA had no beneficial effect on ischemia (29.0 ± 4.7 mm3) and neuronal deficit. Both parameters were similar to those seen in animals with permanent occlusion. Administration of fingolimod in combination with tPA and 24 and 48 h after occlusion reduced infarct volume (22.0 ± 5.9 mm3, p< 0.05), and improved both functional outcome measures.
Effect of fingolimod on hemorrhagic transformation and blood barrier breakdown
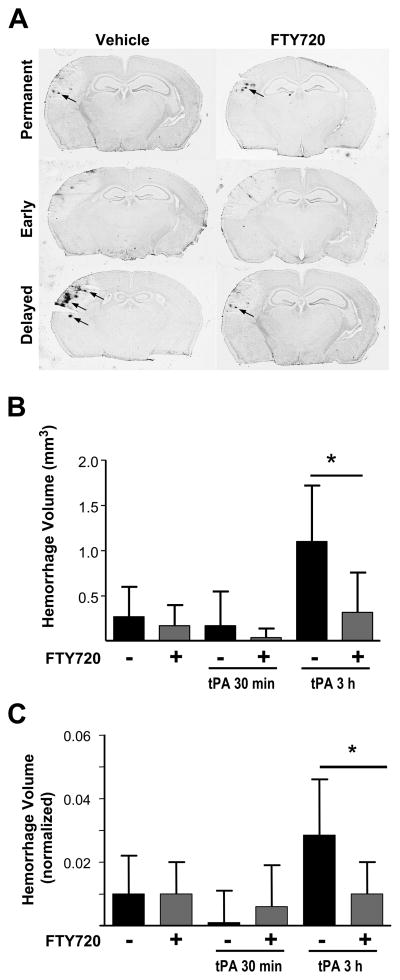
Permanent occlusion induced by thrombin and early recanalization were associated with only minor hemorrhages in the ischemic region at 3 days (total volume: 0.27 ± 0.33 mm3 and 0.17 ± 0.38 mm3 in the permanent and 30 min tPA groups). No significant difference was observed between groups. Fingolimod treatment did not affect hemorrhage volume in either group (Figure 4).
Figure 4.
A) Representative DAB-stained sections illustrating the effect of vehicle or fingolimod (0.5mg/kg FTY720) on hemorrhage volume in animals with permanent occlusion, and animals with early or delayed recanalization. B) The volume of hemorrhages (small arrows in A) was analyzed 3 days after occlusion. Panel C represents the ischemic region normalized for infarct volumes. Data are means ± SD; n=9 to 10; *P<0.05.
Delayed recanalization caused a significant increase in hemorrhage volume (1.10 ± 0.62 mm3) compared with animals not treated with tPA (P < 0.05) and animals treated with tPA 30 min (P < 0.05) after occlusion. Hemorrhagic transformation was significantly reduced (0.32 ± 0.44 mm3) when tPA was administered in combination with fingolimod (p<0.05). The same effects were observed after normalization of these data by infarct volumes.
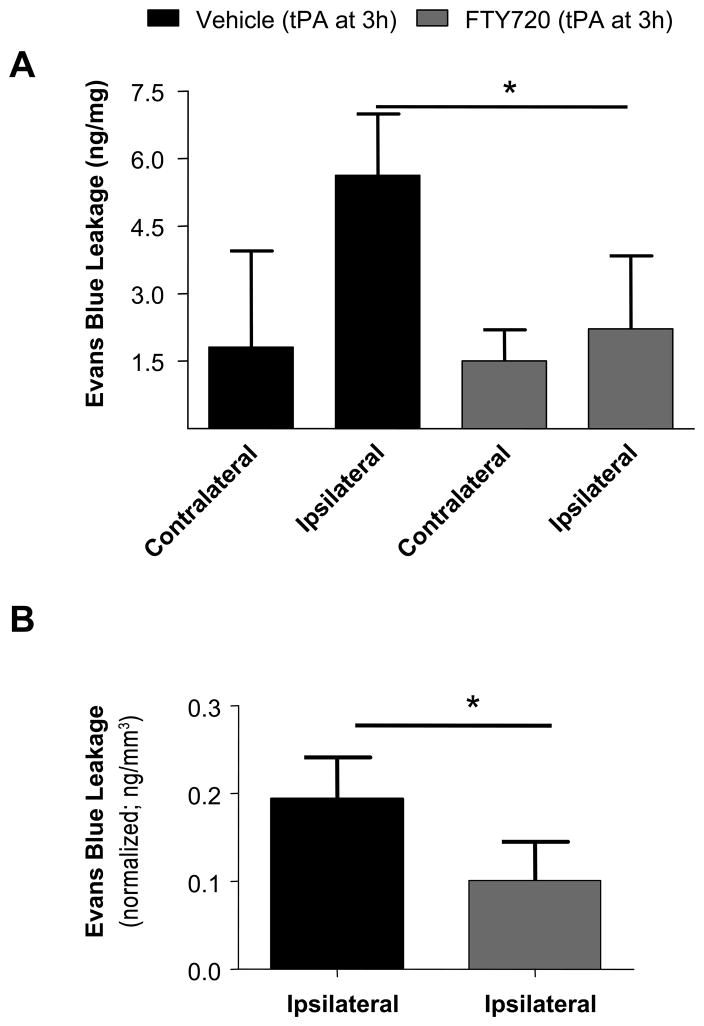
To further examine the effect of fingolimod on vascular integrity, we examined Evans Blue extravasation 24 h after occlusion in mice receiving delayed tPA (Figure 5). We found that tPA increased Evans Blue leakage in the ipsilateral region (5.6 ± 1.4 ng Evans Blue/mg tissue) compared with the contralateral region (1.8 ± 2.1 ng Evans Blue/mg tissue). The ipsilateral increase was not observed when tPA was administered in combination with fingolimod (2.2 ± 1.6 ng Evans Blue/mg tissue, ipsilateral region, p < 0.05).
Figure 5.
A) Effect of fingolimod (0.5 mg/kg FTY720) on Evans Blue extravasation in animals with delayed recanalization determined in contralateral and ipsilateral hemisphere of control and treated group. B shows Evans Blue extravasation in ischemic region normalized for infarct volume. Evans Blue extravasation was determined 24 h after occlusion. Data are means ± SD; n=6; *P<0.05.
The extent of Evans Blue leakage 3 and 48 hours after tPA administration was similar to that observed at 24 h (data no shown) and the effect of fingolimod was only investigated at 24 h.
To demonstrate that the increased Evans Blue leakage was caused by tPA and was not due to ischemic damage or reperfusion injury, we assessed Evans Blue leakage in mice with permanent thrombin-induced occlusion and in mice in which transient occlusion (3 hours) was induced using a vessel clip 15; but we did not observe an ipsilateral increase in Evans blue extravasation, and therefore did not study the effect of fingolimod in either cohort (Figure S3). No differences in the pattern of Evans Blue leakage were observed after normalizing these data for infarct volume.
In vitro analysis interaction of fingolimod/P-fingolimod and tPA activity
In order to expand on the observation that fingolimod treatment did not affect the reperfusion time-course of thrombolytic treatment compared with vehicle group (Figure 1), we used an in vitro assay to confirm that fingolimod or P-fingolimod did not interfere with the enzymatic activity of tPA. Fingolimod and P-fingolimod, tested in a range of concentrations between 0.01 nM and 100 μM, did not modify the activity of tPA, at variance with leupeptin, used as a positive control (Figure 4S).
Discussion
This study shows that fingolimod attenuates the neurological deficit and reduces infarct volume in a model of thromboembolic MCA occlusion. In this model, in combination with thrombolytic therapy, fingolimod improves the neurological outcome with early tPA administration, while with delayed administration of tPA fingolimod both improves neurological outcome and reduces the infarct volume.
Fingomolid has been recently suggested to be a potential new treatment for ischemic brain injury, based on several studies using rodent models of brain ischemia 9–12 (one study however failed to detect an effect 16, suggesting variations possibly due to experimental conditions). When protective effects by fingolimod were observed, the mechanisms of action were not clearly established. Thus, when fingolimod was tested in a rat model of transient ischemia by filament occlusion of the MCA, it reduced neuronal injury and improved behavior by activation of Akt and ERK via S1P1 receptors, preventing neuronal apoptosis 10. In a mouse study, the protective efficacy of fingolimod was associated with a reduction of inflammatory response and a direct neuroprotective effect mediated by inhibition of apoptotic death, but preservation of blood brain–barrier was not observed 9. We have recently described that in a transient mouse model, fingolimod reduced infarct size, neurological deficit and edema 12. This protective effect was accompanied by decreased inflammation. However, at variance with the neuroprotective effect previously described 9–10, fingolimod did not protect primary neurons against glutamate excitotoxicity or hydrogen peroxide, but it decreased ICAM-1 expression in brain endothelial cells stimulated by tumor necrosis factor alpha. The findings of the later study suggest that anti-inflammatory mechanisms, and possibly vasculo-protection, rather than direct effects on neurons, are the most plausible mechanisms that underlie the beneficial effects of fingolimod12.
In the three treatment paradigms used in the present study, fingolimod improved neurological deficit but did not achieve a significant reduction of infarct volume when administered in combination with tPA 30 min after occlusion (Figure 2). Previous clinical studies have observed that tPA treatment administered within 3 h of symptom onset decreases the neuroinflammation that follows stroke 17. Although further studies are necessary to confirm this hypothesis, we speculate that the reduction of inflammatory response induced by early tPA administration might have masked the beneficial effect of fingolimod on infarct size.
Vasculo-protection and preservation of BBB integrity are other important processes associated with the activation of S1P receptors 18–19, and have been implicated in the effects of fingolimod in studies unrelated to stroke 20. S1P receptor activation stimulates the recruitment of endothelial proteins that form adherens junctions, thereby creating a tighter contact between endothelial cells, enhancing BBB integrity 20
Early tPA administration is the optimal therapeutic strategy to rescue still viable ischemic tissue and to improve the outcome in patients with acute ischemic stroke. But studies based on the magnetic resonance imaging (MRI) perfusion/diffusion (“mismatch concept 21) suggest that there might be patients who could benefit from thrombolysis even beyond 3 hours, even if they present a higher risk of BBB breakdown and hemorrhagic transformation. In agreement with these clinical data and also with previous experimental studies in rats and mice, we observed that tPA administered 30 min after thrombin injection (early administration) reduced ischemic injury without BBB damage, while BBB breakdown and hemorrhagic transformation appeared when tPA was given 3 hours after occlusion (delayed administration).
Of note, the dose of 10 mg/kg tPA used in the current study is higher than the clinical dose (0.9 mg/kg). The fibrinolytic system in rodents has been known for 30 years to be about 10 times less sensitive to tPA than in humans22. The vast majority of stroke studies in rodents have therefore been performed with 10 mg/kg tPA. In our study, significant hemorrhages were not seen with early tPA. We therefore assume that the hemorrhages we observed were the consequence of the delayed administration rather than of the high dose of tPA, and we believe that fingolimod-induced prevention of BBB damage caused by delayed tPA might indeed be clinically significant. Similarly, mice were treated with rather high doses of fingolimod (0.5 mg/kg bodyweight/d), but it is a dose commonly used to treat mice with experimental autoimmune encephalomyelitis (a model of multiple sclerosis). 23–24
It is possible that reduced hemorrhagic transformation and Evans blue extravasation could be the mere consequence of reduced infarct volume in fingolimod-treated mice after delayed rtPA, since a direct effect of fingolimod on BBB damage was not demonstrated. But this is unlikely, since normalized data per infarct volume (Figure 4 and 5) or lack of EB leakage in mice with permanent occlusion or mice with transient occlusion (3 hours) induced using a vessel clip (Figure S3) suggest that BBB damage was not associated with infarct size and was mediated by delayed tPA administration and, confirming the vasculo-protective effect of fingolimod.
Several treatments, some promising, have been tested in combination with tPA (see 25 for review) to reduce its neurotoxicity, risk of hemorrhage and reperfusion injury, or to increase neuroprotection and increase therapeutic time window. One of the advantages of fingolimod with respect to other treatments is that in addition to reducing the risk of hemorrhage, fingolimod is itself protective, which is likely to synergize with the benefits of thrombolytic therapy as we have observed in our study.
In conclusion, in this study we have confirmed the protective efficacy of fingolimod as a treatment against ischemic stroke in a novel model thromboembolic occlusion model, and we have shown the potential applicability of this treatment in combination with tPA to reduce the risk of brain hemorrhage.
Supplementary Material
Acknowledgments
Sources of funding
This work was supported by NIH grants (NS049263 and NS055104) to CW and grant P30NS045776 (Interdepartmental Neuroscience Center). Spanish Ministry of Science and Innovation SAF2011-30517 AND RENEVAS (RD06/0026/000). Furthermore, FC is the recipient of a fellowship from the Conselleria de Industria, Xunta de Galicia (Programa Angeles Alvariño).
Footnotes
Disclosures
None
References
- 1.Tomsick TA, Khatri P, Jovin T, Demaerschalk B, Malisch T, Demchuk A, et al. Equipoise among recanalization strategies. Neurology. 2010;74:1069–1076. doi: 10.1212/WNL.0b013e3181d76b8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 3.Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie ET, et al. The proteolytic activity of tissue-plasminogen activator enhances nmda receptor-mediated signaling. Nat Med. 2001;7:59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- 4.Cohen JA, Chun J. Mechanisms of fingolimod’s efficacy and adverse effects in multiple sclerosis. Ann Neurol. 2011;69:759–777. doi: 10.1002/ana.22426. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez C, Gonzalez-Diez M, Badimon L, Martinez-Gonzalez J. Sphingosine-1-phosphate: A bioactive lipid that confers high-density lipoprotein with vasculoprotection mediated by nitric oxide and prostacyclin. Thromb Haemost. 2009;101:665–673. [PubMed] [Google Scholar]
- 6.Soliven B, Miron V, Chun J. The neurobiology of sphingosine 1-phosphate signaling and sphingosine 1-phosphate receptor modulators. Neurology. 2011;76:S9–14. doi: 10.1212/WNL.0b013e31820d9507. [DOI] [PubMed] [Google Scholar]
- 7.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 8.Pelletier D, Hafler DA. Fingolimod for multiple sclerosis. N Engl J Med. 2012;366:339–347. doi: 10.1056/NEJMct1101691. [DOI] [PubMed] [Google Scholar]
- 9.Czech B, Pfeilschifter W, Mazaheri-Omrani N, Strobel MA, Kahles T, Neumann-Haefelin T, et al. The immunomodulatory sphingosine 1-phosphate analog fty720 reduces lesion size and improves neurological outcome in a mouse model of cerebral ischemia. Biochem Biophys Res Commun. 2009;389:251–256. doi: 10.1016/j.bbrc.2009.08.142. [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa Y, Suzuki H, Sozen T, Rolland W, Zhang JH. Activation of sphingosine 1-phosphate receptor-1 by fty720 is neuroprotective after ischemic stroke in rats. Stroke. 2010;41:368–374. doi: 10.1161/STROKEAHA.109.568899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfeilschifter W, Czech-Zechmeister B, Sujak M, Mirceska A, Koch A, Rami A, et al. Activation of sphingosine kinase 2 is an endogenous protective mechanism in cerebral ischemia. Biochem Biophys Res Commun. 2011;413:212–217. doi: 10.1016/j.bbrc.2011.08.070. [DOI] [PubMed] [Google Scholar]
- 12.Wei Y, Yemisci M, Kim HH, Yung LM, Shin HK, Hwang SK, et al. Fingolimod provides long-term protection in rodent models of cerebral ischemia. Ann Neurol. 2011;69:119–129. doi: 10.1002/ana.22186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Yebenes I, Sobrado M, Zarruk JG, Castellanos M, Perez de la Ossa N, Davalos A, et al. A mouse model of hemorrhagic transformation by delayed tissue plasminogen activator administration after in situ thromboembolic stroke. Stroke. 2011;42:196–203. doi: 10.1161/STROKEAHA.110.600452. [DOI] [PubMed] [Google Scholar]
- 14.Orset C, Macrez R, Young AR, Panthou D, Angles-Cano E, Maubert E, et al. Mouse model of in situ thromboembolic stroke and reperfusion. Stroke. 2007;38:2771–2778. doi: 10.1161/STROKEAHA.107.487520. [DOI] [PubMed] [Google Scholar]
- 15.Eikermann-Haerter K, Lee JH, Yuzawa I, Liu CH, Zhou Z, Shin HK, et al. Migraine mutations increase stroke vulnerability by facilitating ischemic depolarizations. Circulation. 2012;125:335–345. doi: 10.1161/CIRCULATIONAHA.111.045096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liesz A, Sun L, Zhou W, Schwarting S, Mracsko E, Zorn M, et al. Fty720 reduces post-ischemic brain lymphocyte influx but does not improve outcome in permanent murine cerebral ischemia. PLoS One. 2011;6:e21312. doi: 10.1371/journal.pone.0021312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montaner J, Salat D, García-Berrocoso T, Molina C, Chacón P, Ribó M, et al. Reperfusion therapy for acute stroke improves outcome by decreasing neuroinflammation. Translational stroke research. 2010;1:261–267. doi: 10.1007/s12975-010-0038-0. [DOI] [PubMed] [Google Scholar]
- 18.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, et al. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu D, Wang Y, Singh I, Bell RD, Deane R, Zhong Z, et al. Protein’s controls hypoxic/ischemic blood-brain barrier disruption through the tam receptor tyro3 and sphingosine 1-phosphate receptor. Blood. 2010;115:4963–4972. doi: 10.1182/blood-2010-01-262386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez T, Estrada-Hernandez T, Paik JH, Wu MT, Venkataraman K, Brinkmann V, et al. Phosphorylation and action of the immunomodulator fty720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J Biol Chem. 2003;278:47281–47290. doi: 10.1074/jbc.M306896200. [DOI] [PubMed] [Google Scholar]
- 21.Jansen O, Schellinger P, Fiebach J, Hacke W, Sartor K. Early recanalisation in acute ischaemic stroke saves tissue at risk defined by mri. Lancet. 1999;353:2036–2037. doi: 10.1016/S0140-6736(99)01146-0. [DOI] [PubMed] [Google Scholar]
- 22.Korninger C, Collen D. Studies on the specific fibrinolytic effect of human extrinsic (tissue-type) plasminogen activator in human blood and in various animal species in vitro. Thromb Haemost. 1981;46:561–565. [PubMed] [Google Scholar]
- 23.Papadopoulos D, Rundle J, Patel R, Marshall I, Stretton J, Eaton R, et al. Fty720 ameliorates mog-induced experimental autoimmune encephalomyelitis by suppressing both cellular and humoral immune responses. J Neurosci Res. 2010;88:346–359. doi: 10.1002/jnr.22196. [DOI] [PubMed] [Google Scholar]
- 24.Fujino M, Funeshima N, Kitazawa Y, Kimura H, Amemiya H, Suzuki S, et al. Amelioration of experimental autoimmune encephalomyelitis in lewis rats by fty720 treatment. J Pharmacol Exp Ther. 2003;305:70–77. doi: 10.1124/jpet.102.045658. [DOI] [PubMed] [Google Scholar]
- 25.Kaur J, Zhao Z, Klein GM, Lo EH, Buchan AM. The neurotoxicity of tissue plasminogen activator? J Cereb Blood Flow Metab. 2004;24:945–963. doi: 10.1097/01.WCB.0000137868.50767.E8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.