Abstract
Evidence from two experiments suggests that negative arousal increases biases in attention that result from differences in visual salience. Participants were exposed to negative arousing or neutral sounds before briefly viewing an array of letters. They reported as many of the letters as they could, and attention was biased to certain letters by increasing salience through visual contrast. Regardless of the type of sound heard, participants were more likely to recall high-salience letters than low-salience letters. However, on arousing trials recall of high-salience letters increased, while recall of low-salience letters did not. These findings indicate that negative emotional arousal increases the selectivity of attention, and provides evidence for arousal-biased competition (ABC) theory (Mather & Sutherland, 2011), which predicts that emotional arousal enhances representations of stimuli that have priority.
Keywords: Emotional Arousal, Salience, Short-Term Memory
Humans are motivated to focus on potential threats and rewards (Lang, 2010; Pessoa & Ungerleider, 2004; Vuilleumier, 2005). Survival without such biases is difficult to imagine, and many previous studies confirm that arousing stimuli are perceptually salient and grab attention (Mather, 2007). People are less likely to perceive neutral stimuli when they are competing spatially or temporally with arousing stimuli (Arnell, Killman, & Fijavz, 2007; Most & Wang, 2011; Sheth & Pham, 2008). This suggests that arousing stimuli consume a disproportionate amount of resources, which comes at the cost of processing neutral stimuli.
But if enough time is given between the presentation of the emotional stimulus and the neutral target, or the emotional stimulus is presented through a different modality, arousal can enhance perception of subsequently presented neutral stimuli (Bocanegra & Zeelenberg, 2009; Ciesielski, Armstrong, Zald, & Olatunji, 2010; Padmala & Pessoa, 2008; Phelps, Ling, & Carrasco, 2006; Zeelenberg & Bocanegra, 2010). In each of these studies perceptual enhancement was observed for a single item.
Similar results have been observed in studies that present emotionally arousing cues immediately before visual search. For example, a fearful face can improve one’s ability to identify the presence of a complex visual target, such as a house, within a large array of complex distracters (Becker, 2009). Fearful faces also enhance search for less complex stimuli like nonsensical shapes (Olatunji, Ciesielski, Armstrong, & Zald, 2011). Thus, emotionally arousing stimuli can enhance detection of an emotionally neutral target. But because visual search paradigms measure performance for only a single target, it is unclear whether arousal leads to a general enhancement in visual processing for everything shown after the arousing stimulus, or if the enhancement is selective to targets because they match top-down goals.
According to arousal-biased competition (ABC) theory (Mather & Sutherland, 2011), arousing stimuli should lead to only selective enhancements in processing subsequent neutral stimuli. Vision research demonstrates that goal-relevance and/or perceptual salience determine which objects out of a cluttered visual scene will be selected for further processing (Desimone & Duncan, 1995; Beck & Kastner, 2009). Selection is competitive, with bottom-up (salience) and top-down (goal-relevance) factors biasing competition and determining stimulus priority (Fecteau & Munoz, 2006). ABC theory predicts that emotional arousal increases the competitive advantage of stimulus priority, leading to ‘winner-take-more’ and ‘loser-take-less’ effects in the competition for limited resources.
Thus, ABC theory predicts that arousal can lead to both enhancement and impairment among neutral stimuli competing for attention. Whether arousing stimuli enhance or impair the processing of a particular subsequent neutral stimulus would be determined by whether the stimulus has high or low priority. As outlined in computational models of vision, bottom-up perceptual salience is determined by the contrast between a stimulus and its surrounding context (Itti & Koch, 2000). Low-level visual features like luminance, color, and the orientation of lines are measured at each location and compared with surrounding locations. The differences between the central location and its surrounding locations are then amplified through an iterative process that simulates center-surround competitive processes among neurons in the primate visual system. The spatial scale of the competing locations gradually increases, ultimately revealing the most salient region in the display. ABC theory predicts that arousal amplifies these competitive processes, such that representations of perceptually salient stimuli gain additional strength, while competing representations are more suppressed than they would be otherwise.
Previous studies showing that arousing stimuli enhance subsequent neutral stimuli involved measures of only a single target (Becker, 2009; Bocanegra & Zeelenberg, 2009; Ciesielski, et al., 2010; Olatunji, et al., 2011; Padmala & Pessoa, 2008; Phelps, et al., 2006; Zeelenberg & Bocanegra, 2010). The goal of the current study was to test the ABC hypothesis by measuring the effects of emotional arousal on subsequent visual processing for an array of multiple targets to be reported. Attention was biased to certain letters by increasing the visual contrast of a subset of the letters in the array. The use of multiple targets and the biasing of attention using visual contrast allowed us to test ABC theory’s prediction that arousal should enhance high priority and suppress low priority stimuli.
General Method
Overview
On each trial in Experiment 1 participants focused on a central fixation cross and listened to a short audio clip. Shortly after the sound clip ended, a circular array of letters briefly appeared around the fixation cross. Participants were immediately cued to recall as many of the letters as possible. Some of the letters were presented in a darker shade of grey and were less numerous than the lighter shaded letters, giving them priority in the competition for selection. Experiment 2 was an attempt to replicate the findings observed in Experiment 1. To our knowledge, previous studies demonstrating subsequent enhancements in visual processing have not explored the limit at which arousal loses its enhancing effects. To explore this limit, a second condition with a longer ISI range (4000–6000 ms instead of 750–3000 ms) was added to Experiment 2. We hypothesized that on arousing trials participants would report more high priority letters and fewer low priority letters, compared with neutral trials. Stimuli
Twenty highly arousing negative sounds and 20 neutral sounds from the International Affective Digital Sound (IADS) system (Bradley & Lang, 2000, 2007) were presented via headphones. The sounds consisted of ecologically valid stimuli such as screams, physical abuse, vomiting and bomb explosions (Table 1).
Table 1.
IADS library numbers for all 40 sounds used in both experiments.
| Stimulus Type | IADS Library Numbers |
|---|---|
| Negative Arousing | 106 115 134 244 255 260 276 279 282 283 289 292 420 501 600 624 626 711 712 730 |
| Neutral | 102 113 130 132 170 225 246 250 252 322 358 373 375 377 382 701 708 720 723 728 |
Each letter in the array was printed in uppercase Arial font 2.5 cm from the center fixation point. The entire circle subtended 11.08 × 14.58 ° of visual arc. The letter ‘I’ was not used, as it resembled the lowercase version of letter ‘L’. High-salience letters had RGB values of 102, 102 and 102, respectively. Low-salience letters possessed RGB values of 204, 204 and 204, respectively. The stimuli were displayed on an iMac monitor with a media white point value of X: 0.9505 Y: 1.0 Z: 1.0891.
Participants
In Experiment 1 all 55 participants (37 female) reported having normal or corrected-to-normal vision and hearing. They ranged from ages 18–29 (M = 20.96). Similarly, all 110 participants (90 female) in Experiment 2 reported having normal or corrected-to-normal vision and hearing, and ranged from ages 18–27 (M = 20.17).
Procedure
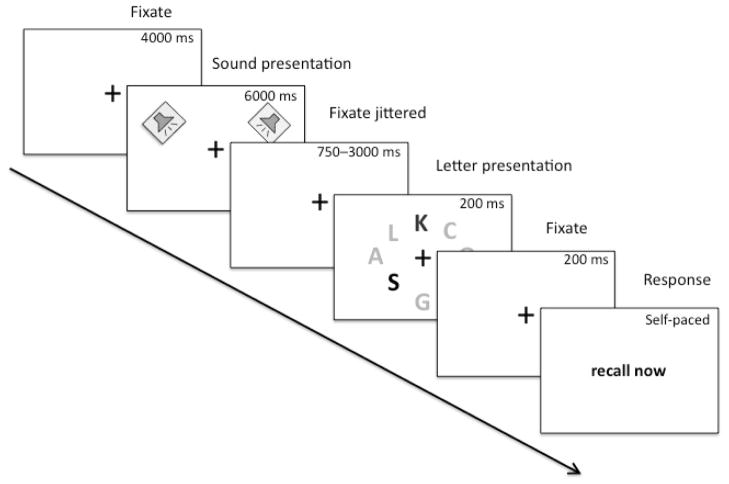
Participants used a chin rest during the experiment, completing five practice trials first. Each of the 40 randomly ordered trials began with a center fixation cross that remained on screen until participants were cued to respond (Figure 1). After 4 seconds of fixation a sound was presented for 6 seconds followed by a brief presentation of 8 letters. The letters were presented for 200 ms to reduce the possibility of eye-movements during the letter display. Each letter array consisted of 3 high-salience letters and 5 low-salience letters. This distribution was based on pilot data indicating that 3 high-salience letters and 5 low-salience letters led to recall scores (percent correct) for each salience type that allowed increases and decreases to be observed due to arousal (avoiding ceiling effects in high salience letter reporting). Once the letters were removed, participants were cued to report the letters they saw via key press. To prevent top-down influences, participants were instructed not to try harder to recall certain letters based on their shade of grey. Finally, due to the difficulty of the task it was emphasized that participants should be less concerned with avoiding errors and more concerned with recalling letters.
Figure 1.
Schematic depiction of the procedure used in Experiment 1 and in condition 1 of Experiment 2.
Data Analysis
To test the hypothesis that emotional arousal increases recall of high priority stimuli at the cost of recalling low priority stimuli, we performed 2 × 2 repeated measures ANOVAs using arousal and salience as within-subject factors. Recall was calculated as percent correct. All Cohen’s d effect size statistics were corrected for inflation biases that occur in repeated measures designs. The inflation occurs as a result of using the pooled variance of the measures to calculate the d statistic (Dunlap, Cortina, Vaslow, & Burke, 1996).
In addition, the use of ecologically valid sounds made it difficult to control for low-level differences (see Bradley & Lang, 2000) in intensity and frequency between the arousing and neutral sound clips. Intensity and frequency are the two primary components that make up an audio stimulus, thus we used hierarchical linear model (HLM) analyses to see if the arousal type of the sounds could predict recall for high and low-salience letters while controlling for differences in peak intensity (dB) and peak frequency (Hz). We also calculated the temporal position of the peak intensity within each sound clip, and controlled for this variable in the HLM. This was done to ensure that it was differences in the arousing content of the sounds, rather than differences in non-semantic features of the audio that were influencing recall. Our dependent variable in this HLM analysis was a difference score indicating the advantage of high salience over low salience letters (high-salience minus low-salience proportion recalled).
Experiment 1
Method
In Experiment 1 the ISIs separating the sounds from the letters were randomized and ranged from 750–3000 ms. A large ISI was used to diminish participant’s ability to predict the onset of the letter array. The inter-trial-interval (ITI) varied, as recall was self-paced.
Results
In Experiment 1 a greater proportion of high-salience letters were recalled compared with low-salience letters, F(1,54) = 69.06, p < 0.001, ηp2 = 0.56. Moreover, we observed a disordinal interaction between arousal and salience, F(1,54) = 5.61, p < 0.05, ηp2 = 0.09, as on arousing trials performance for high-salience letters increased while performance for low-salience letters decreased (Table 2). No main effect of arousal was observed indicating that the arousal level of the sounds had no overall impact on the proportion of letters recalled, F(1,54) = 0.60, p = 0.44, ηp2 = 0.01. In a direct comparison, the proportion of high-salience letters recalled was greater on arousing compared to neutral trials, t(54) = 2.04, p < 0.05, d = 0.15, a “winner-take-more” effect. The “loser-take-less” effect for low-salience letter recall was not quite significant, t(54) = 1.73, p = 0.09, d = 0.10, and the total number of errors did not differ across arousing (M = 14.11, SE = 1.91) and neutral (M = 14.44, SE = 1.92) trials, t(54) = 0.67, p = 0.51, d = 0.02.
Table 2.
Letter recall (mean proportion correct and the standard error of the mean) for Experiments 1 and 2.
| Experiment 1 | Experiment 2 condition 1 | Experiment 2 condition 2 | Exp 1 & Exp 2 condition 1 | |||||
|---|---|---|---|---|---|---|---|---|
| Neutral Low- Salience | M = 0.391 | SE = 0.018 | M = 0.425 | SE = 0.014 | M = 0.405 | SE = 0.016 | M = 0.408 | SE = 0.012 |
| Neutral High- Salience | M = 0.632 | SE = 0.021 | M = 0.579 | SE = 0.017 | M = 0.596 | SE = 0.015 | M = 0.605 | SE = 0.014 |
| Arousing Low- Salience | M = 0.378 | SE = 0.017 | M = 0.412 | SE = 0.013 | M = 0.407 | SE = 0.016 | M = 0.395 | SE = 0.011 |
| Arousing High- Salience | M = 0.654 | SE = 0.021 | M = 0.598 | SE = 0.015 | M = 0.600 | SE = 0.015 | M = 0.626 | SE = 0.013 |
Experiment 2
Method
Experiment 2 consisted of two separate conditions. Condition 1 was a replication of Experiment 1, while in Condition 2 the ISIs separating the sounds from the letters was increased from 750–3000 ms to 4000–6000 ms. The purpose of Condition 2 was to explore the duration of arousal’s impact on recall in this paradigm.
Results
We began by examining whether the results of Condition 1 replicated those of Experiment 1. In Condition 1, participants recalled a greater percentage of high-salience letters compared with low-salience letters, F(1,54) = 55.76, p < 0.001, ηp2 = 0.51. Moreover, the disordinal interaction between arousal and salience replicated the results of Experiment 1; on arousing trials the percentage of high-salience letters recalled increased, while the percentage of low salience letters recalled decreased, F(1,54) = 4.67, p < 0.05, ηp2 = 0.08. The direct comparisons confirmed that the percentage of high-salience letters recalled was greater on arousing versus neutral trials, t(54) = 2.05, p < 0.05, d = 0.15, while the direct comparison of low-salience letter recall was marginally significant, t(54) = 1.76, p = 0.085, d = 0.13. In addition, there was no main effect of arousal, F(1,54) = 0.576, p = 0.45, ηp2 = 0.01, and no difference in the number of errors made on arousing (M = 13.75, SE = 1.41) and neutral (M = 14.07, SE = 1.42) trials, t(54) = 0.45, p = 0.65, d = 0.03.
While Condition 1 replicated the results of Experiment 1, Condition 2 did not. The percentage of high-salience letters recalled was greater than that of low-salience letters, F(1,54) = 74.12, p < 0.001, ηp2 = 0.58, but there was no interaction between arousal and salience, F(1,54) = 0.02, p = 0.88, ηp2 < 0.001. There also was no main effect of arousal, F(1,54) = 0.40, p = 0.53, ηp2 = 0.01, and no difference in the number of errors made on arousing (M = 13.45, SE = 1.87) and neutral (M = 13.36, SE = 1.80) trials, t(54) = 0.127, p = 0.90, d = 0.01. The differences between the arousal × salience interaction’s medium effect size in Condition 1 and its near-zero effect size in Condition 2 were not large enough to yield a significant 2 (arousal) × 2 (salience) × 2 (condition) interaction when the two conditions were analyzed together, F(1,108) = 1.70, p = 0.196, ηp2 = 0.02. It seems that the 3-way interaction was a small effect size that we lacked power to detect, as more than 250 participants would be needed to have 80% power to detect effects of this size (Cohen, 1988).
Next we pooled the data from Experiment 1 and Condition 1 of Experiment 2 to gain power as the procedures used were identical. The pattern remained the same as in the separate experiments, except that now both the “winner-take-more” effect, t(109) = −2.892, p < 0.01, d = 0.15, and the “loser-take-less” effect, t(109) = 2.47, p < 0.05, d = 0.11, were significant (Fig. 2).
Figure 2.
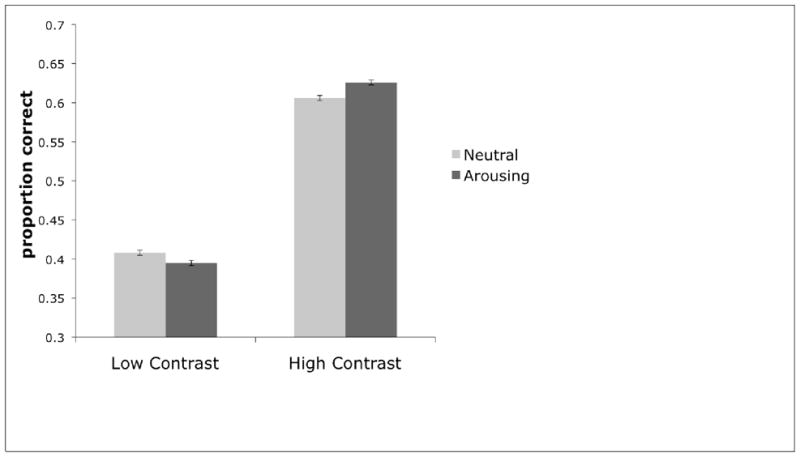
Proportion correct of letters recalled for low and high-salience letters on arousing and neutral trials (n = 110). Data were included from Experiment 1 and condition 1 of Experiment 2, and error bars were calculated as within-subject confidence intervals (Loftus & Masson, 1994).
Finally, the use of ecologically valid sounds made it difficult to control for intensity (dB) and frequency (Hz) differences between the arousing and neutral sound clips. Thus we conducted HLM analyses to determine whether the arousal type of the sounds could predict recall performance in the expected directions while controlling for these low-level differences between the sounds. We again pooled data from Experiment 1 and Condition 1 of Experiment 2 to gain power. We then performed separate analyses for low-salience letters, high-salience letters and difference scores (high-salience minus low-salience). Peak intensity, peak frequency and the temporal location of the peak intensity within the sound clip were added into the model as additional predictors. In all three models arousal type was a significant predictor, while none of the predictors related to the low-level features of the sounds reached significance. This indicates that the arousal type of the sounds, rather than low-level differences in intensity and frequency, underlie the observed interaction between arousal and salience (Table 3).
Table 3.
Hierarchical linear model (HLM) analysis using the arousal type (arousing versus neutral) of the sounds, the peak frequency (Hz) and peak intensity (dB) of the sounds, as well as the temporal location of the peak dB to predict recall rates for (A) high-salience letters, (B) low-salience letters and (C) difference scores (high-salience minus low-salience). Includes results from Experiment 1 and condition 1 of Experiment 2 (n = 110).
| A. High-Salience % Correct | |||||
|---|---|---|---|---|---|
| Effect | b | SE | t | df | p-value |
| Intercept | 0.615909 | 0.012979 | 47.453 | 109 | < 0.001 |
| Arousal | 0.010835 | 0.003986 | 2.718 | 4395 | 0.007 |
| Peak Hz | 0.000000 | 0.000001 | 0.322 | 4395 | 0.747 |
| Peak dB | −0.000167 | 0.000557 | −0.300 | 4395 | 0.764 |
| Peak dB (time) | −0.000794 | 0.002141 | −0.371 | 4395 | 0.711 |
| B. Low-Salience % Correct | |||||
| Effect | b | SE | t | df | p-value |
| Intercept | 0.401409 | 0.010894 | 36.847 | 109 | < 0.001 |
| Arousal | −0.007345 | 0.002953 | −2.487 | 4395 | 0.013 |
| Peak Hz | −0.000000 | 0.000001 | −0.271 | 4395 | 0.786 |
| Peak dB | 0.000283 | 0.000401 | 0.706 | 4395 | 0.480 |
| Peak dB (time) | 0.001802 | 0.001536 | 1.173 | 4395 | 0.241 |
| C. Difference Scores % Correct | |||||
| Effect | b | SE | t | df | p-value |
| Intercept | 0.214500 | 0.019570 | 10.961 | 109 | < 0.001 |
| Arousal | 0.018180 | 0.005862 | 3.101 | 4395 | 0.002 |
| Peak Hz | 0.000000 | 0.000001 | 0.333 | 4395 | 0.739 |
| Peak dB | −0.000450 | 0.000834 | −0.540 | 4395 | 0.589 |
| Peak dB (time) | −0.002595 | 0.003226 | −0.804 | 4395 | 0.421 |
Discussion
In two experiments we tested the hypothesis that negative emotional arousal increases the selectivity of attention, thus impacting immediate recall. In general, regardless of whether an arousing or neutral sound was heard, a greater percentage of high-salience letters was recalled. However, this bias to report high-salience letters increased when participants were exposed to emotionally arousing sounds. A decrease in the reporting of low-salience letters was observed, but only when data from Experiment 1 and Condition 1 of Experiment 2 were pooled together to increase power. These results provide evidence for ABC theory (Mather & Sutherland, 2011), which predicts that emotional arousal enhances selection processes in attention by strengthening representations of high priority stimuli and weakening representations of low priority stimuli. Yet Experiment 2 also indicated there are temporal limits to the effects of arousal. Arousal had no effect on recall in Condition 2 where the ISI was increased from 750 3000 ms to 4000 6000 ms, suggesting that the arousing sounds enhance biased competition processes for only a brief period.
Because we used ecologically valid stimuli, there were low-level differences between emotionally arousing and neutral sounds (see Bradley & Lang, 2000). However, HLM analyses showed that the arousal type of the sounds significantly predicted recall for low-salience and high-salience letters and for difference scores while controlling for differences in intensity (dB) and frequency (Hz). This suggests that it was differences in arousal, rather than differences in low-level features of the sounds, that drove the observed interaction between arousal and salience. A question that should be addressed in future studies is whether positive arousal produces similar effects as the negative arousing stimuli used in this study.
While much of the literature has focused on the mechanisms underlying sensory biases to emotionally arousing stimuli (Adolphs, 2004; Pessoa, 2009; Vuilleumier, 2005), ABC theory focuses on the more general influence arousal has on selective attention processes. Initial studies focusing on arousal’s influence on subsequent visual processing came from rapid-serial-visual-presentation (RSVP) paradigms examining the influence of arousal on a phenomenon known as ‘attentional blink’. In these studies the presentation of an emotional stimulus interfered with identification of a subsequently presented neutral stimulus, leading to an effect known as ‘emotion-induced blindness’ (Arnell, et al., 2007; Ciesielski, et al., 2010; Most, Chun, Widders, & Zald, 2005; Most, Smith, Cooter, Levy, & Zald, 2007; Most & Wang, 2011). However, the duration of time separating the arousing cues from the neutral targets was short (< 500 ms), suggesting that the neutral targets were still competing with the arousing cues for limited resources.
Other studies have shown that emotionally arousing cues increase subsequent perception of neutral stimuli, as long as there is a sufficient amount of time between the arousing cue and the neutral target (Bocanegra & Zeelenberg, 2009). In addition, subsequent enhancement effects can be observed at shorter ISI’s, like 50 ms, with less complex stimuli, like Gabor patches (Phelps, et al., 2006), as well as at longer ISI’s lasting 1000 ms (Padmala & Pessoa, 2008). Furthermore, when the arousing cue is presented auditorily rather than visually, subsequent visual enhancement is immediately observed due to the lack of direct competition between the audio and visual stimuli (Zeelenberg & Bocanegra, 2010). Studies using visual search paradigms to examine the effects of emotional arousal on subsequent attention processes have shown that the brief presentation of a fearful face can enhance subsequent visual search for simple stimuli like nonsensical shapes (Olatunji, et al., 2011) and for more complex stimuli like images of houses (Becker, 2009). ABC theory accounts for these results, predicting that arousal increases attention to stimuli that have priority due to task-relevance or perceptual salience. However these studies do not provide direct evidence for ABC theory because they only measured performance for single targets rather than measuring processing of both high and low priority stimuli.
Our findings extend previous research on how arousal can enhance subsequent processing of neutral stimuli and provide evidence for ABC theory. When multiple stimuli competing for attention vary in bottom-up saliency, the selectivity of attention increases in the following 1–3 s after the arousing cue is removed, as high priority stimuli dominate attention to an even greater extent—an effect we refer to as arousal-biased competition (Mather & Sutherland, 2011). Our design allowed a direct test of ABC theory, as we made low and high-salience letters compete for representation in short-term memory and measured the effects of arousal on recall for each type of letter. Moreover, our findings indicate that arousing cues can influence subsequent processing of neutral information for several seconds after the emotional stimulus is removed, extending findings of previous studies that have observed arousing cues influencing subsequent visual processing for up to one second (Bocanegra & Zeelenberg, 2009; Padmala & Pessoa, 2008). However no influence of arousal on subsequent processing was observed four to six seconds after the cue was removed, suggesting that arousing stimuli’s effect on subsequent visual processing is short lived. These results provide support for ABC theory and suggest that, in order to predict when arousal will enhance and when it will impair subsequent processing, a key factor is the perceptual salience of the stimuli.
References
- Adolphs R. Emotional vision. Nature Neuroscience. 2004;7(11):1167–1168. doi: 10.1038/nn1104-1167. [DOI] [PubMed] [Google Scholar]
- Arnell KM, Killman KV, Fijavz D. Blinded by emotion: Target misses follow attention capture by arousing distractors in RSVP. Emotion. 2007;7(3):465–477. doi: 10.1037/1528-3542.7.3.465. [DOI] [PubMed] [Google Scholar]
- Beck DM, Kastner S. Top-down and bottom-up mechanisms in biasing competition in the human brain. Vision Research. 2009;49(10):1154–1165. doi: 10.1016/j.visres.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker MW. Panic search: Fear produces efficient visual search for nonthreatening objects. Psychological Science. 2009;20(4):435–437. doi: 10.1111/j.1467-9280.2009.02303.x. [DOI] [PubMed] [Google Scholar]
- Bocanegra BR, Zeelenberg R. Dissociating Emotion-Induced Blindness and Hypervision. Emotion. 2009;9(6):865–873. doi: 10.1037/a0017749. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Affective reactions to acoustic stimuli. Psychophysiology. 2000;37(2):204–215. doi: 10.1111/1469-8986.3720204. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. (IADS-2): Affective Ratings of Sounds and Instruction Manual. 2. 2007. The International Affective Digitized Sounds. [Google Scholar]
- Ciesielski BG, Armstrong T, Zald DH, Olatunji BO. Emotion modulation of visual attention: Categorical and temporal characteristics. PLoS ONE. 2010;11(5):e13860. doi: 10.1371/journal.pone.0013860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual-attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dunlap WP, Cortina JM, Vaslow JB, Burke MJ. Meta-analysis of experiments with matched groups or repeated measures designs. Psychological Methods. 1996;1(2):170–177. doi: 10.1037/1082-989X.1.2.170. [DOI] [Google Scholar]
- Fecteau JH, Munoz DP. Salience, relevance, and firing: a priority map for target selection. Trends in Cognitive Sciences. 2006;10(8):382–390. doi: 10.1016/j.tics.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Itti L, Koch C. A saliency-based search mechanism for overt and covert shifts of visual attention. Vision Research. 2000;40(10–12):1489–1506. doi: 10.1016/S0042-6989(99)00163-7. [DOI] [PubMed] [Google Scholar]
- Lang PJ. Emotion and motivation: Toward consensus definitions and a common research purpose. Emotion Review. 2010;2(3):229–233. doi: 10.1177/1754073910361984. [DOI] [Google Scholar]
- Loftus GR, Masson MEJ. Using confidence intervals in within-subject designs. Psychonomic Bulletin & Review. 1994;1(4):476–490. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- Mather M. Emotional arousal and memory binding: An object-based framework. Perspectives on Psychological Science. 2007;2(1):33–52. doi: 10.1111/j.1745- 6916.2007.00028.x. [DOI] [PubMed] [Google Scholar]
- Mather M, Sutherland MR. Arousal-biased competition in perception and memory. Perspectives on Psychological Science. 2011;6(2):114–133. doi: 10.1177/1745691611400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most SB, Chun MM, Widders DM, Zald DH. Attentional rubbernecking: Cognitive control and personality in emotion-induced blindness. Psychonomic Bulletin & Review. 2005;12(4):654–661. doi: 10.3758/bf03196754. [DOI] [PubMed] [Google Scholar]
- Most SB, Smith SD, Cooter AB, Levy BN, Zald DH. The naked truth: Positive, arousing distractors impair rapid target perception. 2007;21(5):964–981. doi: 10.1080/02699930600959340. [DOI] [Google Scholar]
- Most SB, Wang L. Dissociating Spatial Attention and Awareness in Emotion-Induced Blindness. Psychological Science. 2011;22(3):300–305. doi: 10.1177/0956797610397665. [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Ciesielski BG, Armstrong T, Zald DH. Emotional expressions and visual search efficiency: Specificity and effects of anxiety symptoms. Emotion. 2011;11(5):1073–1079. doi: 10.1037/a0021785. [DOI] [PubMed] [Google Scholar]
- Padmala S, Pessoa L. Affective Learning Enhances Visual Detection and Responses in Primary Visual Cortex. The Journal of Neuroscience. 2008;28(24):6202–6210. doi: 10.1523/jneurosci.1233-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control? Trends in Cognitive Sciences. 2009;13(4):160–166. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Ungerleider LG. Neuroimaging studies of attention and the processing of emotion-laden stimuli. In C. A. Heywood, A. D. Milner & C. Blakemore (Eds.) Roots of Visual Awareness - a Festschrift in Honor of Alan Cowey. 2004;144:171–182. doi: 10.1016/S0079-6123(03)14412-3. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Ling S, Carrasco M. Emotion facilitates perception and potentiates the perceptual benefits of attention. Psychological Science. 2006;17(4):292–299. doi: 10.1111/j.1467-9280.2006.01701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth BR, Pham T. How emotional arousal and valence influence access to awareness. Vision Research. 2008;48(23–24):2415–2424. doi: 10.1016/j.visres.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: Neural mechanisms of emotional attention. Trends in Cognitive Sciences. 2005;9(12):585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Zeelenberg R, Bocanegra BR. Auditory emotional cues enhance visual perception. Cognition. 2010;115(1):202–206. doi: 10.1016/j.cognition.2009.12.004. [DOI] [PubMed] [Google Scholar]