Abstract
Context
Inhaled hypertonic saline is recommended as therapy for cystic fibrosis (CF) patients 6 years of age and older, but its efficacy has never been evaluated in CF patients <6 years of age.
Objective
To determine if hypertonic saline reduces the rate of protocol-defined pulmonary exacerbations in CF patients <6 years of age.
Design and Setting
A multicenter, randomized, double-blind placebo-controlled trial was conducted from April 2009 to October 2011 at 30 CF care centers in the United States and Canada.
Participants
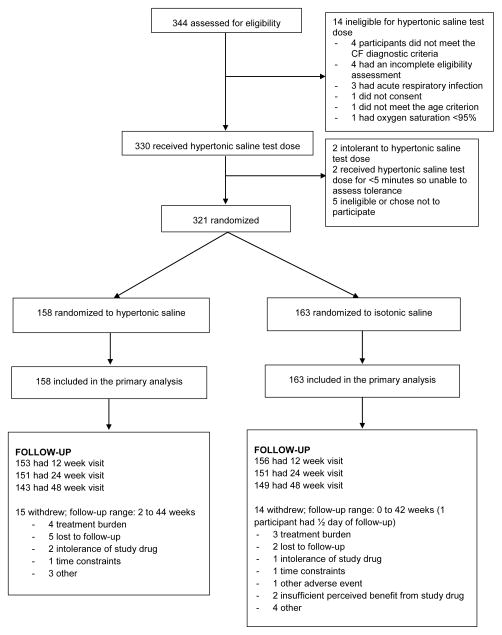
Participants had an established diagnosis of CF and were 4 to 60 months of age. A total of 344 patients were assessed for eligibility; 321 participants were randomized; 29 (9%) withdrew prematurely.
Intervention
The active group (n=158) received 7% hypertonic saline and the control group (n=163) received 0.9% isotonic saline nebulized twice daily for 48 weeks. Both groups received albuterol or levalbuterol prior to each study drug dose.
Main Outcome Measures
the rate of protocol-defined pulmonary exacerbations during the 48 week treatment period treated with oral, inhaled or intravenous antibiotics.
Results
The mean pulmonary exacerbation rate (events/person-year) was 2.3 (95% CI, 2.0, 2.5) in the hypertonic saline group and 2.3 (95% CI, 2.1, 2.6) in the isotonic saline group; the rate ratio was 0.98 (95% CI, 0.84, 1.14)). Among participants with pulmonary exacerbations, the mean number of total antibiotic treatment days for a pulmonary exacerbation was 60 (95% CI 49, 70) in the hypertonic saline group and 52 (95% CI 43, 61) in the isotonic saline group. There was no significant difference in secondary endpoints including height, weight, respiratory rate, oxygen saturation, cough or respiratory symptom scores. Infant pulmonary function testing performed as an exploratory outcome in a subgroup (N=73, with acceptable measurements at 2 visits in 45) did not demonstrate significant differences between groups except for the mean change in forced expiratory volume in 0.5 seconds which was 38 ml greater (95% CI 1, 76) in the hypertonic saline group.
Adherence by returned study drug ampoules was at least 75% in each group. Adverse event profiles were also similar, with the most common adverse event of moderate or severe severity in each group being cough (39% of hypertonic saline group, 38% of isotonic saline group).
Conclusions
Among infants and children with cystic fibrosis less than 6 years old, the use of inhaled hypertonic saline compared with isotonic saline did not reduce the rate of pulmonary exacerbations over 48 weeks of treatment.
Trial Registration
INTRODUCTION
The hallmark features of cystic fibrosis (CF) lung disease include airway infection, inflammation, obstruction, and structural lung damage. These abnormalities begin in infancy, often prior to the onset of symptoms, and progress over the first years of life1–4. Thus, early initiation of effective chronic therapies, an opportunity afforded by newborn screening, could potentially delay or prevent progression of CF lung disease. There are no clinical trials of chronic non-antibiotic maintenance pulmonary therapies in infants and preschoolers with CF, even though this is the population with the greatest potential for long-term benefit.
Dysfunctional ion transport leads to reduced airway surface liquid (ASL) volume in CF and reduction in mucociliary clearance 5. Retained mucus serves as a nidus for chronic infection and exaggerated, sustained neutrophilic inflammation causing progressive airway obstruction and bronchiectasis 6. Hypertonic saline has been demonstrated to increase airway surface liquid in bronchial epithelial cells in vitro and to improve defective mucociliary clearance in CF patients.7, 8
A clinical trial in older children and adults with CF demonstrated modest effects on lung function and a significant decrease in pulmonary exacerbations9, resulting in widespread use of this therapy in older children and adults with CF. Given its mechanism of action, hypertonic saline is an attractive agent for early intervention trials. While 3 short-term safety studies of 7% hypertonic saline have been conducted in CF patients <6 years of age10–12, its efficacy and long-term safety in this population have not been evaluated. Given that hypertonic saline use increased from 6% to 19% among U.S. children with CF 2 to 5 years of age during the enrollment period (2007–2010)13, there was a “window of opportunity” to conduct a clinical trial before hypertonic saline use became widespread in this age range.
We conducted a randomized controlled trial of 7% hypertonic saline among children with CF <6 years of age, to our knowledge the first multicenter clinical trial of a non-antimicrobial chronic CF therapy in this age range. We hypothesized that hypertonic saline would decrease the rate of pulmonary exacerbations and be safe in young children with CF.
METHODS
Overview
This was a 30-center, randomized, parallel group, double blind, controlled trial of 7% hypertonic saline (active drug) vs. 0.9% isotonic saline (control agent) inhaled twice daily for 48 weeks among children with an established diagnosis of CF 4 to 60 months of age at enrollment. The trial was monitored by a Data and Safety Monitoring Board (DSMB) established by the National Heart, Lung, and Blood Institute. IRB approval and written informed consent from parents/guardians were obtained at each participating center. Participant inclusion and exclusion eligibility criteria are detailed in the eMethods. The upper age limit of 60 months was chosen because previous clinical trials of hypertonic saline have enrolled children at least 6 years of age9.
Randomization, Blinding and Treatment Regimen
Participants were randomized 1:1 to 7% hypertonic saline vs. 0.9% isotonic saline, based on random permuted blocks stratified by age (4 to 29 months, 30 to 60 months) and site, via a secure website. Participants, their families, health care providers and research personnel were blinded to treatment assignment. Seven percent hypertonic saline (Hyper-Sal™, PARI Respiratory Equipment, Midlothian, VA) and 0.9% isotonic saline were supplied by Catalent Pharma Solutions (Somerset, NJ) as identically packaged 4 ml blow-fill seal plastic ampoules. Each participant was supplied with a PARI Proneb® Ultra compressor with a PARI LC® Sprint Jr nebulizer equipped with a PARI Baby™ face mask or mouthpiece (PARI Respiratory Equipment, Midlothian, VA).
Clinical Evaluations
Study visits occurred at enrollment/randomization, and 4, 12, 24, 36 and 48 weeks after randomization. At the enrollment visit, after pre-treatment with albuterol or levalbuterol, all participants were evaluated for intolerance to a test dose of 7% hypertonic saline according to pre-defined criteria10 (see eMethods). Participants who tolerated the test dose were randomized. Parents/guardians completed a parent questionnaire weekly, and the Cystic Fibrosis Questionnaire-Revised (CFQ-R)14 and the Treatment Adherence Questionnaire14 quarterly. Further details regarding clinical evaluations are in the eMethods.
Primary and Secondary Outcomes
The primary outcome was the rate of pulmonary exacerbations (events per person-year), defined as treatment with oral, inhaled or intravenous antibiotics for 1 or more pre-specified signs and symptoms within the period 3 days prior to antibiotic start date through antibiotic stop date (which could include 1 or more antibiotics prescribed for the same pulmonary exacerbation). The pre-specified signs and symptoms included: (1) oxygen saturation <90% on room air or ≥5% decline from previous baseline; (2) new lobar infiltrate(s) or atelectasis on chest radiograph; (3) hemoptysis; (4) increased work of breathing or respiratory rate; (5) increased cough; (6) worked harder than usual to breathe during physical activity; (7) increased chest congestion or change in sputum; (8) new or increased adventitial sounds on lung exam; and (9) weight loss ≥5% of body weight or decrease across 1 major percentile in weight percentile for age in past 6 months.
Additional efficacy measures included change in weight, height, resting respiratory rate, room air oxygen saturation and CFQ-R respiratory domain score14 over the 48 week treatment period; and parent report of daytime cough evaluated at the Week 48 visit15. Additional evaluations of pulmonary exacerbations included time to first pulmonary exacerbation as well as number of courses and total number of treatment days with oral, inhaled, and/or intravenous antibiotics for a pulmonary exacerbation or for any indication.
Safety outcomes included the rate of intolerance to the test dose of hypertonic saline at enrollment, adverse events and withdrawal rates, and treatment-emergent respiratory cultures positive for CF pathogens detected through clinical cultures performed at each sites’ microbiology laboratory. All serious adverse events were reviewed by the medical monitor and the DSMB. Adherence to treatment was assessed by: (1) the number of used study drug vials returned, (2) the Treatment Adherence Questionnaire completed quarterly and (3) the weekly parent questionnaire.
Infant Pulmonary Function Testing Substudy
In a substudy at selected sites, infant pulmonary function tests were evaluated as an exploratory endpoint. Additional inclusion criteria for the infant pulmonary function substudy included age ≥4 months and < 16 months at enrollment; additional exclusion criteria included: (1) history of adverse reaction to sedation; (2) clinically significant upper airway obstruction as determined by the site investigator; (3) severe gastroesophageal reflux, defined as persistent frequent emesis despite anti-reflux therapy and (4) acute intercurrent respiratory infection, defined as an increase in cough, wheezing, or respiratory rate with onset in 2 weeks preceding visit. This substudy was performed at 15 sites previously certified to perform research quality infant lung function testing. Subjects underwent an infant pulmonary function test visit a minimum of one day and a maximum of 30 days after the enrollment visit. Infant pulmonary function tests were performed under sedation at this visit and at the 48 week visit. For these subjects, randomization was conducted and study drug was dispensed at the infant pulmonary function test visit rather than at the enrollment visit. Pulmonary function assessments included functional residual capacity (FRC) by body plethysmography 16, 17 and measurements of forced expiratory flows (forced expiratory flow at 75% of vital capacity,FEF75, and mid-maximal forced expiratory flow, FEF25–75) and volumes (forced expiratory volume in 0.5 seconds, FEV0.5, forced vital capacity, FVC) by the raised volume rapid thoracoabdominal compression technique18. Additional lung volumes (residual volume and total lung capacity) were also calculated17. Sites transferred all infant pulmonary function data to the Therapeutics Development Network Infant Pulmonary Function Resource Center at the University of North Carolina for expert over-reading. Acceptable measurements were chosen from the raw data according to published guidelines4, 18, 19.
Sample Size Considerations and Statistical Analysis
For the design, we assumed the rate of pulmonary exacerbations in the isotonic saline (control) group would be 2.22 events/year based on data from a recent large U.S. observational study of children 0–6 years old33. Using this control rate and an O-Brien-Fleming boundary function20 for early stopping with a 0.05 level 2-sided hypothesis test, we calculated a sample size of 150 per group would provide 80% power to detect a rate ratio (hypertonic saline/isotonic saline) less than or equal to 0.80 (or a relative reduction of at least 20%).
The primary outcome, pulmonary exacerbation rate, was compared between groups according to intent-to-treat principles using a Poisson log-linear regression model with log (observation time) as an offset. Observation time was defined as time since randomization to last in-clinic visit or phone-call follow-up. (One participant’s observation time was defined to be ½ day since they did not have an inclinic visit or phone-call follow-up after randomization.) The rate ratio was also analyzed with adjustment for age category and site. The number of treatment days with oral, inhaled, or intravenous antibiotics was compared using a linear regression model of the log of treatment days for participants with greater than 0 treatment days, and estimates were transformed back to the original scale. The probability of remaining free of a pulmonary exacerbation was estimated by the Kaplan-Meier method and the hazard ratio for first pulmonary exacerbation with a proportional hazards regression model. The difference in mean change (week 48 – randomization) in height, weight, respiratory rate, oxygen saturation, and CFQ-R respiratory domain score was estimated by a linear regression model with and without adjustment for age category, site, and baseline measure. The proportion of parental report of daytime cough at week 48 was estimated by a linear regression model with and without adjustment for age category and site. Mixed effects analysis was also used to model repeated measurements of height percentile, weight percentile, respiratory rate and oxygen saturation from all visits. Among infant pulmonary function substudy participants, the differences between groups in mean change in lung function indices were evaluated using linear regression with adjustment for baseline lung function, height, weight, age and gender. Differences in proportions were evaluated by a normal approximation to the binomial distribution. A two-sided significance level of p<0.05 was used without adjustment for multiple comparisons.
Analyses were conducted using R 2.13.0 (www.r-project.org) at the University of Washington Collaborative Health Studies Coordinating Center, Seattle, WA, USA (LB and RK).
RESULTS
Participants Flow and Baseline Characteristics
A total of 321 participants were randomized between April 2009 and October 2010 at 30 sites, 158 to the hypertonic saline group and 163 to the isotonic saline group (Figure 1); these individuals comprised the intent-to-treat population. Fifteen participants (9%) withdrew from the hypertonic saline group and 14 (7%) from the isotonic saline group. Mean duration of study participation was 47 (95% CI, 45, 48) weeks in the hypertonic saline group and 46 (45, 48) weeks in the isotonic saline group. The baseline characteristics of participants were similar in the 2 groups (Table 1). About 60% were <30 months of age at enrollment.
Figure 1.
Trial profile
Table 1.
Baseline Characteristics of Participants by Treatment Group
| N (%) or mean (SD) | ||
|---|---|---|
| Hypertonic saline (N=158) | Isotonic Saline (N = 163) | |
| Age, yrs | 2.2 (1.4) | 2.3 (1.5) |
| Age category | ||
| <30 mo | 95 (60.1%) | 96 (58.9%) |
| ≥30 mo | 63 (39.9%) | 67 (41.1%) |
| Male | 84 (53%) | 92 (56%) |
| CFTR genotype | ||
| N available | 153 | 158 |
| Homozygous DeltaF508 | 82 (53.6%) | 88 (55.7%) |
| Compound heterozygote DeltaF508 | 34 (22.2%) | 36 (22.8%) |
| Other | 37 (24.2%) | 34 (21.5%) |
| Race/Ethnicity | ||
| Non-Hispanic Caucasian | 149 (94.3%) | 153 (93.9%) |
| Hispanic | 6 (3.8%) | 7 (4.3%) |
| Other | 3 (1.9%) | 3 (1.8%) |
| Sweat chloride, mEq/L1 | 95.2 (18.0) | 94.7 (18.9) |
| Weight, kg | 12.2 (4.1) | 12.5 (4.1) |
| Weight percentile | 39.7 (28.1) | 43.0 (29.1) |
| Height, cm | 84.8 (14.8) | 85.7 (15.0) |
| Height percentile | 36.9 (27.0) | 39.9 (28.1) |
| Positive newborn screen2 | 101 (75%) | 92 (68%) |
| Chronic medication use | ||
| Dornase alfa | 61 (39%) | 65 (40%) |
| Albuterol/Levalbuterol | 115 (73%) | 120 (74%) |
| Positive respiratory culture3 | ||
| Pseudomonas aeruginosa | 60 (38.0%) | 69 (42.3%) |
| S. aureus | 98 (62.0%) | 124 (76.1%) |
| MRSA | 5 (3.2%) | 11 (6.8%) |
| S. maltophilia | 25 (15.8%) | 35 (21.5%) |
| A. xylosoxidans | 4 (2.5%) | 3 (1.8%) |
| B. Cepacia | 0 (0%) | 0 (0%) |
| Resting respiratory rate, bpm | 31 (8.8) | 30 (9.2) |
| Oximetry, % | 98 (1.4) | 98 (1.5) |
| N (%) with a parent-reported daytime cough4 | 27 (17.1%) | 24 (14.7%) |
| CFQ-R respiratory domain score5 | 86.9 (13.9) | 87.7 (12.3) |
| Consented to infant pulmonary function substudy | 36 | 37 |
| FRC6 (ml) | 198 (50) | 216 (59) |
| FEV0.5 7(ml) | 276 (68) | 282 (66) |
| FEF75 7(ml/sec) | 303 (132) | 284 (105) |
| FEF25–75 7(ml/sec) | 592 (212) | 578 (172) |
| RV/TLC 8 | 0.3 (0.1) | 0.3 (0.1) |
Data available from 125 participants in hypertonic saline group and 126 in isotonic saline group
Data available from 135 participants in hypertonic saline group and 135 in isotonic saline group
P. aeruginosa isolated from respiratory culture at or at any time prior to randomization. For other organisms, positive culture at or within 24 months prior to randomization.
As per reference15
Scores range from 0 to 100, with a higher score indicating milder symptoms. Data available from 156 participants in hypertonic saline group and 157 participants in isotonic saline group
FRC, functional residual capacity; data available from 36 participants in hypertonic saline group and 37 in isotonic saline group
FEV0.5, forced expiratory volume in 0.5 seconds; FEF75, forced expiratory flow at 75% of vital capacity; FEF25–75, mean forced expiratory flow between the 25th and 75th percent of vital capacity; data available from 29 participants in hypertonic saline group and 32 in the isotonic saline group
RV/TLC, ratio of residual volume to total lung capacity; data available from 27 participants in hypertonic saline group and 29 in isotonic saline group
Pulmonary exacerbations and secondary efficacy endpoints
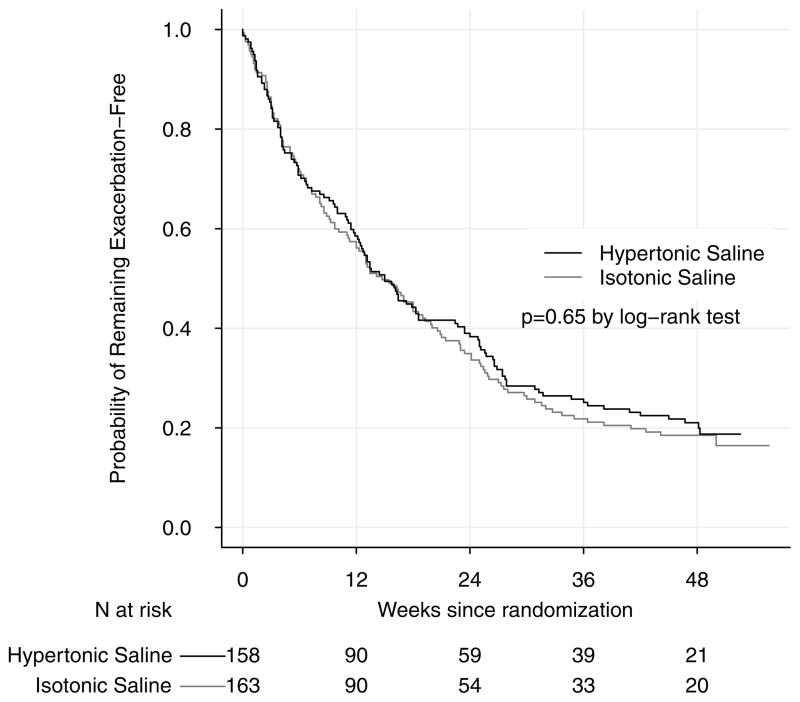
The pulmonary exacerbation rate was 2.3 (95% CI, 2.0, 2.5) per person-year among participants randomized to hypertonic saline and 2.3 (95% CI, 2.1, 2.6) per person-year among participants randomized to isotonic saline. The ratio of the mean pulmonary exacerbation rate in the hypertonic saline group compared to the isotonic saline group was 0.97 (95% CI, 0.83, 1.13) (Table 2). A Kaplan-Meier plot of time to first pulmonary exacerbation for both groups is displayed in Figure 2. The hazard ratio for time to first pulmonary exacerbation in the hypertonic saline group compared to the isotonic saline group was 0.95 (95% CI, 0.74, 1.21) (Table 2). Among participants with pulmonary exacerbations, the mean number of total antibiotic treatment days for pulmonary exacerbations was 60 (95% CI 49, 70) in the hypertonic saline group and 52 (95% CI 43, 61) in the isotonic saline group; the median (25th–75th percentiles) was 41 (24, 71) for the hypertonic saline group and 35 (21, 56) for the isotonic saline group. The ratio of mean total number of antibiotic treatment days for a pulmonary exacerbation in the hypertonic saline group compared to the isotonic saline group was 1.13 (95% CI 0.91, 1.40).
Table 2.
Comparison of pulmonary exacerbation rates and related endpoints
| Hypertonic saline / isotonic saline ratio (95% CI) | ||||
|---|---|---|---|---|
| Hypertonic saline | Isotonic saline | Unadjusted | Adjusted1 | |
| Pulmonary exacerbations rate2, events/person-year (95% CI). 124 participants randomized to hypertonic saline had 321 pulmonary exacerbations during 142 person-years of follow-up; 129 participants randomized to isotonic saline had 338 pulmonary exacerbations during 145 person-years of follow-up. | 2.3 (2.0, 2.5) | 2.3 (2.1, 2.6) | 0.97 (0.83,1.1) | 0.98 (0.84, 1.15) |
| Total number of treatment days for a pulmonary exacerbation3, mean (95% CI) | 60 (49, 70) | 52 (63, 71) | 1.13 (0.91,1.40) | 1.11 (0.89,1.37) |
| Hazard ratio for first pulmonary exacerbation, hypertonic saline/isotonic saline (95% CI) | 0.94 (0.74, 1.21) | 0.94 (0.73, 1.22) | ||
Adjusted for age category and site.
Includes log of observation time as an offset in the Poisson regression model
Among participants with pulmonary exacerbations
Figure 2.
Kaplan-Meier Plot of Time to First Exacerbation by Treatment Group
Of the 659 total number of pulmonary exacerbations, 636 (96.6%) were treated with oral, 50 (7.6%) with inhaled and 45 (6.8%) by intravenous antibiotics (not mutually exclusive; eTable 1). There was no difference between groups in the rates of pulmonary exacerbations treated by oral, inhaled or intravenous antibiotics as separate categories, or in the number of courses of oral, inhaled or intravenous antibiotics administered for any indication (eTable 1). Similarly, the rates of pulmonary exacerbations were similar in the hypertonic saline and isotonic saline groups among participants <30 months of age and ≥30 months of age at enrollment (eTable 1).
Significant differences were not detected between groups in weight, height, respiratory rate, room air oxygen saturation during the study, or in the CFQ-R respiratory domain score or parent report of daytime cough at the final study visit (Table 3, eTable 2).
Table 3.
Summary of Secondary Endpoints
| Hypertonic Saline | Isotonic Saline | Difference1 (95% CI) | ||||
|---|---|---|---|---|---|---|
| N2 | Mean or % (95% CI) | N2 | Mean or % (95% CI) | Unadjusted | Adjusted | |
| Change3 in weight percentile | 143 | 5.7 (3.1,8.2) | 149 | 3.2 (0.7, 5.7) | 2.5 (−1.1, 6.1) | 1.6 (−1.9, 5.0) |
| Change in height percentile | 143 | 8.2 (5.2, 11.2) | 149 | 4.5 (2.0, 7.0) | 3.7 (−0.2, 7.5) | 2.3 (−1.4, 6.0) |
| Change in resting respiratory rate, bpm | 143 | −3.8 (−5.1, −2.4) | 146 | −3.6 (−5.2, −2.1) | −0.2 (−2.2, 1.9) | 0.6 (−0.5, 1.8) |
| Change in room air oximetry, % | 143 | −0.1 (−0.4, 0.2) | 148 | 0.1 (−0.2, 0.3) | −0.2 (−0.6, 0.2) | −0.1 (−0.4, 0.2) |
| Change in CFQ-R respiratory score | 133 | 0.5 (−2.1,3.1) | 139 | −3.2 (−6.3, 0) | 3.7 (−0.5, 7.8) | 3.3 (0.0, 6.7) |
| % with parent report of cough at Week 48 | 143 | 18 (12, 25) | 149 | 25 (18, 32) | −7 (−16, 3) | −6 (−15, 4) |
Difference in mean change or proportion, estimated from linear regression model with and without adjustment for age category, site, and measure at randomization (cough is adjusted for age category and site only)
N: number of participants with measurements
Change: measurement at week 48 – measurement at randomization
Seventy three participants consented to participate in the infant pulmonary function substudy. The baseline lung function measures of the substudy participants were similar in the hypertonic saline and isotonic saline groups (Table 1). Acceptable measurements at the enrollment and final study visit were obtained in 62 (85%) participants for functional residual capacity, 45 (62%) for raised volume forced expiratory flows and volumes, and 36 (49%) for residual volume. No significant differences between the hypertonic saline and isotonic saline groups were detected in the raw change from baseline to Week 48 in any of the pulmonary function measures (Table 4). After adjustment for baseline lung function, gender, age, height and weight, the mean change in FEV0.5 was 38 ml greater (95% CI 1, 76) in the hypertonic saline group compared to the isotonic saline group (Table 4).
Table 4.
Summary of Infant Pulmonary Function Measures
| Hypertonic Saline | Isotonic Saline | ||||
|---|---|---|---|---|---|
| Measure1 | N2 | Mean or % (95% CI) | N2 | Mean or % (95% CI) | Difference3 (95% CI) |
| Change4 in FEV0.5 (ml) | 22 | 162 (132, 193) | 23 | 121 (91, 152) | 38 (1, 76) |
| Change in FEF75 (ml/sec) | 22 | 163 (105, 220) | 23 | 111 (46, 175) | 46 (−29, 121) |
| Change in FEF25–75 (ml/sec) | 22 | 269 (191, 348) | 23 | 186 (91, 281) | 99 (−7, 204) |
| Change in FRC (ml) | 35 | 106 (90, 122) | 27 | 105 (79, 131) | −2 (−30, 26) |
| Change in RV/TLC (%) | 18 | 0.7 (−3.4, 4.9) | 18 | 1.5 (−2.7, 5.6) | −1.1 (−4.8, 2.6) |
For FEV0.5, FEF75 and FEF25–75, a positive treatment effect would manifest as a larger change; for FRC and RV/TLC, a positive treatment effect would manifest as a smaller change.
N: number of participants with measurements
Difference in mean change between hypertonic saline and isotonic saline groups, estimated from linear regression model with adjustment for baseline lung function, gender, age, height and weight
Change: measurement at week 48 – measurement at randomization
Adherence
Mean adherence to study medications was 75.2% (95% CI, 72.2, 78.2) based on returned study drug vials among 311 participants. Based on the weekly parent questionnaire (available from 309 participants), mean adherence to twice daily dosing was 91% (95% CI, 89, 93), and to at least once daily dosing was 96% (95% CI, 95, 98). Based on the quarterly treatment adherence questionnaire (available from 312 participants), mean adherence to twice daily dosing was 88% (95% CI 85, 90), to using at least 6 days per week was 86% (95% CI 83, 89), and to nebulizing 10 or more minutes per treatment was 89% (95% CI 86, 92). Adherence was similar between the 2 groups (eTable 3).
Safety
Of the participants who received the test dose of 7% hypertonic saline at enrollment, 2 were found to be intolerant and were not randomized. Serious adverse events are shown in Table 5. In the hypertonic saline group, there were 56 serious adverse events among 33 participants. In the isotonic saline group, there were 74 events among 43 participants. No significant differences between groups in the proportion of participants with serious adverse events of each category were detected. The most common serious adverse event in both groups was cough or increased cough, occurring in 8% of participants in the hypertonic saline group and 10% of participants in the isotonic saline group.
Table 5.
Serious Adverse Events by Treatment Group
| Adverse Event Category | Hypertonic Saline (N=158) | Isotonic Saline (N=163) | ||||
|---|---|---|---|---|---|---|
| Participants | % | Events | Participants | % | Events | |
| All | 33 | 21% | 56 | 43 | 26% | 74 |
| Abdominal distension | 2 | 1% | 2 | 1 | 1% | 1 |
| Abdominal pain or stomach ache | 3 | 2% | 3 | 4 | 2% | 4 |
| Burkholderia cepacia | 2 | 1% | 2 | 2 | 1% | 2 |
| C. difficile colitis | 2 | 1% | 3 | 0 | 0% | 0 |
| Constipation | 1 | 1% | 1 | 2 | 1% | 2 |
| Cough or Increased cough | 13 | 8% | 16 | 16 | 10% | 19 |
| Decreased appetite | 0 | 0% | 0 | 2 | 1% | 2 |
| Diarrhea | 0 | 0% | 0 | 1 | 1% | 1 |
| Distal intestinal obstructive syndrome | 1 | 1% | 1 | 2 | 1% | 2 |
| Fever | 1 | 1% | 2 | 4 | 2% | 4 |
| Nasal congestion or stuffy nose | 2 | 1% | 2 | 3 | 2% | 3 |
| Poor growth and/or need for gastrostomy | 5 | 3% | 6 | 5 | 3% | 5 |
| Pseudomonas aeruginosa eradication | 4 | 3% | 4 | 2 | 1% | 2 |
| Pulmonary congestion or chest congestion | 0 | 0% | 0 | 2 | 1% | 2 |
| Rectal prolapse | 0 | 0% | 0 | 1 | 1% | 2 |
| RSV | 1 | 1% | 1 | 1 | 1% | 1 |
| Vomiting or emesis | 3 | 2% | 5 | 6 | 4% | 6 |
| Wheezing | 4 | 3% | 4 | 2 | 1% | 2 |
| Other | 4 | 3% | 4 | 11 | 7% | 13 |
% is the number of participants with event divided by the number in each group multiplied by 100
A significant difference between groups was not detected in the proportion of adverse events of moderate or severe severity occurring in >10% of participants in either group (eTable 4). The proportion of participants with new isolation of bacteria from respiratory cultures during the study period is shown in eTable 5; statistically significant differences between groups were not detected.
DISCUSSION
This is to our knowledge the first clinical trial assessing a chronic non-antimicrobial pulmonary therapy in children with CF < 6 years of age. Hypertonic saline did not reduce the rate of pulmonary exacerbations in these young children. In addition, hypertonic saline did not demonstrate any significant effects on secondary endpoints including weight, height, respiratory rate, oxygen saturation, antibiotic use, or parent report of respiratory signs and symptoms.
Previous studies in older children and adults with CF have documented benefits of inhaled hypertonic saline 7–9, 21, 22. In the multi-center Australian study in patients >6 years of age, treatment with hypertonic saline did not demonstrate a significant effect on the primary outcome measure, the rate of change of lung function, but was associated with a significant reduction in the rate of pulmonary exacerbations9. Pulmonary exacerbation rate was chosen as the primary outcome in the current trial because of the important effect observed in the Australian hypertonic saline trial9 and because pulmonary exacerbations are a clinical endpoint (affecting how a person feels, functions or survives)23 that have been associated with survival in CF24, 25. The pulmonary exacerbation definition in the current study differed from that in the Australian study, in which pulmonary exacerbations were defined as treatment with intravenous antibiotics for pre-defined signs and symptoms, or the occurrence of those signs and symptoms independent of treatment. Our definition, similar to that used in 2 prior studies in young CF patients26, 27, was designed to capture all events in which several days of new respiratory signs or symptoms triggered treatment with oral, inhaled or intravenous antibiotics, the standard clinical practice for CF patients in this age range. In the current study, while the vast majority of pulmonary exacerbations were treated with oral antibiotics, there was no difference between the 2 groups in the rate of exacerbations even if limited to those treated with intravenous antibiotics, nor in respiratory symptoms. Thus, it is unlikely that the difference in our results is due to a different pulmonary exacerbation definition.
As opposed to older patients, pulmonary exacerbations in infants and young children are frequently triggered by viral infections. It is thus possible that hypertonic saline has less ability to prevent exacerbations in children <6 years of age than in older CF patients. Previous studies have demonstrated that viral infections occur at similar rates in CF and non CF infants, but that the severity and duration of symptoms is increased in CF patients28. Thus hypertonic saline, even if not affecting the rate of pulmonary exacerbations in young children with CF, might reduce the severity and duration of symptoms, similar to its observed effect in non-CF infants with bronchiolitis29. However, the current study provides no evidence that the severity or duration of pulmonary exacerbations was influenced by hypertonic saline, as parent-reported respiratory signs and symptoms and days of antibiotic therapy did not differ between groups.
We estimated the expected pulmonary exacerbation rate based on data from an ongoing U.S. observational study of early CF lung disease, the EPIC Observational Study30. The rate of pulmonary exacerbations in the current study (mean 2.3 events per person-year) was very similar to that observed in the EPIC Observational Study (2.22 per person-year), indicating that the trial was adequately designed to observe the predefined treatment effect. In addition, the participants in the current study had baseline characteristics (Table 1) similar to the overall patient population in the U.S. CF National Patient Registry in this age range, suggesting that our findings are generalizable to the overall CF population <6 years of age.
This study was designed to primarily demonstrate an effect on clinically meaningful events rather than on prevention of lung disease progression. Our choice of endpoints was limited by the fact that validated outcome measures commonly used in older patients are lacking for very young children with CF. It could be argued that an intervention targeting mucociliary clearance in a population with limited clinical lung disease is unlikely to improve any short-term clinical outcome measure and that a more realistic goal would be to slow progression of structural airway damage or improve lung function. We conducted a substudy of infant pulmonary function tests as an exploratory endpoint at selected sites in order to gain information to adequately power future studies using this endpoint. Interestingly, the mean change in FEV0.5 over the treatment period was significantly greater in the hypertonic saline group compared to the isotonic saline group. While these findings may be due to chance, they also may reflect improvement in airflow limitation in the hypertonic saline group that was not detectable with our primary or secondary outcome measures. Due to the relatively silent nature of early CF lung disease, sensitive endpoints are critical.
When the current study was being planned, protocols for chest CT and multiple breath washout for multiple age ranges were not adequately developed and multi-center experience in infants and young children with these techniques was limited. The availability of appropriate multi-center protocols and networks as well as increased expertise in these techniques suggests that adequately powered trials using physiologic measures as outcomes may be conducted. Future studies of hypertonic saline in young children utilizing these or other endpoints will allow evaluation of the effects of this treatment on early structural airway damage and lung function, including ventilation inhomogeneity.
In both the current study and the Australian hypertonic saline trial, isotonic saline served as the control agent. It is possible that isotonic saline has a more pronounced effect on mucus hydration in the very young than in older patients. In addition, participants in both arms received albuterol prior to each dose of study drug. Both of these factors might have limited our ability to detect a difference in outcomes between the 2 groups. The fact that the exacerbation rate in the control group was very similar to that in an untreated historical cohort would suggest that there was not an important effect of isotonic saline on the primary endpoint. Unfortunately, it is not feasible to perform a true placebo controlled study of hypertonic saline as no inhaled agent is completely inert.
Treatment with hypertonic saline was well tolerated and adherence to therapy was overall high. Chronic inhaled therapy could pose a risk of new acquisition of bacterial pathogens if nebulizers are not properly cleaned and disinfected31. As this study did not include an untreated control group, this potential side effect of inhalation therapy cannot be excluded. However, the rate of new acquisition of organisms did not differ significantly from that reported in the CF Registry or in the EPIC Observational Study32. Therefore, while not showing a decrease in pulmonary exacerbation rate, this study supports previous smaller series demonstrating that inhalation of hypertonic saline is safe in infants and young children.
In conclusion, among infants and children with CF less than 6 years old, the use of inhaled hypertonic saline compared with isotonic saline did not reduce the rate of pulmonary exacerbations over 48 weeks of treatment. Further study with physiologic endpoints is warranted to better understand how this drug may slow progression of structural airway damage or improve lung function in the youngest population.
Supplementary Material
Acknowledgments
Funding/support: This study was jointly funded by NIH (U01 HL 092931, UL1RR025014) and CF Foundation Therapeutics, Inc (ISIS07K1), the subsidiary of the not-for-profit Cystic Fibrosis Foundation that sponsors research grants and contracts.. Study drug and placebo were supplied by Pari Respiratory and Catalent, respectively, which were not otherwise involved in any aspect of the study.
Role of the sponsors: NIH and the NIH-appointed Data Safety Monitoring Board oversaw study conduct and reviewed and approved the manuscript. CFFT, Pari and Catalent did not participate in the design or conduct of the study; the collection, management or interpretation of the data; nor the preparation, review, or approval of the manuscript.
Footnotes
Author contributions: Drs. Kronmal and Brumback had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Rosenfeld, Ratjen, Brumback, Kronmal, Davis
Analysis and interpretation of data: Rosenfeld, Ratjen, Brumback, Kronmal, Davis
Acquisition of data: all authors
Analysis and interpretation of data: Rosenfeld, Ratjen, Brumback, Stephens, Johnson, Kronmal, Davis
Drafting of the manuscript: Rosenfeld, Ratjen, Brumback, Kronmal, Davis
Critical revision of the manuscript for important intellectual content: Rosenfeld, Ratjen, Brumback, Kronmal, Davis
Statistical analysis: Brumback, Kronmal
Obtained funding: Rosenfeld, Ratjen, Brumback, Kronmal, Davis
Administrative, technical or material support: Rowbotham, Daniel, McNamara, Johnson
Study supervision: all authors
Financial disclosures: Dr. Rosenfeld reports receipt of funding (institutional) from CF Foundation Therapeutics, Inc and the National Institutes of Health and having served as a consultant for Vertex Pharmaceuticals and on the North American Scientific Advisory Group of the Epidemiologic Study of CF sponsored by Genentech. Dr. Ratjen receipt of funding (institutional) from CF Foundation Therapeutics, Inc, the National Institutes of Health, CF Canada, Canadian Institute for Child Health and Inspire, Inc, and having served as consultant for Inspire, Inc, Vertex Pharmaceuticals, Novartis, Bayer, Talecris, CSL Behring, Roche and Gilead. He has also served on a speakers board for Genentech and had meeting expenses paid by Pari. Mr. Rowbotham reports no financial disclosures. Ms. McNamara reports no financial disclosures. Ms. Johnson serves as a consultant for nSpire Health. Dr. Brumback, Dr. Daniel, and Dr. Kronmal report receipt of funding (institutional) from NHLBI and CFFT. Dr. Davis reports receipt of funding (institutional) from CF Foundation Therapeutics, Inc and the National Institutes of Health and having served as a consultant or on the advisory board for Vertex Pharmaceuticals, Novartis and Inspire, Inc.
References
- 1.Ranganathan SC, Stocks J, Dezateux C, et al. The evolution of airway function in early childhood following clinical diagnosis of cystic fibrosis. Am J Respir Crit Care Med. 2004;169:928–33. doi: 10.1164/rccm.200309-1344OC. [DOI] [PubMed] [Google Scholar]
- 2.Davis SD, Fordham LA, Brody AS, et al. Computed tomography reflects lower airway inflammation and tracks changes in early cystic fibrosis. Am J Respir Crit Care Med. 2007;175:943–50. doi: 10.1164/rccm.200603-343OC. [DOI] [PubMed] [Google Scholar]
- 3.Mott LS, Park J, Murray CP, et al. Progression of early structural lung disease in young children with cystic fibrosis assessed using CT. Thorax. 2011 doi: 10.1136/thoraxjnl-2011-200912. [DOI] [PubMed] [Google Scholar]
- 4.Davis SD, Rosenfeld M, Kerby GS, et al. Multicenter Evaluation of Infant Lung Function Tests as Cystic Fibrosis Clinical Trial Endpoints. Am J Respir Crit Care Med. 2010;182:1387–97. doi: 10.1164/rccm.200908-1236OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boucher RC. Evidence for airway surface dehydration as the initiating event in CF airway disease. J Intern Med. 2007;261:5–16. doi: 10.1111/j.1365-2796.2006.01744.x. [DOI] [PubMed] [Google Scholar]
- 6.Livraghi A, Randell SH. Cystic fibrosis and other respiratory diseases of impaired mucus clearance. Toxicol Pathol. 2007;35:116–29. doi: 10.1080/01926230601060025. [DOI] [PubMed] [Google Scholar]
- 7.Donaldson S, Bennett W, Zeman K, Knowles M, Tarran R, Boucher R. Mucus Clearance and Lung Function in Cystic Fibrosis with Hypertonic Saline. N Engl J Med. 2006;354:241–50. doi: 10.1056/NEJMoa043891. [DOI] [PubMed] [Google Scholar]
- 8.Wark P, McDonald VM. Nebulised hypertonic saline for cystic fibrosis. Cochrane Database Syst Rev. 2009:CD001506. doi: 10.1002/14651858.CD001506.pub3. [DOI] [PubMed] [Google Scholar]
- 9.Elkins MR, Robinson M, Rose BR, et al. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354:229–40. doi: 10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]
- 10.Rosenfeld M, Davis S, Brumback L, et al. Inhaled hypertonic saline in infants and toddlers with cystic fibrosis: short-term tolerability, adherence, and safety. Pediatr Pulmonol. 2011;46:666–71. doi: 10.1002/ppul.21425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dellon EP, Donaldson SH, Johnson R, Davis SD. Safety and tolerability of inhaled hypertonic saline in young children with cystic fibrosis. Pediatr Pulmonol. 2008;43:1100–6. doi: 10.1002/ppul.20909. [DOI] [PubMed] [Google Scholar]
- 12.Subbarao P, Balkovec S, Solomon M, Ratjen F. Pilot study of safety and tolerability of inhaled hypertonic saline in infants with cystic fibrosis. Pediatr Pulmonol. 2007;42:471–6. doi: 10.1002/ppul.20603. [DOI] [PubMed] [Google Scholar]
- 13.Cystic Fibrosis Foundation. National Patient Registry 2010 Annual Data Report. Bethesda, Maryland: 2011. [Google Scholar]
- 14.Quittner AL, Sweeny S, Watrous M, et al. Translation and linguistic validation of a disease-specific quality of life measure for cystic fibrosis. J Pediatr Psychol. 2000;25:403–14. doi: 10.1093/jpepsy/25.6.403. [DOI] [PubMed] [Google Scholar]
- 15.West SE, Zeng L, Lee BL, et al. Respiratory infections with Pseudomonas aeruginosa in children with cystic fibrosis: early detection by serology and assessment of risk factors. JAMA. 2002;287:2958–67. doi: 10.1001/jama.287.22.2958. [DOI] [PubMed] [Google Scholar]
- 16.Stocks J, Godfrey S, Beardsmore C, Bar-Yishay E, Castile R. Plethysmographic measurements of lung volume and airway resistance. ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. European Respiratory Society/ American Thoracic Society. Eur Respir J. 2001;17:302–12. doi: 10.1183/09031936.01.17203020. [DOI] [PubMed] [Google Scholar]
- 17.Castile R, Filbrun D, Flucke R, Franklin W, McCoy K. Adult-type pulmonary function tests in infants without respiratory disease. Pediatr Pulmonol. 2000;30:215–27. doi: 10.1002/1099-0496(200009)30:3<215::aid-ppul6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 18.ATS/ERS statement: raised volume forced expirations in infants: guidelines for current practice. Am J Respir Crit Care Med. 2005;172:1463–71. doi: 10.1164/rccm.200408-1141ST. [DOI] [PubMed] [Google Scholar]
- 19.Stocks J, Godfrey S, Beardsmore C, Bar-Yishay E, Castile R ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. European Respiratory Society/American Thoracic Society. Plethysmographic measurements of lung volume and airway resistance. ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. European Respiratory Society/ American Thoracic Society. Eur Respir J. 2001;17:302–12. doi: 10.1183/09031936.01.17203020. [DOI] [PubMed] [Google Scholar]
- 20.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–56. [PubMed] [Google Scholar]
- 21.Eng PA, Morton J, Douglass JA, Riedler J, Wilson J, Robertson CF. Short-term efficacy of ultrasonically nebulized hypertonic saline in cystic fibrosis. Pediatr Pulmonol. 1996;21:77–83. doi: 10.1002/(SICI)1099-0496(199602)21:2<77::AID-PPUL3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 22.Dmello D, Nayak RP, Matuschak GM. Stratified assessment of the role of inhaled hypertonic saline in reducing cystic fibrosis pulmonary exacerbations: a retrospective analysis. BMJ Open. 2011;1:e000019. doi: 10.1136/bmjopen-2010-000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayer-Hamblett N, Ramsey BW, Kronmal RA. Advancing outcome measures for the new era of drug development in cystic fibrosis. Proc Am Thorac Soc. 2007;4:370–7. doi: 10.1513/pats.200703-040BR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayer-Hamblett N, Rosenfeld M, Emerson J, Goss CH, Aitken ML. Developing cystic fibrosis lung transplant referral criteria using predictors of 2-year mortality. Am J Respir Crit Care Med. 2002;166:1550–5. doi: 10.1164/rccm.200202-087OC. [DOI] [PubMed] [Google Scholar]
- 25.Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol. 2001;153:345–52. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saiman L, Anstead M, Mayer-Hamblett N, et al. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2010;303:1707–15. doi: 10.1001/jama.2010.563. [DOI] [PubMed] [Google Scholar]
- 27.Treggiari MM, Retsch-Bogart G, Mayer-Hamblett N, et al. Comparative efficacy and safety of 4 randomized regimens to treat early Pseudomonas aeruginosa infection in children with cystic fibrosis. Arch Pediatr Adolesc Med. 2011;165:847–56. doi: 10.1001/archpediatrics.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Ewijk BE, van der Zalm MM, Wolfs TF, van der Ent CK. Viral respiratory infections in cystic fibrosis. J Cyst Fibros. 2005;4 (Suppl 2):31–6. doi: 10.1016/j.jcf.2005.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Mendoza-Sassi RA, Wainwright C, Klassen TP. Nebulized hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database Syst Rev. 2008:CD006458. doi: 10.1002/14651858.CD006458.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Treggiari MM, Rosenfeld M, Mayer-Hamblett N, et al. Early anti-pseudomonal acquisition in young patients with cystic fibrosis: rationale and design of the EPIC clinical trial and observational study’. Contemp Clin Trials. 2009;30:256–68. doi: 10.1016/j.cct.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saiman L, Siegel J. Infection control recommendations for patients with cystic fibrosis: microbiology, important pathogens, and infection control practices to prevent patient-to-patient transmission. Infect Control Hosp Epidemiol. 2003;24:S6–52. doi: 10.1086/503485. [DOI] [PubMed] [Google Scholar]
- 32.Rosenfeld M, Emerson J, McNamara S, et al. Baseline characteristics and factors associated with nutritional and pulmonary status at enrollment in the cystic fibrosis EPIC observational cohort. Pediatr Pulmonol. 2010;45:934–44. doi: 10.1002/ppul.21279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.