Sub-clinical gut inflammation is present in two-thirds of adult and pediatric spondyloarthritis (SpA) patients (1, 2) and predicts a chronic course of arthritis (2, 3). Thus, there may be value in evaluating the gut in SpA patients. However, commonly used tests, such as colonoscopy, barium studies and computed tomography, are limited by expense, invasiveness, or radiation exposure (4), prompting a need for non-invasive surrogate markers. Serologic markers revealed a large number of false positive tests (5).
Fecal calprotectin is a sensitive and specific marker for the presence of IBD or other intestinal illnesses (6). This test has not heretofore been used to assess for sub-clinical gut inflammation in arthritis patients. In this study, we measured fecal calprotectin levels in children with ERA, comparing them to children with non-SpA subtypes of JIA, as well as children with unrelated connective tissue diseases (CTD) and non-inflammatory control subjects.
We enrolled four groups of children: (1) 9 children with the ERA subtype of JIA, (2) 17 children with other subtypes of JIA (persistent oligoarticular, n = 6; extended oligo-articular, n = 1; RF− poly-articular, n = 8; RF+ poly-articular, n = 2), (3) 9 children with unrelated CTD (dermatomyositis, n = 3; localized scleroderma, n = 5; microscopic polyangiitis, n = 1); (4) 6 non-inflammatory control subjects (e.g. hypermobility syndrome.) All of the children with JIA met the International League of Associations for Rheumatology criteria (7). All of the children with ERA met the criteria on the basis of a history of arthritis in an HLA-B27+ male with onset after the sixth birthday; additional SpA features include clinically diagnosed enthesitis in two, sacroiliitis in three, family history in two, and acute anterior uveitis in one. We excluded children with acute onset (< 30 days) of gastrointestinal (GI) symptoms as well as those with prior abnormal studies of the GI tract. We defined GI symptoms as being one or more of a history of chronic abdominal pain that interfered with activity, hematochezia, persistent diarrhea, or poor growth (8). Active arthritis was defined by having at least one joint with swelling or limitation accompanied by joint pain or tenderness (7). For children with unrelated CTD, disease activity was assessed clinically by the attending physician. Each enrolled child submitted a stool specimen for measurement of calprotectin; the specimen was provided either on the day of the visit, was hand-delivered to the hospital, or was shipped overnight to the hospital by a commercial carrier. This study was approved by the Institutional Review Board at UT Southwestern Medical Center. Informed consent was obtained from each subject’s legal guardian, and assent was obtained in children age 10 or older.
Once received by the clinical laboratory, stool specimens were aliquoted into transport media provided by a commercial laboratory (ARUP, Salt Lake City, UT) and were stored at −25°C and shipped frozen overnight to ARUP for measurement of calprotectin, which was performed via ELISA. Calprotectin is stable at room temperature for up to seven days (9).
Calprotectin was measured on a continuous scale. Comparisons between the groups were made with the Kruskal-Wallis test. The values were also dichotomized to below or greater than (or equal) to 121 micrograms/gm, the cutoff suggested by ARUP. Comparisons of the dichotomized values were performed via the Chi squared test.
The patient population is shown in Table 1. GI symptoms were reported in two patients with ERA (abdominal pain and loose stools in one; abdominal pain and weight loss in another) and in one patient with polyarticular JIA (chronic poor weight gain.)
Table 1.
Study subjects.
| Characteristic | Non-inflammatory controls | CTD controls | JIA controls | ERA | p-value |
|---|---|---|---|---|---|
| n | 6 | 9 | 17 | 9 | N/A |
| Demographics | |||||
| Age (years)† | 3.8 (3.0 – 9.6) | 11 (8.0 – 14) | 6.2 (2.8 – 11) | 12 (9.5 – 14) | 0.014 |
| % Female | 67 | 56 | 82 | 0 | 0.001 |
| Race/ethnicity (%) | 0.070 | ||||
| Latino | 17 | 56 | 12 | 0 | |
| Non-Latino White | 67 | 33 | 82 | 67 | |
| African-American | 17 | 0 | 6 | 22 | |
| Other | 0 | 11 | 0 | 11 | |
| Treatment (%) | |||||
| NSAIDs | 33 | 0 | 65 | 56 | 0.013 |
| Systemic CS | 0 | 33 | 0 | 11 | 0.042 |
| Methotrexate | 0 | 78 | 65 | 56 | 0.019 |
| Etanercept | 0 | 0 | 5.9 | 33 | 0.055 |
| Other DMARD* | 0 | 33 | 0 | 0 | 0.009 |
| Active disease | 0 | 67 | 100 | 78 | < 0.001 |
| GI symptoms (%) | 0 | 0 | 5.9 | 22 | 0.242 |
| Fecal calprotectin (mcg/gm)† | 80 (28 – 120) | 26 (0 – 38) | 51 (36 – 98) | 171 (34 – 280) | 0.034 |
| Fecal calprotectin > 120 mcg/gm (%) | 17 | 11 | 18 | 67 | 0.024 |
Continuous data is presented as medians (intraquartile range).
Other DMARD: one patient was taking mycophenolate; another was taking both mycophenolate and hydroxychloroquine, and a third was taking 6-mercaptopurine.
Abbreviations: CS = corticosteroids, DMARD = disease-modifying anti-rheumatic drug.
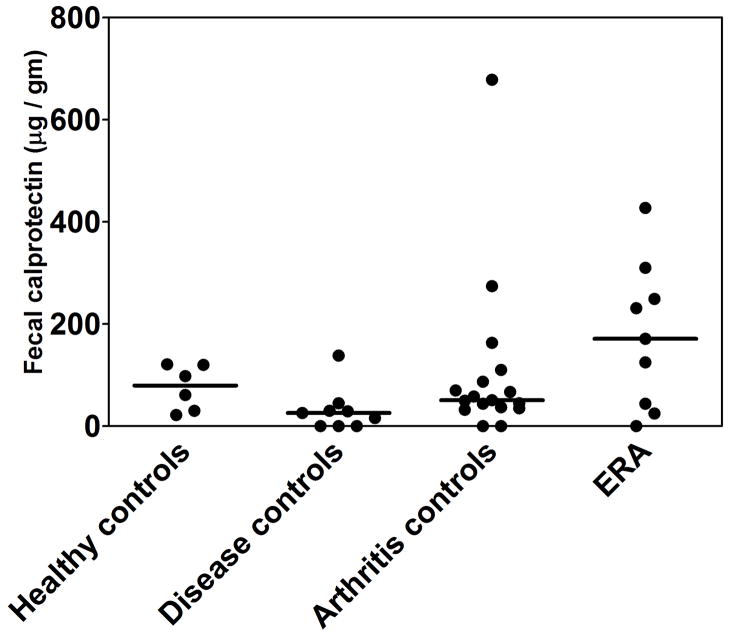
Fecal calprotectin levels are shown in Table 1 and Figure 1. Median levels were highest in the children with ERA, and lowest in the CTD controls (p = 0.034) Among patients with ERA, NSAID use was not associated with an elevated fecal calprotectin level (125 vs 201, p = 1.000). Of the three patients who reported GI symptoms, two of them (one with ERA, and one with poly-articular JIA) had normal calprotectin levels, while one ERA patient had a calprotectin level of 310. We dichotomized values to less than or at least equal to 121 micrograms/gm, the manufacturer’s suggested cutoff. The majority of ERA patients but a minority of patients in each of the other groups had abnormal levels (Table 1; p = 0.024).
Fig 1.
Calprotectin levels in children with ERA, arthritis controls (oligo-articular and poly-articular JIA)., non-inflammatory controls, and disease controls (unrelated CTD).
The primary implication of this study is that a safe and non-invasive test can be used to evaluate for sub-clinical gut inflammation in arthritis patients. In addition, we confirm prior studies showing evidence of gut inflammation in children with SpA (2).
This study has several limitations. Our sample size is relatively small, and reflected patients who were heterogeneous with respect to treatment. Several of the JIA patients were receiving NSAIDs, which could potentially have influenced the fecal calprotectin levels (10), albeit no clear association was observed in this study. Additionally, although fecal calprotectin is stable for up to seven days at room temperature (9), several of the specimens were shipped to us over the summer, where temperatures in Texas exceeded room temperature. The possibility for selection bias exists, in that patients with GI symptoms may have been more likely to participate in the study. However, no obvious relationship was observed between GI symptoms and calprotectin levels. All of the subjects with ERA were male, introducing a potential confounding factor. However, levels were similar between male and female patients in each of the other groups (data not shown), suggesting that gender is not likely to be an independent predictive factor. Finally, fecal calprotectin levels were only obtained at a single point in time.
In summary, we show for the first time that stool biomarkers may be used to evaluate for intestinal inflammation in children with JIA. Future studies should be performed using validated gold standard assessments to confirm the significance of elevated fecal calprotectin levels in this population, and also towards defining the place of routine assessments of fecal inflammation in children and adults with SpA.
Acknowledgments
Grant support: Dr. Stoll was supported by Grant Number UL1RR024982, titled, “North and Central Texas Clinical and Translational Science Initiative” (Milton Packer, M.D., PI) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research.
The authors wish to thank the patients and families for participating in this study.
References
- 1.Mielants H, Veys EM, Goemaere S, Goethals K, Cuvelier C, De Vos M. Gut inflammation in the spondyloarthropathies: clinical, radiologic, biologic and genetic features in relation to the type of histology. A prospective study. J Rheumatol. 1991;18(10):1542–51. [PubMed] [Google Scholar]
- 2.Mielants H, Veys EM, Cuvelier C, De Vos M, Goemaere S, Maertens M, et al. Gut inflammation in children with late onset pauciarticular juvenile chronic arthritis and evolution to adult spondyloarthropathy--a prospective study. J Rheumatol. 1993;20(9):1567–72. [PubMed] [Google Scholar]
- 3.Mielants H, Veys EM, Cuvelier C, De Vos M, Goemaere S, De Clercq L, et al. The evolution of spondyloarthropathies in relation to gut histology. III. Relation between gut and joint. J Rheumatol. 1995;22(12):2279–84. [PubMed] [Google Scholar]
- 4.Sinha R, Nwokolo C, Murphy PD. Magnetic resonance imaging in Crohn’s disease. BMJ. 2008;336(7638):273–6. doi: 10.1136/bmj.39456.527419.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Vries M, van der Horst-Bruinsma I, van Hoogstraten I, van Bodegraven A, von Blomberg BM, Ratnawati H, et al. pANCA, ASCA, and OmpC antibodies in patients with ankylosing spondylitis without inflammatory bowel disease. J Rheumatol. 2010;37(11):2340–4. doi: 10.3899/jrheum.100269. [DOI] [PubMed] [Google Scholar]
- 6.Costa F, Mumolo MG, Bellini M, Romano MR, Ceccarelli L, Arpe P, et al. Role of faecal calprotectin as non-invasive marker of intestinal inflammation. Dig Liver Dis. 2003;35(9):642–7. doi: 10.1016/s1590-8658(03)00381-5. [DOI] [PubMed] [Google Scholar]
- 7.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–2. [PubMed] [Google Scholar]
- 8.Wyllie R, Hyams JS, Kay M, editors. Pediatric Gastrointestinal and Liver Diseases. 3. Elsevier; 2006. [Google Scholar]
- 9.Bunn SK, Bisset WM, Main MJ, Golden BE. Fecal calprotectin as a measure of disease activity in childhood inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2001;32(2):171–7. doi: 10.1097/00005176-200102000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Tibble JA, Sigthorsson G, Foster R, Scott D, Fagerhol MK, Roseth A, et al. High prevalence of NSAID enteropathy as shown by a simple faecal test. Gut. 1999;45(3):362–6. doi: 10.1136/gut.45.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]