Abstract
Acetaminophen (APAP) hepatotoxicity because of overdose is the most frequent cause of acute liver failure in the western world. Metabolic activation of APAP and protein adduct formation, mitochondrial dysfunction, oxidant stress, peroxynitrite formation and nuclear DNA fragmentation are critical intracellular events in hepatocytes. However, the early cell necrosis causes the release of a number of mediators such as high-mobility group box 1 protein, DNA fragments, heat shock proteins (HSPs) and others (collectively named damage-associated molecular patterns), which can be recognized by toll-like receptors on macrophages, and leads to their activation with cytokine and chemokine formation. Although pro-inflammatory mediators recruit inflammatory cells (neutrophils, monocytes) into the liver, neither the infiltrating cells nor the activated resident macrophages cause any direct cytotoxicity. In contrast, pro- and anti-inflammatory cytokines and chemokines can directly promote intracellular injury mechanisms by inducing nitric oxide synthase or inhibit cell death mechanisms by the expression of acute-phase proteins (HSPs, heme oxygenase-1) and promote hepatocyte proliferation. In addition, the newly recruited macrophages (M2) and potentially neutrophils are involved in the removal of necrotic cell debris in preparation for tissue repair and resolution of the inflammatory response. Thus, as discussed in detail in this review, the preponderance of experimental evidence suggests that the extensive sterile inflammatory response during APAP hepatotoxicity is predominantly beneficial by limiting the formation and the impact of pro-inflammatory mediators and by promoting tissue repair.
Keywords: acetaminophen, hepatotoxicity, inflammation
Acetaminophen (APAP) overdose is currently the most frequent cause of acute liver failure in USA, the UK and many other countries (1). APAP tablets are often used in suicide attempts. However, there are an increasing number of cases of unintentional overdosing because of the consumption of multiple-drug preparations containing APAP. APAP hepatotoxicity has been recognized since the 1960s (2) and, shortly thereafter, a mouse model of APAP-induced liver injury was introduced (3–5). Early mechanistic insight into the pathophysiology of APAP hepatotoxicity obtained with this model included the formation of a reactive metabolite, hepatic glutathione (GSH) depletion and protein binding, which correlated with liver injury (3–5). The recognition that GSH is a critical defence against the reactive metabolite led to the introduction of N-acetylcysteine (NAC) as an antidote against APAP hepatotoxicity in clinical practice (6). NAC is still used today as the only approved drug to treat APAP overdose patients (7).
Although the focus of research in understanding the mechanisms of APAP-induced liver injury was always on intracellular events in hepatocytes, there is an increasing awareness that nonparenchymal cells of the liver and infiltrating inflammatory cells may be involved in the pathogenesis (8, 9). However, in contrast to the widely accepted contribution of inflammatory cells in the pathophysiology of hepatic ischaemia–reperfusion injury, obstructive cholestasis and endotoxaemia (10–12), the contribution of inflammation in the mechanism of APAP-induced liver injury is highly controversial (13). The objective of the current review was to critically evaluate recent findings on the role of sterile inflammation and innate immune cells in APAP-induced liver injury and repair and to try to reconcile many of these observations into a coherent hypothesis.
Intracellular mechanisms of acetaminophen-induced hepatocyte cell death
A fundamental principle of sterile inflammation is the requirement for necrotic cell death (14). Thus, it needs to be kept in mind that a substantial initial cell death is necessary to trigger an inflammatory response that may modulate the early injury.
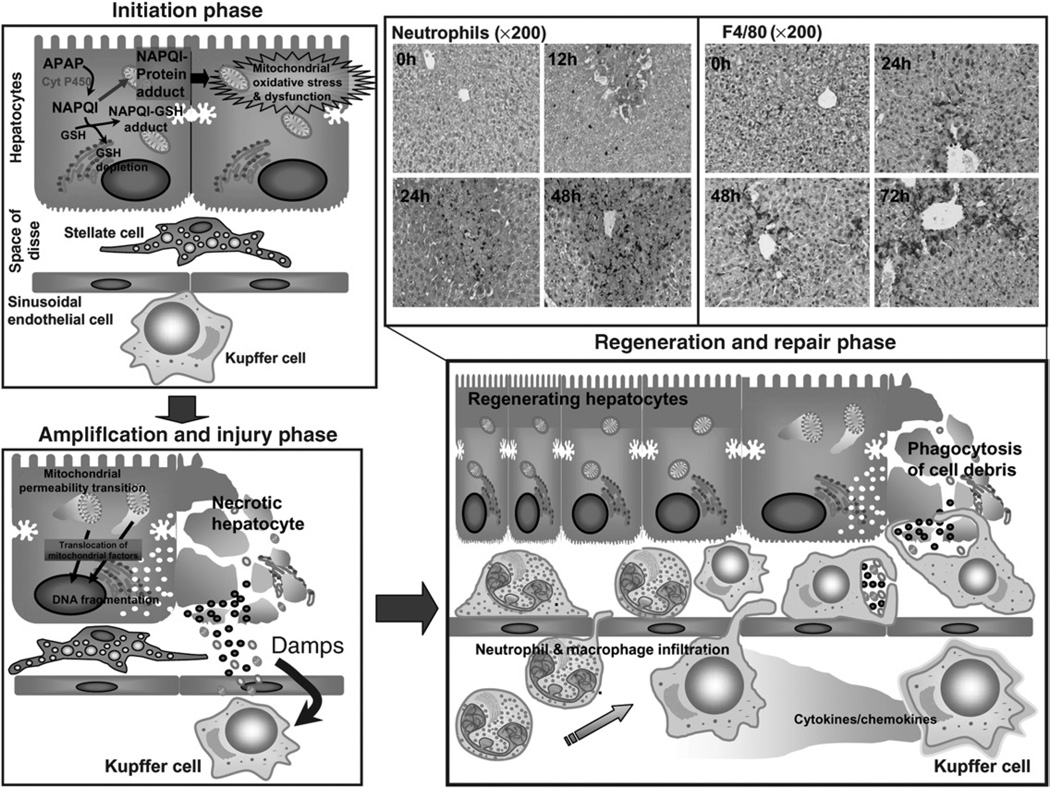
Hepatocyte cell death: initiation phase (Fig. 1)
Fig. 1.
Phases of acetaminophen (APAP)-induced hepatotoxicity. APAP overdose results in metabolic activation of the drug to the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI), which depletes glutathione and forms APAP protein adducts triggering the initiation of the injury process with mitochondrial oxidative and nitrosative stress and compromised respiratory function. In the subsequent amplification phase, these mitochondrial events result in activation of mediators such as c-jun-N-terminal kinase (JNK), followed by initiation of the mitochondrial permeability transition with resultant translocation of mitochondrial proteins such as apoptosis-inducing factor and endonuclease G to the nucleus, producing DNA fragmentation and necrotic cell death. A number of cellular components released during necrosis including nuclear DNA fragments, formyl peptides and HMGB1 can act as damage-associated molecular patterns (DAMPs) to activate resident liver macrophages (Kupffer cells). In the regeneration and repair phase, chemokine and cytokine secretion because of Kupffer cell activation results in homing and transmigration of neutrophils and macrophages into the damaged tissue to facilitate removal of dead cells and activate regenerative pathways.
Mechanistic work by Mitchell and coworkers (3–5) established the fact that APAP-induced liver injury depends on the metabolism of a small fraction of the overall dose of APAP (generally < 10%) through the P450 system (Cyp). This reaction, which is catalysed mainly by CYP2E1, yields the formation of a reactive metabolite, N-acetyl-p-benzoquinone imine (NAPQI), which reacts either spontaneously, or via catalysis by glutathione-S-transferases, with GSH (15). If the formation of NAPQI exceeds the capacity of GSH to eliminate this metabolite, NAPQI can react with protein sulphhydryl groups, which leads to the formation of protein adducts (4, 5). During the early days, it was assumed that covalent binding of APAP to proteins was the direct cause of cell death (3–5). Although cell death correlates with early protein binding (16), it was obvious that the total amount of adducts is limited and may be insufficient to cause cell death. Therefore, it was hypothesized that NAPQI may selectively affect vital proteins within the cell. During the next decade, a substantial number of adducted proteins were identified (17, 18). However, none of the proteins proved to be absolutely vital such that a moderate loss of enzyme activity could explain the rapid cell death. Work with the nonhepatotoxic regioisomer of APAP, 3′-hydroxyacetanilide, suggested that not the overall amount of protein binding was critical for cell death but the capability to bind to mitochondrial proteins (19, 20). The parallel recognition that APAP overdose can inhibit mitochondrial respiration (21, 22) and causes a selective mitochondrial oxidant stress (23) led to the concept that early protein binding is the initiator of toxicity, which requires amplification and propagation in order to cause cell death (24, 25). The basis of this concept is that any intervention that may affect the early metabolic activation of APAP will modulate all subsequent events including the inflammatory response. In other words, it is critical to evaluate these early events for any pharmacological (drug, chemical, antibody, siRNA, etc.) or any genetic (gene knock-out or transgenic mouse) intervention applied to this model.
Hepatocyte cell death: amplification and injury phase (Fig. 1)
Central to amplifying the initial stress of protein adduct formation is the mitochondria. Although some of the mechanistic details are still unknown, it is well established that the mitochondrial translocation of bax is a very early event (25–27). Bax together with Bak forms pores in the outer mitochondrial membrane that leads to the release of intermembrane proteins including cytochrome c, the second mitochondrial activator of caspases (smac), endonuclease G and apoptosis-inducing factor (AIF) (27). Endonuclease G and AIF translocate to the nucleus (28) and contribute to the characteristic nuclear DNA fragmentation and cell death (27). Independent of bax, mitochondrial protein binding triggers an inhibition of the mitochondrial respiration, which causes a selective oxidant stress and peroxynitrite formation in the mitochondria (29, 30). The oxidant stress and peroxynitrite do not cause relevant lipid peroxidation in vivo (31) but are responsible for mitochondrial DNA damage (30) and the opening of the mitochondrial membrane permeability transition pore (MPT) (32–34), which triggers the collapse of the membrane potential and cessation of ATP formation. The resulting mitochondrial swelling leads to the rupture of the outer membrane with the release of intermembrane proteins and subsequent nuclear DNA fragmentation (27). The selective scavenging of mitochondrial peroxynitrite by accelerating the recovery of mitochondrial GSH levels documented the critical role of peroxynitrite in the pathophysiology (35, 36). In addition, the supply of large doses of NAC and GSH supports mitochondrial function by providing substrates for ATP synthesis (37). Together, the emerging evidence is very strong that dysfunction of the mitochondria and the resulting energy crisis and nuclear DNA damage are key events in causing oncotic necrotic cell death (38).
Sterile inflammation: cytokine formation and activation of the inflammasome
The formation of cytokines such as tumour necrosis factor (TNF)-α, interleukin (IL)-1β and others has been well described after APAP overdose (39–42) but the initiating mechanisms emerged only recently. Damage-associated molecular patterns (DAMPs) are molecules released from dying cells that are ligands for toll-like receptors (TLRs) on macrophages and other cell types (43, 44). DAMPs, identified to be released during APAP hepatotoxicity, include high-mobility group box 1 protein (HMGB1), heat shock proteins (HSPs), DNA fragments (Fig. 1) and others (14, 45–47). A hypo-acetylated form of HMGB1 can be passively released by necrotic cells (47) and a hyperacetylated form of HMGB1 is secreted by activated macrophages and indicates an inflammatory response (48). Both hyper- and hypo-acetylated forms of HMGB1 are found in the plasma after an APAP overdose (47). However, it appears that HMGB1 alone is less effective as a pro-inflammatory mediator; combinations of HMGB1 with other DAMPS such as DNA fragments are the most potent inflammagens (49). Although these DAMPs are clearly released into the plasma during APAP-induced liver injury, the impact on injury mechanisms is controversial. Antibodies against HMGB1 have been shown to reduce hepatic neutrophil accumulation without effect on injury (14). However, other authors reported a minor reduction in liver injury (50) or drastically reduced liver injury in the presence of HMGB1 antibodies (51). A caveat of comparing these experimental results is that there were differences in the strains and age of mice used, the nutritional status of the mice (fed vs fasted) and the source of the neutralizing antibody. In particular, the use of 1-day-old mouse pups in one study showing no protection with HMGB1 antibodies (14) raises concerns about the relevance of these findings. HMGB1 antibodies attenuated cytokine and chemokine [TNF-α, monocyte chemo-attractant protein (MCP)-1, IL-6] formation (50, 51) supporting the hypothesis that HMGB1 is an important mediator of the inflammatory response after APAP overdose. Mice deficient in TLR4, a receptor for HMGB1, showed a moderate reduction in an APAP-induced injury (52) but mice deficient in TNF-α (53) were not protected. In contrast, TNF receptor 1-deficient mice had exaggerated liver injury, which correlated with accelerated iNOS induction and peroxynitrite formation (54).
DNA fragmentation is a characteristic feature of APAP-induced cell death (Fig. 2) (30, 55, 56). DNA fragments released during APAP-induced necrosis (45, 57) can be recognized by TLR9 (58). The pathophysiological relevance of TLR9 has been implicated by the protective effect of TLR9 antagonists and in TLR9-deficient mice (58). TLR9 stimulation can activate cytokine formation through the activation of nuclear factor-κB (NF-κB) (58). It was hypothesized that IL-1α and IL-1β are critical mediators of APAP hepatotoxicity based on the observation that IL-1 receptor-deficient (IL-1R1−/−) mice are completely protected (59) and the reports that mice deficient in components of the Nalp3 inflammasome show substantially reduced injury (58). This protein complex consists of Nalp3 (NACHT, LRR and pyrin domain-containing protein 3), ASC (apoptosis-associated speck-like protein containing a CARD) and caspase-1 (60). Based on the reduced injury in Nalp3−/−, ASC−/− and caspase-1−/− mice, it was concluded that processing of pro-IL-1β and pro-IL-18 to the active cytokines is critical for APAP-induced liver injury (58). Although these are interesting ideas, there are numerous concerns. Firstly, IL-1R1−/− mice are not protected against APAP-induced liver injury (61), which questions the relevance of IL-1α or IL-1β in the pathophysiology. Secondly, the amount of IL-1β produced after APAP overdose is very small and not enough to activate neutrophils (61). In fact, even a massive overdose of IL-1β administered after APAP recruits more neutrophils into the liver but does not enhance APAP-induced liver injury (61) strongly arguing against a role of IL-1β in APAP hepatotoxicity. Thirdly, although a caspase inhibitor effectively prevented mature IL-1β formation, the inhibitor affected neither hepatic neutrophil recruitment nor liver injury (61). Fourth, in our hands, Nalp3−/−, ASC−/− and caspase-1−/− mice were not protected against APAP-induced liver injury (62). Fifth, the lack of a cytoplasmic death domain in the IL-1R makes it impossible for IL-1α or IL-1β to directly induce cell death (63). Thus, a cytotoxic effect of these cytokines would depend on inflammatory cell activation (64). However, as discussed later, there is strong evidence against any direct involvement of neutrophils or macrophages in cell killing during APAP hepatotoxicity.
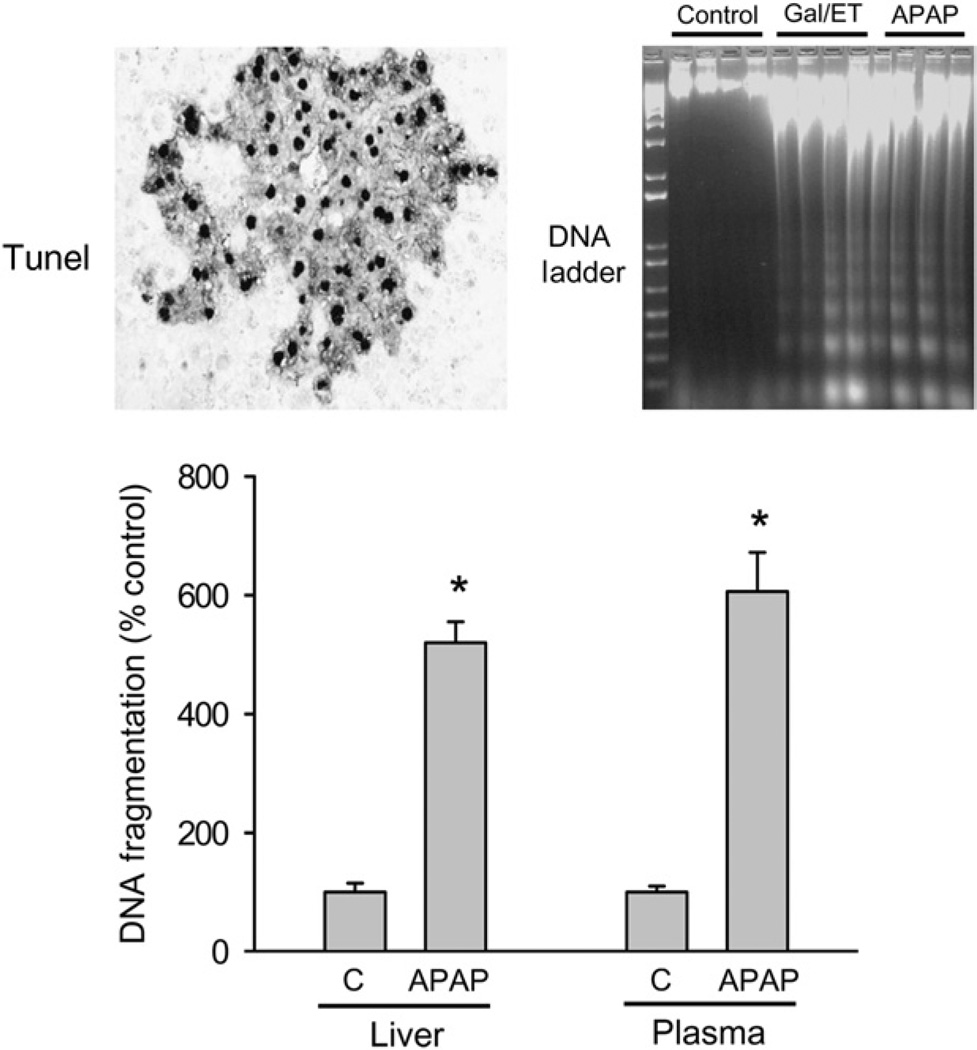
Fig. 2.
Nuclear DNA damage and release of DNA fragments into plasma. DNA damage was assessed after 300 mg/kg acetaminophen (APAP) in mice using the TUNEL assay (indicates DNA strand breaks), DNA fragments in an agarose gel (DNA Ladder) (represents mono- and polyoligonucleosomes generated by endonucleases) and the DNA fragmentation assay (measures oligonucleosomes in cytosol or plasma based on detection of histone proteins). The data indicate early DNA strandbreaks (TUNEL assay at 3 h) and nuclear DNA degradation at 6 h (DNA ladder, DNA fragmentation assay). DNA ladder caused by APAP is indistinguishable from apoptosis-mediated DNA fragmentation after galactosamine/LPS. At 6 h after APAP treatment, a substantial amount of DNA fragments is released into the plasma. Data adapted from Cover et al. (30) (reproduced with permission).
Taken together, there is established release of various DAMPs after APAP overdose triggering the formation of a number of cytokines and chemokines and initiating the recruitment of neutrophils and monocytes into the liver. However, there is no convincing evidence to suggest that the pro-inflammatory mediator formation results in direct cell death (apoptosis) (38) or a neutrophil- or a macrophage-mediated injury (13). In contrast, inflammatory cytokines are able to modulate intracellular events within hepatocytes, thereby altering toxicity.
Role of Kupffer cells in acetaminophen hepatotoxicity
The resident macrophages of the liver (Kupffer cells) are activated within 1–2 h after an APAP overdose in mice as indicated by the formation of cytokines (39–42, 46, 65). In this respect, it is important to recognize that the first report on macrophage activation after APAP used a rat model (66). In this case, the resident Kupffer cells and mononuclear cells accumulating in the centrilobular area after 24 h were isolated. Pretreatment with gadolinium chloride (GdCl3), which selectively reduces the capacity of Kupffer cells to generate reactive oxygen (67), attenuated APAP-induced liver injury in rats after 24 h (68). Again, the main effect in this rat model of APAP-induced liver injury appears to be on infiltrating macrophages, and not on Kupffer cells. However, these studies were never followed up with more detailed mechanistic investigations and it was never ruled out that the beneficial effect of GdCl3 was secondary to some protective effect on Kupffer cell activation that modulated the hepatocellular injury. In general, the rat model is not used much because even a massive APAP overdose produces only a relatively mild injury, which does not reflect the human overdose situation.
A few years after the first study in the rat, a similar investigation using GdCl3 was repeated in the mouse (69). In this report, GdCl3 completely eliminated the oxidant stress, peroxynitrite formation and liver injury during the first 8 h after APAP (69). In this case, GdCl3 clearly acted on Kupffer cells as infiltrating monocytes are not detectable before 12–24 h in the mouse (70). However, the main conclusions of this manuscript, i.e. that Kupffer cell-derived oxidants are responsible for the centrilobular injury (69), are highly questionable. Firstly, the most active Kupffer cells in terms of reactive oxygen formation are located in the periportal area as part of the liver’s vital host defence function (71, 72). Thus, it is unlikely that oxidants formed in the periportal area cause selective damage to centrilobular hepatocytes. Secondly, mice with a genetic deficiency of a functional nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, the enzyme by which phagocytes such as Kupffer cells produce reactive oxygen, do not show reduced oxidant stress or peroxynitrite formation and liver injury after an APAP overdose (73). Likewise, inhibitors of NADPH oxidase do not protect against APAP hepatotoxicity (74). Thus, there is no credible experimental evidence to support the conclusion that Kupffer cells are involved in the pathophysiology of APAP-induced liver injury by directly causing cell injury through reactive oxygen and peroxynitrite formation. In support of these findings, several subsequent studies using GdCl3 found either very little or even no protection (65, 75, 76). Even the sinusoidal endothelial cell injury and haemorrhage that occurs early after APAP overdose in certain mouse strains appears to be related to APAP toxicity in endothelial cells rather than indirect cytotoxicity of Kupffer cells (29, 40, 65, 75, 77). However, the most important finding related to Kupffer cells was reported by Ju et al. (76). These investigators clearly demonstrated that the elimination of Kupffer cells by clodronate liposomes actually increased APAP toxicity. The most likely explanation is related to the elimination of the formation of IL-10, IL-6 and other cytokines and of cyclo-oxygenase products (76). IL-10 was shown to protect against APAP toxicity by downregulation of iNOS expression and peroxynitrite formation (78). In addition, cyclo-oxygenase products induce HSPs (79). HSP70 (80) and HSP32 (heme oxygenase-1) (81) are induced after APAP and protect against APAP toxicity. Together, these data demonstrate that the dominant effect of Kupffer cell activation after APAP is not to cause cytotoxicity by oxidant formation but to limit toxicity by preventing excessive iNOS induction, by promoting cyto-protective gene expression and by supporting regeneration.
Role of neutrophils in acetaminophen hepatotoxicity
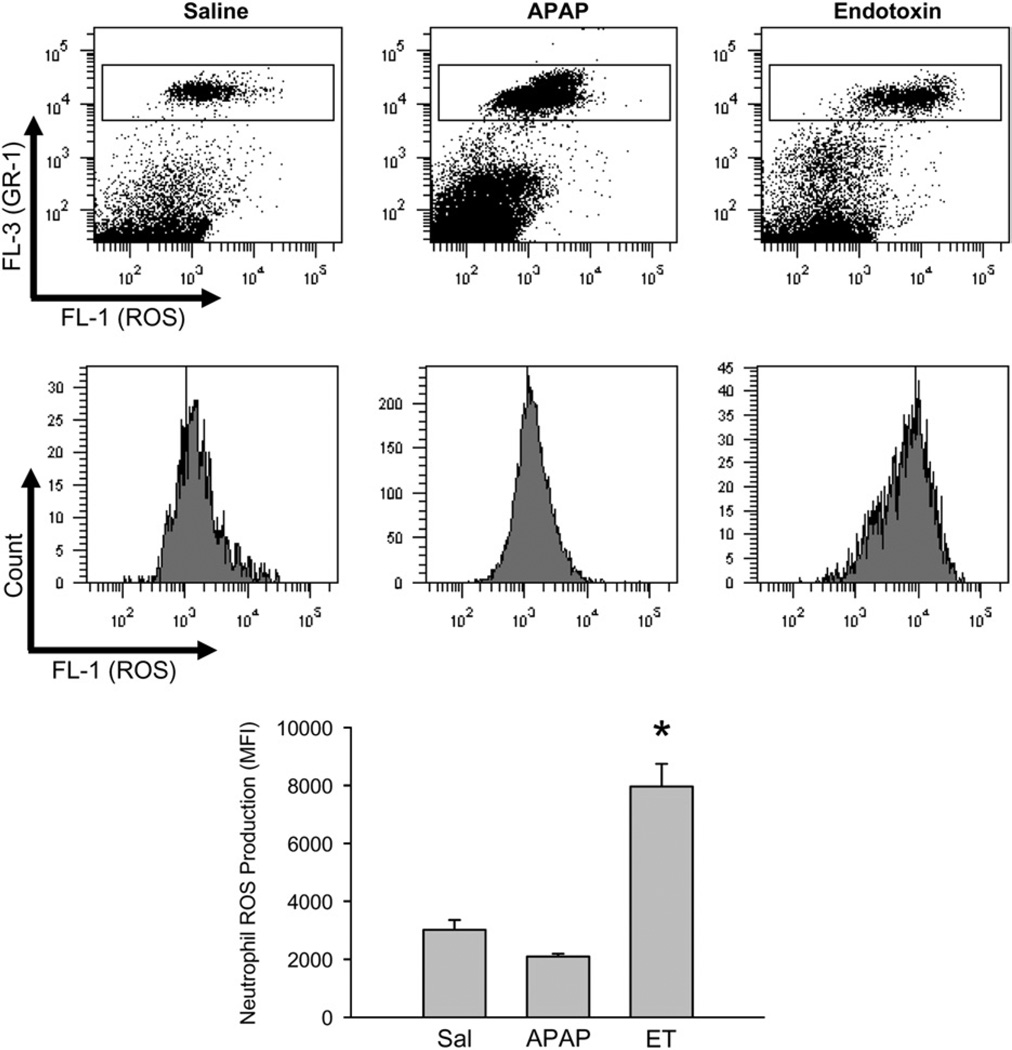
The early release of DAMPs and the formation of cytokines during the sterile inflammatory response after APAP overdose lead to the recruitment of neutrophils into the hepatic vasculature (14, 40). As has been demonstrated in a number of cases, given the appropriate chemotactic signal from the parenchyma, these leucocytes can extravasate and seriously aggravate liver injury (10–12). Despite the initial results arguing against an active role of neutrophils in APAP hepatotoxicity (40), two studies using the identical experimental approach suggested that neutrophils are responsible in part for APAP-induced liver injury (82, 83). This conclusion was mainly based on the use of a neutropenia-inducing antibody, which was injected 24 h before APAP (82, 83). However, when animals were treated with the same antibody after APAP administration, but still before the onset of injury (early necrosis), neutropenia did not protect (74). The reason for these contradictory results using the same reagent was that the pretreatment regimen caused not only neutrophil depletion but also triggered a preconditioning effect because of the accumulation of the antibody-tagged neutrophils in the liver and the attempts of Kupffer cells to remove them (84, 85). Phagocytosis of inactivated neutrophils causes Kupffer cell activation (84) and triggers a stress response in hepatocytes including the induction of inflammatory genes and a number of protective genes, e.g. metallothionein, heme oxygenase-1 and others (85). Consequently, hepatocytes from animals pretreated with this neutropenia antibody were more resistant to APAP toxicity independent of the lack of neutrophils in blood. This conclusion is further supported by the lack of protection of mice deficient in intercellular adhesion molecule (ICAM)-1 (74), CD18 (86) and phox91 (73) as well as the ineffectiveness of pharmacological inhibitors of NADPH oxidase (74). In fact, neutrophils recruited into the liver after APAP overdose are not even primed or activated (Fig. 3) (86). However, if neutrophils are activated by an injection of endotoxin or IL-1β after an APAP, there are substantially more neutrophils in the liver that are primed for reactive oxygen species (ROS) formation, but there is still no aggravation of the injury (61, 86). Together, these data strongly suggest that neutrophils do not contribute to the liver injury after an APAP overdose (Table 1); the few manuscripts that suggest a role for neutrophils have to be interpreted cautiously as off-target effects of the neutropenia interventions can explain the protection independent of neutrophils.
Fig. 3.
Priming of liver accumulated neutrophils for reactive oxygen formation. Mice were treated with saline, 100 µg endotoxin/kg for 90 min or 300 mg APAP/kg for 6 h. Hepatic nonparenchymal cells were isolated and then stimulated ex vivo with phorbol ester (PMA). Upon PMA-induced ROS production DHR-123 is converted to R-123 and quantified in neutrophils by flow cytometry. Representative ROS histograms or mean fluorescence intensities for saline, the positive control endotoxin and APAP are shown. *P < 0.05 (compared with saline control). (Figure reproduced from Williams et al. (86) with permission.)
Table 1.
Role of neutrophils in acetaminophen toxicity
| Evidence against neutrophil involvement in the injury process | Evidence for a role of neutrophils in the injury process |
|---|---|
| NADPH oxidase-deficient mice: no protection (73) | |
| NADPH oxidase inhibitors: no protection (74) | |
| ICAM-1-deficient mice: no protection (74) | |
| Post-APAP neutropenia antibody: no protection (74) | Pre-APAP (24 h) neutropenia antibody: protection but concerns regarding off-target cytoprotective effects (82, 83) |
| CD18 neutralizing antibody: no protection (40) | |
| CD18-deficient mice: no protection (86) | |
| Enhanced recruitment of activated hepatic neutrophils with LPS or IL-1β: unaltered injury (61, 86) | |
| No APAP-induced activation of blood and liver neutrophils (CD11b, ROS) (40, 61, 86) | |
| Limited presence of neutrophils in areas of injury (74, 86) |
APAP, acetaminophen; ICAM, intercellular adhesion molecule; IL, interleukin; LPS, lipopolysaccharide; NADPH, nicotinamide adenine dinucleotide phosphate; ROS, reactive oxygen species.
Role of natural killer and natural killer T cells in acetaminophen hepatotoxicity
It was reported that the depletion of natural killer (NK) and NK T cells in the liver exerted a protective effect against APAP hepatotoxicity (87). The increase in a large number of cytokines and chemokines including interferon-γ (IFN-γ) after APAP treatment (500 mg/kg) was substantially reduced in NK and NK T cell-depleted mice (87). In addition, the authors observed a downregulation of the Fas receptor and reduced neutrophil accumulation in the NK/NK T cell-depleted mice (87). Interestingly, the effect of APAP on cytokine and chemokine formation, Fas receptor expression and neutrophil infiltration could also be reproduced in IFN-γ-deficient mice (87, 88). Because IFN-γ formation was mainly attributed to NK and NK T cells, it was concluded that these cell types are responsible for IFN-γ production and the more severe injury after an APAP overdose (87). It was later determined that the presence of DMSO as a solvent in these studies activated NK and NK T cells, which does not occur without DMSO (89). Overall, it was found that DMSO used in these initial studies increased the activation of these NK and NK T cells and in particular enhanced granzyme B and IFN-γ production. The depletion of NK and NK T cells does not alter APAP-induced injury unless these cell types are pre-activated with DMSO (89). Despite the limitations, the initial study (87) did demonstrate that IFN-γ has the potential to modulate APAP-induced toxicity, even if it was induced under nonphysiological conditions. Further confirming the potential of IFN-γ to alter APAP toxicity, IFN-γ−/− mice (87, 88) and wild-type mice treated with IFN-γ-neutralizing antibody were used (88). Depending on the time after APAP overdose, IFN-γ−/− mice showed a two- to eight-fold reduction in plasma ALT activities and the neutralizing antibody reduced ALT levels approximately three-fold at 24 h (88). The reduction in injury also correlated with reduced immune cell infiltration, cytokine and chemokine formation and liver Fas receptor expression (88).
During APAP-induced sterile inflammation, various immune cells are recruited into the liver, many of which highly express the Fas ligand (FasL), and it is well established that hepatocytes express the Fas receptor. Increased circulating levels of Fas have been observed after an APAP overdose in humans (90). The Fas/FasL interaction has the potential to modulate APAP-induced injury by affecting intracellular signalling mechanisms. Although the easiest interaction between FasL and the Fas receptor would be to trigger apoptosis, there is no evidence that apoptotic cell death makes a relevant contribution to the overall liver injury after an APAP overdose (38). In addition, APAP does not induce caspase activation and caspase inhibitors do not protect (56, 61, 91). The only exception is a limited and temporary caspase activation in fed CD-1 mice; however, a caspase inhibitor did not reduce liver injury in these mice either (50). In contrast, the mitochondrial dysfunction induced by APAP in hepatocytes interrupts the critical mitochondrial signalling pathway and eliminates Fas-mediated apoptosis (56, 92). Nevertheless, several lines of evidence exist linking the Fas receptor and/or FasL to altered APAP toxicity. An in vivo study in mice showed that knocking down the Fas receptor protected against 300 mg/kg APAP overdose but the protection was lost when the dose was increased to 700 mg/kg (93). These experiments utilized antisense or scrambled oligonucleotides, which were injected once per day for 4 days before an APAP overdose (93). In this study, potential alterations in APAP metabolism and/or GSH levels were not evaluated (93). This raises the possibility that the protection may have been an indirect effect. A different study demonstrated that subliminal activation of Fas by the Fas-activating antibody, Jo-2, enhanced APAP-induced injury (94). In these experiments, the very low dose of Jo-2 itself caused no plasma ALT increase or any detectable caspase activation in the liver. If administered before APAP, however, Jo-2 caused a more than two-fold increase in APAP-induced injury (94). In this study, it was shown that the subliminal Fas activation increased iNOS induction, which then enhanced APAP-induced injury (94), linking Fas receptor activation to a critical intracellular signalling event. In a related study, it was demonstrated that mice with defective Fas receptor (lpr mice) or Fas ligand (gld mice) were partially protected from APAP-induced injury (87). Both lpr and gld mice had a four- to five-fold reduction in plasma ALT with enhanced survival (87). However, these experiments were again performed in the presence of DMSO, which might have affected the results. Thus, three independent studies demonstrated three unique ways in which Fas receptor activation can modulate APAP toxicity. Potential Fas activation mediated through infiltrating leucocytes (i.e. NK and NK T cells) alters intracellular signalling within the hepatocyte to make them more vulnerable to APAP-induced toxicity by a mechanism independent of the Fas/FasL apoptotic cell death pathway.
Bacterial and viral infections and acetaminophen toxicity
Bacterial and viral infections can modulate the susceptibility to APAP-induced liver injury. Treatment with lipopolysaccharide (LPS) has been used as a model to study the effect of bacterial infection on drug metabolism. The simultaneous treatment of cocultures of rat Kupffer cells and hepatocytes with LPS and phenobarbital resulted in a strong downregulation (85%) of the phenobarbital-induced cytochrome P450 isoform Cyp2b1 in hepatocytes, which was mediated by TNF release from Kupffer cells (95). Hepatic cytochrome P450 enzyme activities were also depressed in mice treated with LPS in vivo (96) and a number of studies have demonstrated that pretreatment of rats or mice with LPS for 24 h (0.1–4 mg/kg) resulted in a decrease of APAP metabolism and protection from injury (68, 97, 98). The suppression of P450 activities and hepatoprotection against an APAP overdose was eliminated in TLR4-defective mice, suggesting that Kupffer cell-derived cytokines were responsible for this effect in vivo (98). However, if LPS (0.1 mg/kg) or IL-1β is administered 3 h after APAP, there is an increase in neutrophil accumulation in the liver but no aggravation of injury (61, 86). Thus, even enhanced recruitment of primed neutrophils by LPS or cytokines does not affect APAP-induced injury suggesting no direct cytotoxicity by these innate immune cells. However, low levels of endogenous LPS have also been implicated in APAP-induced liver injury, with studies showing that mice either lacking LPS-binding protein (LBP) or administration of a synthetic peptide to block interaction of LPS and LBP were protective against APAP hepatotoxicity (99, 100). Together, these data suggest that Kupffer cell activation by endogenous LPS may promote cell death mechanisms by effects such as promoting iNOS induction.
In contrast, a recent study using a 2-h pretreatment with 4–5 mg/kg LPS triggered injury at 24 h after a noninjurious dose of APAP (175 mg/kg) (101). The authors concluded that bacterial-mediated inflammation renders a noninjurious dose of APAP hepatotoxic (101). However, this conclusion is highly questionable. The time course of injury in the LPS/APAP group was different compared with a toxic dose of APAP (101). The high LPS dose administered recruits primed and activated neutrophils into the liver. However, it is well known that these cells do not cause any injury without a signal for transmigration (102). In this case, the signal was generated by causing a subtoxic cellular stress with APAP, which triggered a neutrophil-mediated injury (101). This mechanism is very similar to the galactosamine/LPS model, where LPS-generated cytokines prime neutrophils and the subsequent apoptosis causes neutrophil transmigration and aggravation of liver injury (103). Thus, the combination of LPS/APAP is a typical neutrophil-mediated injury model with little relevance for APAP hepatotoxicity.
The possible effect of viral infections on drug metabolism has been recognized for decades (104, 105) and recent epidemiological data indicate that hepatitis C virus (HCV) infection predisposes patients to APAP-induced acute liver injury (106, 107). Viral infections result in elevated levels of type I IFNs (108) and the IFN inducer poly rI:rC has been used in a number of studies evaluating immune modulation of APAP toxicity. The treatment of BALB/cJ mice with poly rI:rC was found to depress the levels of total thiol adduct excretion subsequent to APAP treatment, indicating that it decreases the metabolic activation of the drug (109). Mice treated with poly rI:rC also had significantly lower mortality with doses of APAP up to 900 mg/kg, with a decrease in necrosis but without any effect on glutathione levels (110). Acute infection with a recombinant adenovirus has been shown to inhibit Cyp3a2, resulting in a depression of docetaxel metabolism (111), and a recent study using mice infected with a replication-deficient adenovirus showed decreased expression of Cyp1a2 and Cyp2e1 mRNA, accompanied by protection against subsequent APAP hepatotoxicity (112). However, activities of the major human cytochrome P450s were found to be similar in both noninfected and chimeric mice with humanized liver (PXB mice) infected with HCV (113) and mice overexpressing the HCV core protein in liver mitochondria showed no change in Cyp2e1 protein levels (A. Ramachandran and H. Jaeschke, unpublished data). It is possible that acute viral infection affects cytochrome P450 enzyme levels, which then adapt at later time points with chronic infection. It has been suggested that long-term changes in drug metabolism in response to viral infections start from the time the virus enters the circulation, are reinforced by virus binding to cellular targets and are further solidified by changes in cellular processes long after the virus is cleared (114).
Innate immunity and liver regeneration
In addition to the injury mechanisms, initiation of regeneration is critical for the repair of the damaged liver tissue and the recovery of the patient (115). Vascular endothelial growth factor, IL-6, TNF-α and other mediators have been implicated in promoting tissue regeneration after an APAP overdose (54, 116–119). Dividing hepatocytes closest to the area of necrosis are replacing the dead cells (36). However, a prerequisite of hepatocyte proliferation is the removal of necrotic cells by phagocytes. Both neutrophils and monocyte-derived macrophages are recruited into the area of necrosis (Fig. 1). The infiltrating macrophages (M2) are distinct from activated resident macrophages of the liver (M1; Kupffer cells) in terms of their cytokine profile produced (120, 121). M2 macrophages generate IL-10 and other cytokines that downregulate inflammation and promote tissue repair and have a high capacity for phagocytosis (120, 121). M2 macrophages are recruited into the liver within 12–24 h after an APAP overdose, i.e. after the peak of injury (70). The recruitment of M2 macrophages specifically into the area of necrosis occurs through the formation of MCP-1 generated by injured hepatocytes and recruited macrophages (122). Animals deficient in the C-C chemokine receptor 2 (CCR2), the receptor for MCP-1 on monocytes, experienced reduced M2 accumulation during APAP hepatotoxicity and consequently a substantial delay in tissue repair (70, 122). These data support the hypothesis that M2 macrophages are critical for the removal of necrotic cells and for tissue repair. In addition, M2 macrophages can induce apoptosis of neutrophils, which contributes to the resolution of the inflammatory response after tissue injury (70). The prevention of M2 infiltration in CCR2−/− mice led to an increase in hepatic neutrophil numbers (70). Although the role of neutrophils in tissue regeneration has not been specifically investigated, these data are not consistent with a vital importance of neutrophils in this process. Clearly, M2 macrophages appear to be the most critical phagocytes for clearing necrotic cell debris and shutting down inflammation.
Mouse strains and humans
Most of the experimental findings discussed in this review were obtained in rats and in various inbred and outbred strains of mice. It has to be considered that some differences in the results and potential mechanistic conclusions may have been caused by the different strains of mice used. Although the variations in susceptibility to an APAP overdose between various mouse strains (123) are not well understood, basal differences in gene expression including drug metabolism genes, stress response genes and differences in innate immune responses should be considered. For example, it is well known that some mouse strains, including C3He/FeJ and CD-1 mice, demonstrate significant hepatic haemorrhage after an APAP overdose, while others, such as C57Bl/6 mice, do not (29, 40, 47, 50, 77). This could possibly be explained by differences in Cyp2e1 expression in sinusoidal endothelial cells, which has been shown to correlate with the susceptibility to develop vascular injury and haemorrhage (77).
The vast majority of experiments involving APAP toxicity use animals fasted for 12–15 h. Fasting reduces the hepatic GSH content and eliminates its diurnal variation (124). Therefore, moderate APAP doses can be used to achieve more uniform toxicity. However, fasting also decimates glycogen stores and partially reduces hepatic ATP levels, which may be the reason for the complete absence of apoptotic cell death in fasted animals (50). Antoine et al. (47, 50) demonstrated recently that there was evidence for limited caspase activation and apoptotic cell death between 3 and 5 h after an APAP overdose in fed CD-1 mice. In addition, caspase activation correlated with the presence of an oxidized form of HMGB1 in plasma and a reduced inflammatory response (50). The oxidation of cysteine 106 of HMGB1 by caspases from apoptotic cells prevents binding of HMGB1 to TLRs on macrophages and eliminates cytokine formation (125, 126). Antoine et al. (50) concluded that lower ATP levels in hepatocytes of fasted animals prevents apoptosis and facilitates necrotic cell death with the release of reduced HMGB1, which triggers cytokine formation and the recruitment of inflammatory cells. Our own studies of this effect confirmed evidence of limited caspase activation in fed but not in fasted Swiss Webster mice, another outbred strain, at 3–5 h after APAP (127). However, this effect was not observed in fed or fasted C57Bl/6 (127) or C3He/FeJ mice (38). These findings suggest that the temporary and limited caspase activation may occur predominantly in fed mice of outbred strains (CD-1, Swiss Webster) and may not depend on the nutritional status (127). In addition, we observed hepatic neutrophil accumulation in both fed and fasted mice of all strains as long as liver injury was present, indicating that the temporary caspase activation in certain strains of mice did not affect induction of the inflammatory response. However, the impact of mouse strain and nutritional status on the mechanisms of APAP-induced liver injury and the resulting innate immune response may have important implications for translating the experimental results to the clinic and hence clearly require more detailed studies. In addition, it needs to be kept in mind that there are differences between the innate immune system of mice and humans (128). Thus, ultimately the mechanistic conclusions derived from these rodent studies need to be investigated in APAP overdose patients before new clinical intervention strategies can be proposed.
Summary and conclusions
The mechanism of APAP hepatotoxicity is dominated by intracellular events including the formation of a reactive metabolite, GSH depletion and protein adduct formation, which initiates a mitochondrial oxidant stress and peroxynitrite formation (Fig. 1). Ultimately, this oxidant stress and peroxynitrite are responsible for the MPT, nuclear DNA fragmentation and necrotic cell death. The subsequent release of DAMPs results in the activation of resident macrophages (Kupffer cells) with cytokine and chemokine formation and the recruitment of neutrophils and monocytes into the liver. Despite the substantial sterile inflammatory response after the initial cell death, there is no convincing experimental evidence to support the hypothesis that Kupffer cells, neutrophils or monocytes directly cause cell injury by producing cytotoxic mediators. In contrast, pro- and anti-inflammatory cytokines produced mainly by activated Kupffer cells (M1) modulate intracellular mechanisms of cell death by regulating the gene expression of iNOS, HSPs, heme oxygenase and others. In addition, monocyte-derived macrophages (M2) and potentially neutrophils are instrumental in removing necrotic cell debris and promoting hepatocyte proliferation, ultimately resulting in tissue repair and resolution of the inflammatory response. Thus, in contrast to other acute injury models, e.g. hepatic ischaemia–reperfusion injury, the innate immune response after APAP-induced liver cell injury is mainly beneficial and is well orchestrated to aid in the repair of the tissue damage.
Given the relatively solid picture that is emerging on the role of the innate immunity in APAP toxicity, why are there so many controversies in this field? A significant part of the problem is that most immunological interventions and even many pharmacological treatment strategies have a high risk for off-target effects in this model. In order to avoid misinterpretations, the entire spectrum of the pathophysiology including metabolism and disposition, intracellular signalling events and the innate immunity needs to be considered. Awareness of the numerous potential pitfalls affecting the results in this model is absolutely essential. Furthermore, it is vital that novel mechanistic discoveries be verified by several independent approaches and be critically compared with the entire existing knowledge on APAP hepatotoxicity. Only then can we make real progress in the understanding of the mechanisms of APAP-induced liver injury, which have relevance for the human pathophysiology and may eventually lead to novel treatment strategies.
Acknowledgements
Research in the authors’ laboratory is supported in part by the National Institutes of Health Grants R01 DK070195 and R01 AA12916 (to H. J.) and by grants P20 RR016475 and P20 RR021940 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health. C. D. Williams was supported by the ‘Training Program in Environmental Toxicology’ (T32 ES007079-26A2) from the National Institute of Environmental Health Sciences.
Footnotes
Conflict of interest statement: The authors declare that there are no conflicts of interest.
References
- 1.Larson AM, Polson J, Fontana RJ, et al. Acute Liver Failure Study Group. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 2.Thomson JS, Prescott LF. Liver damage and impaired glucose tolerance after paracetamol overdosage. Br Med J. 1966;2:506–507. doi: 10.1136/bmj.2.5512.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell JR, Jollow DJ, Potter WZ, et al. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther. 1973;187:185–194. [PubMed] [Google Scholar]
- 4.Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973;187:211–217. [PubMed] [Google Scholar]
- 5.Jollow DJ, Mitchell JR, Potter WZ, et al. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther. 1973;187:195–202. [PubMed] [Google Scholar]
- 6.Prescott LF, Park J, Ballantyne A, Adriaenssens P, Proudfoot AT. Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet. 1977;2:432–434. doi: 10.1016/s0140-6736(77)90612-2. [DOI] [PubMed] [Google Scholar]
- 7.Polson J, Lee WM. American Association for the Study of Liver Disease. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179–1197. doi: 10.1002/hep.20703. [DOI] [PubMed] [Google Scholar]
- 8.Jaeschke H. Role of inflammation in the mechanism of acetaminophen-induced hepatotoxicity. Expert Opin Drug Metabol Toxicol. 2005;1:389–397. doi: 10.1517/17425255.1.3.389. [DOI] [PubMed] [Google Scholar]
- 9.Liu ZX, Kaplowitz N. Role of innate immunity in acetaminophen-induced hepatotoxicity. Expert Opin Drug Metabol Toxicol. 2006;2:493–503. doi: 10.1517/17425255.2.4.493. [DOI] [PubMed] [Google Scholar]
- 10.Jaeschke H. Molecular mechanisms of hepatic ischemia–reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–G26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 11.Jaeschke H. Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia–reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1083–G1088. doi: 10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- 12.Jaeschke H, Hasegawa T. Role of neutrophils in acute inflammatory liver injury. Liver Int. 2006;26:912–919. doi: 10.1111/j.1478-3231.2006.01327.x. [DOI] [PubMed] [Google Scholar]
- 13.Jaeschke H. Innate immunity and acetaminophen-induced liver injury: why so many controversies? Hepatology. 2008;48:699–701. doi: 10.1002/hep.22556. [DOI] [PubMed] [Google Scholar]
- 14.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 15.Dahlin DC, Miwa GT, Lu AY, Nelson SD. N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci USA. 1984;81:1327–1331. doi: 10.1073/pnas.81.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts DW, Bucci TJ, Benson RW, et al. Immunohistochemical localization and quantification of the 3-(cystein-S-yl)-acetaminophen protein adduct in acetaminophen hepatotoxicity. Am J Pathol. 1991;138:359–371. [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen SD, Pumford NR, Khairallah EA, et al. Selective protein covalent binding and target organ toxicity. Toxicol Appl Pharmacol. 1997;143:1–12. doi: 10.1006/taap.1996.8074. [DOI] [PubMed] [Google Scholar]
- 18.Qiu Y, Benet LZ, Burlingame AL. Identification of the hepatic protein targets of reactive metabolites of acetaminophen in vivo in mice using two-dimensional gel electrophoresis and mass spectrometry. J Biol Chem. 1998;273:17940–17953. doi: 10.1074/jbc.273.28.17940. [DOI] [PubMed] [Google Scholar]
- 19.Qiu Y, Benet LZ, Burlingame AL. Identification of hepatic protein targets of the reactive metabolites of the non-hepatotoxic regioisomer of acetaminophen, 3′-hydroxyacetanilide, in the mouse in vivo using two-dimensional gel electrophoresis and mass spectrometry. Adv Exp Med Biol. 2001;500:663–673. doi: 10.1007/978-1-4615-0667-6_99. [DOI] [PubMed] [Google Scholar]
- 20.Tirmenstein MA, Nelson SD. Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3′-hydroxyacetanilide, in mouse liver. J Biol Chem. 1989;264:9814–9819. [PubMed] [Google Scholar]
- 21.Meyers LL, Beierschmitt WP, Khairallah EA, Cohen SD. Acetaminophen-induced inhibition of hepatic mitochondrial respiration in mice. Toxicol Appl Pharmacol. 1988;93:378–387. doi: 10.1016/0041-008x(88)90040-3. [DOI] [PubMed] [Google Scholar]
- 22.Ramsay RR, Rashed MS, Nelson SD. In vitro effects of acetaminophen metabolites and analogs on the respiration of mouse liver mitochondria. Arch Biochem Biophys. 1989;273:449–457. doi: 10.1016/0003-9861(89)90504-3. [DOI] [PubMed] [Google Scholar]
- 23.Jaeschke H. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J Pharmacol Exp Ther. 1990;255:335–341. [PubMed] [Google Scholar]
- 24.Jaeschke H, Knight TR, Bajt ML. The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol Lett. 2003;144:279–288. doi: 10.1016/s0378-4274(03)00239-x. [DOI] [PubMed] [Google Scholar]
- 25.Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- 26.Adams ML, Pierce RH, Vail ME, et al. Enhanced acetaminophen hepatotoxicity in transgenic mice overexpressing BCL-2. Mol Pharmacol. 2001;60:907–915. doi: 10.1124/mol.60.5.907. [DOI] [PubMed] [Google Scholar]
- 27.Bajt ML, Farhood A, Lemasters JJ, Jaeschke H. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2008;324:8–14. doi: 10.1124/jpet.107.129445. [DOI] [PubMed] [Google Scholar]
- 28.Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol Sci. 2006;94:217–225. doi: 10.1093/toxsci/kfl077. [DOI] [PubMed] [Google Scholar]
- 29.Knight TR, Kurtz A, Bajt ML, Hinson JA, Jaeschke H. Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stress. Toxicol Sci. 2001;62:212–220. doi: 10.1093/toxsci/62.2.212. [DOI] [PubMed] [Google Scholar]
- 30.Cover C, Mansouri A, Knight TR, et al. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2005;315:879–887. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- 31.Knight TR, Fariss MW, Farhood A, Jaeschke H. Role of lipid peroxidation as a mechanism of liver injury after acetaminophen overdose in mice. Toxicol Sci. 2003;76:229–236. doi: 10.1093/toxsci/kfg220. [DOI] [PubMed] [Google Scholar]
- 32.Masubuchi Y, Suda C, Horie T. Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J Hepatol. 2005;42:110–116. doi: 10.1016/j.jhep.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 33.Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- 34.Ramachandran A, Lebofsky M, Baines CP, Lemasters JJ, Jaeschke H. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic Res. 2011;45:156–164. doi: 10.3109/10715762.2010.520319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knight TR, Ho YS, Farhood A, Jaeschke H. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: protection by glutathione. J Pharmacol Exp Ther. 2002;303:468–475. doi: 10.1124/jpet.102.038968. [DOI] [PubMed] [Google Scholar]
- 36.Bajt ML, Knight TR, Farhood A, Jaeschke H. Scavenging peroxynitrite with glutathione promotes regeneration and enhances survival during acetaminophen-induced liver injury in mice. J Pharmacol Exp Ther. 2003;307:67–73. doi: 10.1124/jpet.103.052506. [DOI] [PubMed] [Google Scholar]
- 37.Saito C, Zwingmann C, Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology. 2010;51:246–254. doi: 10.1002/hep.23267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci. 2002;67:322–328. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- 39.Blazka ME, Wilmer JL, Holladay SD, Wilson RE, Luster MI. Role of proinflammatory cytokines in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 1995;133:43–52. doi: 10.1006/taap.1995.1125. [DOI] [PubMed] [Google Scholar]
- 40.Lawson JA, Farhood A, Hopper RD, Bajt ML, Jaeschke H. The hepatic inflammatory response after acetaminophen overdose: role of neutrophils. Toxicol Sci. 2000;54:509–516. doi: 10.1093/toxsci/54.2.509. [DOI] [PubMed] [Google Scholar]
- 41.Gardner CR, Laskin JD, Dambach DM, et al. Exaggerated hepatotoxicity of acetaminophen in mice lacking tumor necrosis factor receptor-1. Potential role of inflammatory mediators. Toxicol Appl Pharmacol. 2003;192:119–130. doi: 10.1016/s0041-008x(03)00273-4. [DOI] [PubMed] [Google Scholar]
- 42.James LP, Kurten RC, Lamps LW, McCullough S, Hinson JA. Tumour necrosis factor receptor 1 and hepatocyte regeneration in acetaminophen toxicity: a kinetic study of proliferating cell nuclear antigen and cytokine expression. Basic Clin Pharmacol Toxicol. 2005;97:8–14. doi: 10.1111/j.1742-7843.2005.pto_97102.x. [DOI] [PubMed] [Google Scholar]
- 43.Schwabe RF, Seki E, Brenner DA. Toll-like receptor signaling in the liver. Gastroenterology. 2006;130:1886–1900. doi: 10.1053/j.gastro.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 44.Jeannin P, Jaillon S, Delneste Y. Pattern recognition receptors in the immune response against dying cells. Curr Opin Immunol. 2008;20:530–537. doi: 10.1016/j.coi.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 45.Jahr S, Hentze H, Englisch S, et al. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61:1659–1665. [PubMed] [Google Scholar]
- 46.Martin-Murphy BV, Holt MP, Ju C. The role of damage associated molecular pattern molecules in acetaminophen-induced liver injury in mice. Toxicol Lett. 2010;192:387–394. doi: 10.1016/j.toxlet.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Antoine DJ, Williams DP, Kipar A, et al. High-mobility group box-1 protein and keratin-18, circulating serum proteins informative of acetaminophen-induced necrosis and apoptosis in vivo. Toxicol Sci. 2009;112:521–531. doi: 10.1093/toxsci/kfp235. [DOI] [PubMed] [Google Scholar]
- 48.Bonaldi T, Talamo F, Scaffidi P, et al. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bianchi ME. HMGB1 loves company. J Leukoc Biol. 2009;86:573–576. doi: 10.1189/jlb.1008585. [DOI] [PubMed] [Google Scholar]
- 50.Antoine DJ, Williams DP, Kipar A, Laverty H, Park BK. Diet restriction inhibits apoptosis and HMGB1 oxidation and promotes inflammatory cell recruitment during acetaminophen hepatotoxicity. Mol Med. 2010;16:479–490. doi: 10.2119/molmed.2010.00126. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yohe HC, O’Hara KA, Hunt JA, et al. Involvement of Toll-like receptor 4 in acetaminophen hepatotoxicity. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1269–G1279. doi: 10.1152/ajpgi.00239.2005. [DOI] [PubMed] [Google Scholar]
- 53.Boess F, Bopst M, Althaus R, et al. Acetaminophen hepatotoxicity in tumor necrosis factor/lymphotoxin-alpha gene knockout mice. Hepatology. 1998;27:1021–1029. doi: 10.1002/hep.510270418. [DOI] [PubMed] [Google Scholar]
- 54.Chiu H, Gardner CR, Dambach DM, et al. Role of tumor necrosis factor receptor 1 (p55) in hepatocyte proliferation during acetaminophen-induced toxicity in mice. Toxicol Appl Pharmacol. 2003;193:218–227. doi: 10.1016/j.taap.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 55.Ray SD, Sorge CL, Raucy JL, Corcoran GB. Early loss of large genomic DNA in vivo with accumulation of Ca2+ in the nucleus during acetaminophen-induced liver injury. Toxicol Appl Pharmacol. 1990;106:346–351. doi: 10.1016/0041-008x(90)90254-r. [DOI] [PubMed] [Google Scholar]
- 56.Lawson JA, Fisher MA, Simmons CA, Farhood A, Jaeschke H. Inhibition of Fas receptor (CD95)-induced hepatic caspase activation and apoptosis by acetaminophen in mice. Toxicol Appl Pharmacol. 1999;156:179–186. doi: 10.1006/taap.1999.8635. [DOI] [PubMed] [Google Scholar]
- 57.McGill MR, Sharpe MR, Williams CD, Taha M, Jaeschke H. Acetaminophen hepatotoxicity in humans: mitochondrial injury and DNA fragmentation in overdose patients (abstract) Toxicol Sci. 2011;120(Suppl. 2):97. [Google Scholar]
- 58.Imaeda AB, Watanabe A, Sohail MA, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen CJ, Kono H, Golenbock D, et al. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 60.Lamkanfi M, Dixit VM. Inflammasomes: guardians of cytosolic sanctity. Immunol Rev. 2009;227:95–105. doi: 10.1111/j.1600-065X.2008.00730.x. [DOI] [PubMed] [Google Scholar]
- 61.Williams CD, Farhood A, Jaeschke H. Role of caspase-1 and interleukin-1beta in acetaminophen-induced hepatic inflammation and liver injury. Toxicol Appl Pharmacol. 2010;247:169–178. doi: 10.1016/j.taap.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams CD, Shaw PJ, Jaeschke H. Role of the NALP3 inflammasome in neutrophil activation and cell injury during acetaminophen-induced hepatotoxicity (abstract) Toxicol Sci. 2011;120(Suppl. 2):97. [Google Scholar]
- 63.Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 64.Bajt ML, Farhood A, Jaeschke H. Effects of CXC chemokines on neutrophil activation and sequestration in hepatic vasculature. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1188–G1195. doi: 10.1152/ajpgi.2001.281.5.G1188. [DOI] [PubMed] [Google Scholar]
- 65.Ito Y, Bethea NW, Abril ER, McCuskey RS. Early hepatic microvascular injury in response to acetaminophen toxicity. Microcirculation. 2003;10:391–400. doi: 10.1038/sj.mn.7800204. [DOI] [PubMed] [Google Scholar]
- 66.Laskin DL, Pilaro AM. Potential role of activated macrophages in acetaminophen hepatotoxicity. I. Isolation and characterization of activated macrophages from rat liver. Toxicol Appl Pharmacol. 1986;86:204–215. doi: 10.1016/0041-008x(86)90051-7. [DOI] [PubMed] [Google Scholar]
- 67.Liu P, McGuire GM, Fisher MA, et al. Activation of Kupffer cells and neutrophils for reactive oxygen formation is responsible for endotoxin-enhanced liver injury after hepatic ischemia. Shock. 1995;3:56–62. [PubMed] [Google Scholar]
- 68.Laskin DL, Gardner CR, Price VF, Jollow DJ. Modulation of macrophage functioning abrogates the acute hepatotoxicity of acetaminophen. Hepatology. 1995;21:1045–1050. [PubMed] [Google Scholar]
- 69.Michael SL, Pumford NR, Mayeux PR, Niesman MR, Hinson JA. Pretreatment of mice with macrophage in-activators decreases acetaminophen hepatotoxicity and the formation of reactive oxygen and nitrogen species. Hepatology. 1999;30:186–195. doi: 10.1002/hep.510300104. [DOI] [PubMed] [Google Scholar]
- 70.Holt MP, Cheng L, Ju C. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J Leukoc Biol. 2008;84:1410–1421. doi: 10.1189/jlb.0308173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bautista AP, Mészáros K, Bojta J, Spitzer JJ. Superoxide anion generation in the liver during the early stage of endotoxemia in rats. J Leukoc Biol. 1990;48:123–128. doi: 10.1002/jlb.48.2.123. [DOI] [PubMed] [Google Scholar]
- 72.Jaeschke H, Bautista AP, Spolarics Z, Spitzer JJ. Superoxide generation by Kupffer cells and priming of neutrophils during reperfusion after hepatic ischemia. Free Radic Res Commun. 1991;15:277–284. doi: 10.3109/10715769109105223. [DOI] [PubMed] [Google Scholar]
- 73.James LP, McCullough SS, Knight TR, Jaeschke H, Hinson JA. Acetaminophen toxicity in mice lacking NADPH oxidase activity: role of peroxynitrite formation and mitochondrial oxidant stress. Free Radic Res. 2003;37:1289–1297. doi: 10.1080/10715760310001617776. [DOI] [PubMed] [Google Scholar]
- 74.Cover C, Liu J, Farhood A, et al. Pathophysiological role of the acute inflammatory response during acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2006;216:98–107. doi: 10.1016/j.taap.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 75.Knight TR, Jaeschke H. Peroxynitrite formation and sinusoidal endothelial cell injury during acetaminophen-induced hepatotoxicity in mice. Comp Hepatol. 2004;3(Suppl. 1):S46. doi: 10.1186/1476-5926-2-S1-S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ju C, Reilly TP, Bourdi M, et al. Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice. Chem Res Toxicol. 2002;15:1504–1513. doi: 10.1021/tx0255976. [DOI] [PubMed] [Google Scholar]
- 77.Yin H, Cheng L, Holt M, et al. Lactoferrin protects against acetaminophen-induced liver injury in mice. Hepatology. 2010;51:1007–1016. doi: 10.1002/hep.23476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bourdi M, Masubuchi Y, Reilly TP, et al. Protection against acetaminophen-induced liver injury and lethality by interleukin 10: role of inducible nitric oxide synthase. Hepatology. 2002;35:289–298. doi: 10.1053/jhep.2002.30956. [DOI] [PubMed] [Google Scholar]
- 79.Reilly TP, Brady JN, Marchick MR, et al. A protective role for cyclooxygenase-2 in drug-induced liver injury in mice. Chem Res Toxicol. 2001;14:1620–1628. doi: 10.1021/tx0155505. [DOI] [PubMed] [Google Scholar]
- 80.Tolson JK, Dix DJ, Voellmy RW, Roberts SM. Increased hepatotoxicity of acetaminophen in Hsp70i knockout mice. Toxicol Appl Pharmacol. 2006;210:157–162. doi: 10.1016/j.taap.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 81.Chiu H, Brittingham JA, Laskin DL. Differential induction of heme oxygenase-1 in macrophages and hepatocytes during acetaminophen-induced hepatotoxicity in the rat: effects of hemin and biliverdin. Toxicol Appl Pharmacol. 2002;181:106–115. doi: 10.1006/taap.2002.9409. [DOI] [PubMed] [Google Scholar]
- 82.Liu ZX, Han D, Gunawan B, Kaplowitz N. Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology. 2006;43:1220–1230. doi: 10.1002/hep.21175. [DOI] [PubMed] [Google Scholar]
- 83.Ishida Y, Kondo T, Kimura A, et al. Opposite roles of neutrophils and macrophages in the pathogenesis of acetaminophen-induced acute liver injury. Eur J Immunol. 2006;36:1028–1038. doi: 10.1002/eji.200535261. [DOI] [PubMed] [Google Scholar]
- 84.Bautista AP, Spolarics Z, Jaeschke H, Smith CW, Spitzer JJ. Antineutrophil monoclonal antibody (1F12) alters superoxide anion release by neutrophils and Kupffer cells. J Leukoc Biol. 1994;55:328–335. doi: 10.1002/jlb.55.3.328. [DOI] [PubMed] [Google Scholar]
- 85.Jaeschke H, Liu J. Neutrophil depletion protects against murine acetaminophen hepatotoxicity: another perspective (letter) Hepatology. 2007;45:1588–1589. doi: 10.1002/hep.21549. [DOI] [PubMed] [Google Scholar]
- 86.Williams CD, Bajt ML, Farhood A, Jaeschke H. Acetaminophen-induced hepatic neutrophil accumulation and inflammatory liver injury in CD18-deficient mice. Liver Int. 2010;30:1280–1292. doi: 10.1111/j.1478-3231.2010.02284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu ZX, Govindarajan S, Kaplowitz N. Innate immune system plays a critical role in determining the progression and severity of acetaminophen hepatotoxicity. Gastroenterology. 2004;127:1760–1774. doi: 10.1053/j.gastro.2004.08.053. [DOI] [PubMed] [Google Scholar]
- 88.Ishida Y, Kondo T, Ohshima T, et al. A pivotal involvement of IFN-gamma in the pathogenesis of acetaminophen-induced acute liver injury. FASEB J. 2002;16:1227–1236. doi: 10.1096/fj.02-0046com. [DOI] [PubMed] [Google Scholar]
- 89.Masson MJ, Carpenter LD, Graf ML, Pohl LR. Pathogenic role of natural killer T and natural killer cells in acetaminophen-induced liver injury in mice is dependent on the presence of dimethyl sulfoxide. Hepatology. 2008;48:889–897. doi: 10.1002/hep.22400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tagami A, Ohnishi H, Hughes RD. Increased serum soluble Fas in patients with acute liver failure due to paracetamol overdose. Hepatogastroenterology. 2003;50:742–745. [PubMed] [Google Scholar]
- 91.Jaeschke H, Cover C, Bajt ML. Role of caspases in acetaminophen- induced liver injury. Life Sci. 2006;78:1670–1676. doi: 10.1016/j.lfs.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 92.Knight TR, Jaeschke H. Acetaminophen-induced inhibition of Fas receptor-mediated liver cell apoptosis: mitochondrial dysfunction versus glutathione depletion. Toxicol Appl Pharmacol. 2002;181:133–141. doi: 10.1006/taap.2002.9407. [DOI] [PubMed] [Google Scholar]
- 93.Zhang H, Cook J, Nickel J, et al. Reduction of liver Fas expression by an antisense oligonucleotide protects mice from fulminant hepatitis. Nat Biotechnol. 2000;18:862–867. doi: 10.1038/78475. [DOI] [PubMed] [Google Scholar]
- 94.Tinel M, Berson A, Vadrot N, et al. Subliminal Fas stimulation increases the hepatotoxicity of acetaminophen and bromobenzene in mice. Hepatology. 2004;39:655–666. doi: 10.1002/hep.20094. [DOI] [PubMed] [Google Scholar]
- 95.Milosevic N, Schawalder H, Maier P. Kupffer cell-mediated differential down-regulation of cytochrome P450 metabolism in rat hepatocytes. Eur J Pharmacol. 1999;368:75–87. doi: 10.1016/s0014-2999(98)00988-1. [DOI] [PubMed] [Google Scholar]
- 96.Bertini R, Gervasi PG, Longo V, Ghezzi P. Depression of hepatic drug metabolism in endotoxin-treated and sarcoma-bearing mice. Res Commun Chem Pathol Pharmacol. 1992;76:223–231. [PubMed] [Google Scholar]
- 97.Ishikawa M, Tanno K, Sasaki M, Takayanagi Y, Sasaki K. Antidotal effect of lipopolysaccharide against acetaminophen-induced mortality in mice. Pharmacol Toxicol. 1990;67:387–391. doi: 10.1111/j.1600-0773.1990.tb00850.x. [DOI] [PubMed] [Google Scholar]
- 98.Liu J, Sendelbach LE, Parkinson A, Klaassen CD. Endotoxin pretreatment protects against the hepatotoxicity of acetaminophen and carbon tetrachloride: role of cytochrome P450 suppression. Toxicology. 2000;147:167–176. doi: 10.1016/s0300-483x(00)00193-1. [DOI] [PubMed] [Google Scholar]
- 99.Su GL, Hoesel LM, Bayliss J, Hemmila MR, Wang SC. Lipopolysaccharide binding protein inhibitory peptide protects against acetaminophen-induced hepatotoxicity. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1319–G1325. doi: 10.1152/ajpgi.00140.2010. [DOI] [PubMed] [Google Scholar]
- 100.Su GL, Gong KQ, Fan MH, et al. Lipopolysaccharide-binding protein modulates acetaminophen-induced liver injury in mice. Hepatology. 2005;41:187–195. doi: 10.1002/hep.20533. [DOI] [PubMed] [Google Scholar]
- 101.Maddox JF, Amuzie CJ, Li M, et al. Bacterial- and viral-induced inflammation increases sensitivity to acetaminophen hepatotoxicity. J Toxicol Environ Health A. 2010;73:58–73. doi: 10.1080/15287390903249057. [DOI] [PubMed] [Google Scholar]
- 102.Chosay JG, Essani NA, Dunn CJ, Jaeschke H. Neutrophil margination and extravasation in sinusoids and venules of liver during endotoxin-induced injury. Am J Physiol. 1997;272:G1195–G1200. doi: 10.1152/ajpgi.1997.272.5.G1195. [DOI] [PubMed] [Google Scholar]
- 103.Lawson JA, Fisher MA, Simmons CA, Farhood A, Jaeschke H. Parenchymal cell apoptosis as a signal for sinusoidal sequestration and transendothelial migration of neutrophils in murine models of endotoxin and Fas-antibody-induced liver injury. Hepatology. 1998;28:761–767. doi: 10.1002/hep.510280324. [DOI] [PubMed] [Google Scholar]
- 104.Jacknowitz AI. Possible effect of viral infections on drug metabolism. JAMA. 1984;251:2084–2085. doi: 10.1001/jama.1984.03340400020013. [DOI] [PubMed] [Google Scholar]
- 105.Chang KC, Bell TD, Lauer BA, Chai H. Altered theophylline pharmacokinetics during acute respiratory viral illness. Lancet. 1978;1:1132–1133. doi: 10.1016/S0140-6736(78)90305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nguyen GC, Sam J, Thuluvath PJ. Hepatitis C is a predictor of acute liver injury among hospitalizations for acetaminophen overdose in the United States: a nationwide analysis. Hepatology. 2008;48:1336–1341. doi: 10.1002/hep.22536. [DOI] [PubMed] [Google Scholar]
- 107.Myers RP, Shaheen AA. Hepatitis C, alcohol abuse, and unintentional overdoses are risk factors for acetaminophen-related hepatotoxicity. Hepatology. 2009;49:1399–1400. doi: 10.1002/hep.22798. [DOI] [PubMed] [Google Scholar]
- 108.Pichlmair A, Reis e Sousa C. Innate recognition of viruses. Immunity. 2007;27:370–383. doi: 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 109.Dolphin CT, Caldwell J, Smith RL. Effect of poly rI:rC treatment upon the metabolism of [14C]-paracetamol in the BALB/cJ mouse. Biochem Pharmacol. 1987;36:3835–3840. doi: 10.1016/0006-2952(87)90446-1. [DOI] [PubMed] [Google Scholar]
- 110.Renton KW, Dickson G. The prevention of acetaminophen-induced hepatotoxicity by the interferon inducer poly(rI. rC) Toxicol Appl Pharmacol. 1984;72:40–45. doi: 10.1016/0041-008x(84)90247-3. [DOI] [PubMed] [Google Scholar]
- 111.Wonganan P, Zamboni WC, Strychor S, Dekker JD, Croyle MA. Drug-virus interaction: effect of administration of recombinant adenoviruses on the pharmacokinetics of docetaxel in a rat model. Cancer Gene Ther. 2009;16:405–414. doi: 10.1038/cgt.2008.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Getachew Y, James L, Lee WM, Thiele DL, Miller BC. Susceptibility to acetaminophen (APAP) toxicity unexpectedly is decreased during acute viral hepatitis in mice. Biochem Pharmacol. 2010;79:1363–1371. doi: 10.1016/j.bcp.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kikuchi R, McCown M, Olson P, et al. Effect of hepatitis C virus infection on the mRNA expression of drug transporters and cytochrome p450 enzymes in chimeric mice with humanized liver. Drug Metab Dispos. 2010;38:1954–1961. doi: 10.1124/dmd.109.031732. [DOI] [PubMed] [Google Scholar]
- 114.Croyle MA. Long-term virus-induced alterations of CYP3A-mediated drug metabolism: a look at the virology, immunology and molecular biology of a multi-faceted problem. Expert Opin Drug Metab Toxicol. 2009;5:1189–1211. doi: 10.1517/17425250903136748. [DOI] [PubMed] [Google Scholar]
- 115.Mehendale HM. Tissue repair: an important determinant of final outcome of toxicant-induced injury. Toxicol Pathol. 2005;33:41–51. doi: 10.1080/01926230590881808. [DOI] [PubMed] [Google Scholar]
- 116.Donahower B, McCullough SS, Kurten R, et al. Vascular endothelial growth factor and hepatocyte regeneration in acetaminophen toxicity. Am J Physiol Gastrointest Liver Physiol. 2006;291:G102–G109. doi: 10.1152/ajpgi.00575.2005. [DOI] [PubMed] [Google Scholar]
- 117.Donahower BC, McCullough SS, Hennings L, et al. Human recombinant vascular endothelial growth factor reduces necrosis and enhances hepatocyte regeneration in a mouse model of acetaminophen toxicity. J Pharmacol Exp Ther. 2010;334:33–43. doi: 10.1124/jpet.109.163840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kato T, Ito Y, Hosono K, et al. Vascular endothelial growth factor receptor-1 signaling promotes liver repair through restoration of liver microvasculature after acetaminophen hepatotoxicity. Toxicol Sci. 2010;120:218–229. doi: 10.1093/toxsci/kfq366. [DOI] [PubMed] [Google Scholar]
- 119.James LP, Lamps LW, McCullough S, Hinson JA. Interleukin 6 and hepatocyte regeneration in acetaminophen toxicity in the mouse. Biochem Biophys Res Commun. 2003;309:857–863. doi: 10.1016/j.bbrc.2003.08.085. [DOI] [PubMed] [Google Scholar]
- 120.Laskin DL. Macrophages and inflammatory mediators in chemical toxicity: a battle of forces. Chem Res Toxicol. 2009;22:1376–1385. doi: 10.1021/tx900086v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Adams DH, Ju C, Ramaiah SK, Uetrecht J, Jaeschke H. Mechanisms of immune-mediated liver injury. Toxicol Sci. 2010;115:307–321. doi: 10.1093/toxsci/kfq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dambach DM, Watson LM, Gray KR, Durham SK, Laskin DL. Role of CCR2 in macrophage migration into the liver during acetaminophen-induced hepatotoxicity in the mouse. Hepatology. 2002;35:1093–1103. doi: 10.1053/jhep.2002.33162. [DOI] [PubMed] [Google Scholar]
- 123.Harrill AH, Watkins PB, Su S, et al. Mouse population-guided resequencing reveals that variants in CD44 contribute to acetaminophen-induced liver injury in humans. Genome Res. 2009;19:1507–1515. doi: 10.1101/gr.090241.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jaeschke H, Wendel A. Diurnal fluctuation and pharmacological alteration of mouse organ glutathione content. Biochem Pharmacol. 1985;34:1029–1033. doi: 10.1016/0006-2952(85)90606-9. [DOI] [PubMed] [Google Scholar]
- 125.Kazama H, Ricci JE, Herndon JM, et al. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity. 2008;29:21–32. doi: 10.1016/j.immuni.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yang H, Hreggvidsdottir HS, Palmblad K, et al. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci USA. 2010;107:11942–11947. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jaeschke H, Koerner M, Williams CD. Activation of caspases during acetaminophen toxicity is a strain dependent phenomenon (abstract) Toxicol Sci. 2011;120(Suppl. 2):96. [Google Scholar]
- 128.Fairbairn L, Kapetanovic R, Sester DP, Hume DA. The mononuclear phagocyte system of the pig as a model for understanding human innate immunity and disease. J Leukoc Biol. 2011 doi: 10.1189/jlb.1110607. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]