Abstract
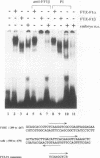
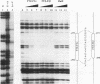
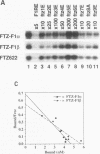
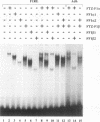
The striped pattern of fushi tarazu (ftz) expression found in the blastoderm of the Drosophila melanogaster embryo is generated largely through complex interactions between multiple transcription factors that bind to the zebra element of the ftz gene. A motif in the zebra element, the FTZ-F1 recognition element (F1RE), has been shown to bind a transcription factor, FTZ-F1 alpha, that is a member of the nuclear receptor family. We recently identified a second, related member of this family, FTZ-F1 beta, that also binds to this motif. To investigate the possibility that FTZ-F1 alpha and FTZ-F1 beta coregulate ftz transcription through the F1RE, we have studied the DNA binding properties of FTZ-F1 alpha and FTZ-F1 beta. We demonstrate that recombinant FTZ-F1 alpha and FTZ-F1 beta proteins produce similar in vitro DNase I footprint patterns on a 14-nucleotide region of the zebra element and bind to this site with similar affinities and sequence specificities. Using wild-type and N-terminally truncated receptors, we have determined that FTZ-F1 alpha and FTZ-F1 beta both bind as monomers to the 9-bp F1RE in the zebra element, as well as to an imperfect inverted F1RE repeat present in the Drosophila alcohol dehydrogenase gene. A polyclonal antibody raised against FTZ-F1 beta identifies a predominant F1RE-binding component in embryonic nuclear extracts. Although FTZ-F1 alpha is also present in these extracts, FTZ-F1 alpha and FTZ-F1 beta do not appear to form heterodimers with each other. Cotransfection assays in mammalian cell culture indicate that both receptors contribute to the net transcriptional activity of a reporter gene through their direct interaction with the F1RE. These data suggest that FTZ-F1 alpha and FTZ-F1 beta likely coregulate common target genes by competition for binding to a 9-bp recognition element.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akam M. The molecular basis for metameric pattern in the Drosophila embryo. Development. 1987 Sep;101(1):1–22. [PubMed] [Google Scholar]
- Amero S. A., Kretsinger R. H., Moncrief N. D., Yamamoto K. R., Pearson W. R. The origin of nuclear receptor proteins: a single precursor distinct from other transcription factors. Mol Endocrinol. 1992 Jan;6(1):3–7. doi: 10.1210/mend.6.1.1738368. [DOI] [PubMed] [Google Scholar]
- Ayer S., Benyajati C. The binding site of a steroid hormone receptor-like protein within the Drosophila Adh adult enhancer is required for high levels of tissue-specific alcohol dehydrogenase expression. Mol Cell Biol. 1992 Feb;12(2):661–673. doi: 10.1128/mcb.12.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayer S., Walker N., Mosammaparast M., Nelson J. P., Shilo B. Z., Benyajati C. Activation and repression of Drosophila alcohol dehydrogenase distal transcription by two steroid hormone receptor superfamily members binding to a common response element. Nucleic Acids Res. 1993 Apr 11;21(7):1619–1627. doi: 10.1093/nar/21.7.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. L., Sonoda S., Ueda H., Scott M. P., Wu C. Repression of the Drosophila fushi tarazu (ftz) segmentation gene. EMBO J. 1991 Mar;10(3):665–674. doi: 10.1002/j.1460-2075.1991.tb07995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson A. M., King D. L., Hatzivassiliou E., Casas J. E., Hallenbeck P. L., Nikodem V. M., Mitsialis S. A., Kafatos F. C. DNA binding and heteromerization of the Drosophila transcription factor chorion factor 1/ultraspiracle. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11503–11507. doi: 10.1073/pnas.89.23.11503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearolf C. R., Topol J., Parker C. S. The caudal gene product is a direct activator of fushi tarazu transcription during Drosophila embryogenesis. Nature. 1989 Sep 28;341(6240):340–343. doi: 10.1038/341340a0. [DOI] [PubMed] [Google Scholar]
- Dearolf C. R., Topol J., Parker C. S. Transcriptional control of Drosophila fushi tarazu zebra stripe expression. Genes Dev. 1989 Mar;3(3):384–398. doi: 10.1101/gad.3.3.384. [DOI] [PubMed] [Google Scholar]
- Fawell S. E., Lees J. A., White R., Parker M. G. Characterization and colocalization of steroid binding and dimerization activities in the mouse estrogen receptor. Cell. 1990 Mar 23;60(6):953–962. doi: 10.1016/0092-8674(90)90343-d. [DOI] [PubMed] [Google Scholar]
- Forman B. M., Samuels H. H. Interactions among a subfamily of nuclear hormone receptors: the regulatory zipper model. Mol Endocrinol. 1990 Sep;4(9):1293–1301. doi: 10.1210/mend-4-9-1293. [DOI] [PubMed] [Google Scholar]
- Green S., Issemann I., Sheer E. A versatile in vivo and in vitro eukaryotic expression vector for protein engineering. Nucleic Acids Res. 1988 Jan 11;16(1):369–369. doi: 10.1093/nar/16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafen E., Kuroiwa A., Gehring W. J. Spatial distribution of transcripts from the segmentation gene fushi tarazu during Drosophila embryonic development. Cell. 1984 Jul;37(3):833–841. doi: 10.1016/0092-8674(84)90418-5. [DOI] [PubMed] [Google Scholar]
- Han W., Yu Y., Altan N., Pick L. Multiple proteins interact with the fushi tarazu proximal enhancer. Mol Cell Biol. 1993 Sep;13(9):5549–5559. doi: 10.1128/mcb.13.9.5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. D., Travers A. A. Identification of the binding sites for potential regulatory proteins in the upstream enhancer element of the Drosophila fushi tarazu gene. Nucleic Acids Res. 1988 Dec 23;16(24):11403–11416. doi: 10.1093/nar/16.24.11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. D., Travers A. A. The tramtrack gene encodes a Drosophila finger protein that interacts with the ftz transcriptional regulatory region and shows a novel embryonic expression pattern. EMBO J. 1990 Jan;9(1):207–216. doi: 10.1002/j.1460-2075.1990.tb08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich V. C., Sliter T. J., Lubahn D. B., MacIntyre A., Gilbert L. I. A steroid/thyroid hormone receptor superfamily member in Drosophila melanogaster that shares extensive sequence similarity with a mammalian homologue. Nucleic Acids Res. 1990 Jul 25;18(14):4143–4148. doi: 10.1093/nar/18.14.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiromi Y., Gehring W. J. Regulation and function of the Drosophila segmentation gene fushi tarazu. Cell. 1987 Sep 11;50(6):963–974. doi: 10.1016/0092-8674(87)90523-x. [DOI] [PubMed] [Google Scholar]
- Hiromi Y., Kuroiwa A., Gehring W. J. Control elements of the Drosophila segmentation gene fushi tarazu. Cell. 1985 Dec;43(3 Pt 2):603–613. doi: 10.1016/0092-8674(85)90232-6. [DOI] [PubMed] [Google Scholar]
- Honda S., Morohashi K., Nomura M., Takeya H., Kitajima M., Omura T. Ad4BP regulating steroidogenic P-450 gene is a member of steroid hormone receptor superfamily. J Biol Chem. 1993 Apr 5;268(10):7494–7502. [PubMed] [Google Scholar]
- Izumo S., Mahdavi V. Thyroid hormone receptor alpha isoforms generated by alternative splicing differentially activate myosin HC gene transcription. Nature. 1988 Aug 11;334(6182):539–542. doi: 10.1038/334539a0. [DOI] [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Lala D. S., Rice D. A., Parker K. L. Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. Mol Endocrinol. 1992 Aug;6(8):1249–1258. doi: 10.1210/mend.6.8.1406703. [DOI] [PubMed] [Google Scholar]
- Langeland J. A., Carroll S. B. Conservation of regulatory elements controlling hairy pair-rule stripe formation. Development. 1993 Feb;117(2):585–596. doi: 10.1242/dev.117.2.585. [DOI] [PubMed] [Google Scholar]
- Laudet V., Hänni C., Coll J., Catzeflis F., Stéhelin D. Evolution of the nuclear receptor gene superfamily. EMBO J. 1992 Mar;11(3):1003–1013. doi: 10.1002/j.1460-2075.1992.tb05139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavorgna G., Karim F. D., Thummel C. S., Wu C. Potential role for a FTZ-F1 steroid receptor superfamily member in the control of Drosophila metamorphosis. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):3004–3008. doi: 10.1073/pnas.90.7.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavorgna G., Ueda H., Clos J., Wu C. FTZ-F1, a steroid hormone receptor-like protein implicated in the activation of fushi tarazu. Science. 1991 May 10;252(5007):848–851. doi: 10.1126/science.1709303. [DOI] [PubMed] [Google Scholar]
- Lee M. S., Kliewer S. A., Provencal J., Wright P. E., Evans R. M. Structure of the retinoid X receptor alpha DNA binding domain: a helix required for homodimeric DNA binding. Science. 1993 May 21;260(5111):1117–1121. doi: 10.1126/science.8388124. [DOI] [PubMed] [Google Scholar]
- Luisi B. F., Xu W. X., Otwinowski Z., Freedman L. P., Yamamoto K. R., Sigler P. B. Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA. Nature. 1991 Aug 8;352(6335):497–505. doi: 10.1038/352497a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Müller J., Bienz M. Sharp anterior boundary of homeotic gene expression conferred by the fushi tarazu protein. EMBO J. 1992 Oct;11(10):3653–3661. doi: 10.1002/j.1460-2075.1992.tb05450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüsslein-Volhard C., Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980 Oct 30;287(5785):795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Ohno C. K., Petkovich M. FTZ-F1 beta, a novel member of the Drosophila nuclear receptor family. Mech Dev. 1993 Jan;40(1-2):13–24. doi: 10.1016/0925-4773(93)90084-b. [DOI] [PubMed] [Google Scholar]
- Oro A. E., McKeown M., Evans R. M. Relationship between the product of the Drosophila ultraspiracle locus and the vertebrate retinoid X receptor. Nature. 1990 Sep 20;347(6290):298–301. doi: 10.1038/347298a0. [DOI] [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Petkovich M. Regulation of gene expression by vitamin A: the role of nuclear retinoic acid receptors. Annu Rev Nutr. 1992;12:443–471. doi: 10.1146/annurev.nu.12.070192.002303. [DOI] [PubMed] [Google Scholar]
- Rosenthal N. Identification of regulatory elements of cloned genes with functional assays. Methods Enzymol. 1987;152:704–720. doi: 10.1016/0076-6879(87)52075-4. [DOI] [PubMed] [Google Scholar]
- Scott M. P., Carroll S. B. The segmentation and homeotic gene network in early Drosophila development. Cell. 1987 Dec 4;51(5):689–698. doi: 10.1016/0092-8674(87)90092-4. [DOI] [PubMed] [Google Scholar]
- Segraves W. A. Something old, some things new: the steroid receptor superfamily in Drosophila. Cell. 1991 Oct 18;67(2):225–228. doi: 10.1016/0092-8674(91)90172-u. [DOI] [PubMed] [Google Scholar]
- Shea M. J., King D. L., Conboy M. J., Mariani B. D., Kafatos F. C. Proteins that bind to Drosophila chorion cis-regulatory elements: a new C2H2 zinc finger protein and a C2C2 steroid receptor-like component. Genes Dev. 1990 Jul;4(7):1128–1140. doi: 10.1101/gad.4.7.1128. [DOI] [PubMed] [Google Scholar]
- Thomas H. E., Stunnenberg H. G., Stewart A. F. Heterodimerization of the Drosophila ecdysone receptor with retinoid X receptor and ultraspiracle. Nature. 1993 Apr 1;362(6419):471–475. doi: 10.1038/362471a0. [DOI] [PubMed] [Google Scholar]
- Topol J., Dearolf C. R., Prakash K., Parker C. S. Synthetic oligonucleotides recreate Drosophila fushi tarazu zebra-stripe expression. Genes Dev. 1991 May;5(5):855–867. doi: 10.1101/gad.5.5.855. [DOI] [PubMed] [Google Scholar]
- Tora L., Gronemeyer H., Turcotte B., Gaub M. P., Chambon P. The N-terminal region of the chicken progesterone receptor specifies target gene activation. Nature. 1988 May 12;333(6169):185–188. doi: 10.1038/333185a0. [DOI] [PubMed] [Google Scholar]
- Tsukiyama T., Ueda H., Hirose S., Niwa O. Embryonal long terminal repeat-binding protein is a murine homolog of FTZ-F1, a member of the steroid receptor superfamily. Mol Cell Biol. 1992 Mar;12(3):1286–1291. doi: 10.1128/mcb.12.3.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H., Hirose S. Defining the sequence recognized with BmFTZ-F1, a sequence specific DNA binding factor in the silkworm, Bombyx mori, as revealed by direct sequencing of bound oligonucleotides and gel mobility shift competition analysis. Nucleic Acids Res. 1991 Jul 11;19(13):3689–3693. doi: 10.1093/nar/19.13.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H., Sonoda S., Brown J. L., Scott M. P., Wu C. A sequence-specific DNA-binding protein that activates fushi tarazu segmentation gene expression. Genes Dev. 1990 Apr;4(4):624–635. doi: 10.1101/gad.4.4.624. [DOI] [PubMed] [Google Scholar]
- Ueda H., Sun G. C., Murata T., Hirose S. A novel DNA-binding motif abuts the zinc finger domain of insect nuclear hormone receptor FTZ-F1 and mouse embryonal long terminal repeat-binding protein. Mol Cell Biol. 1992 Dec;12(12):5667–5672. doi: 10.1128/mcb.12.12.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesono K., Evans R. M. Determinants of target gene specificity for steroid/thyroid hormone receptors. Cell. 1989 Jun 30;57(7):1139–1146. doi: 10.1016/0092-8674(89)90051-2. [DOI] [PubMed] [Google Scholar]
- Wilson T. E., Fahrner T. J., Milbrandt J. The orphan receptors NGFI-B and steroidogenic factor 1 establish monomer binding as a third paradigm of nuclear receptor-DNA interaction. Mol Cell Biol. 1993 Sep;13(9):5794–5804. doi: 10.1128/mcb.13.9.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T. E., Paulsen R. E., Padgett K. A., Milbrandt J. Participation of non-zinc finger residues in DNA binding by two nuclear orphan receptors. Science. 1992 Apr 3;256(5053):107–110. doi: 10.1126/science.1314418. [DOI] [PubMed] [Google Scholar]
- Yao T. P., Segraves W. A., Oro A. E., McKeown M., Evans R. M. Drosophila ultraspiracle modulates ecdysone receptor function via heterodimer formation. Cell. 1992 Oct 2;71(1):63–72. doi: 10.1016/0092-8674(92)90266-f. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]