Abstract
Objective(s)
The objective of this study was to prepare, characterize and evaluate the nanoliposomes containing safranal as a natural sunscreen and moisturizer factor.
Materials and Methods
The experimental formulations included homosalate reference, nanoliposomes containing 0.25, 0.5, 1, 2, 4 and 8% safranal and empty liposomes. The liposomes were prepared using fusion method and homogenization. Homosalate reference was prepared according to FDA standard. Sun protection factors (SPF) of the formulations were determined by two in vitro methods; diluted solution transmittance method and transpore tape method. Studies of in vitro penetration of the formulations across mouse skin were carried out with diffusion cells. The percentage of safranal penetrated and retained in the skin was determined for the formulations up to 24 hr. The amount of the moisture contents of the skin before application and after 30-minute, 1, 3 and 5 hr post-application of the formulations were measured in human volunteers using Corneometer.
Results
The results indicated that, the SPF of liposomes containing 8% safranal (Lip-Safranal 8%) was significantly higher than 8% homosalate reference. The proportion of Lip-Safranal 1% that penetrated the skin was low. There was no significant difference between the skin moisture contents after application of Lip-Safranal 1 and 4% and empty liposomes during the 7 hr post-application period.
Conclusion
These results showed that in equal concentrations, Lip-Safranal could act as a better antisolar agent compared to homosalate and have no moisturizing effect in 1 and 4% concentrations.
Key Words: Liposomes, Moisture, Saffron, Safranal, SunProtection Factor
Introduction
The harmful effects of solar radiation are caused by ultraviolet radiation (UVR) part of the solar rays. UVA and UVB are mainly responsible for skin pathologies such as sunburns, cutaneous degeneration, photosensitivity, phototoxicity, photo-aging, immunosuppression and skin cancer (1-3).
The growing awareness of the damage that UVR might cause on human health has led to increasing use of sunscreen products (1,2). The efficacy of a sunscreen is usually expressed by the Sun Protection Factor (SPF). Higher SPF values result in more effective products in preventing sunburn. Sunscreen products are usually applied superficially to large skin areas; therefore, penetration of the sunscreen’s ingredients may occur, which is not desirable (4-6). Irritation may also occur with some chemical sunscreens (7).
Nowadays, using the natural products that can absorb UVR is of great interest in sunscreen products. This is because of the benefits of these products, more acceptability by the users; also the low probability of the systemic absorption. (8). Natural substances extracted from plants have recently been considered as potential sunscreen resources because of their UV absorption and their antioxidant activity (8-13).
Saffron is the dried stigmas of a flower scientifically identified as Crocus sativus. It is a perennial stemless herb widely cultivated in Iran and some other countries such as India, Spain and Greece (14). Pharmacological studies have revealed that saffron extract has antitumor, radical scavenging properties (15-17), as well as antinociceptive, antiinflammatory (18), anticonvulsant (19), and antidepressant effects (20,21). The main aroma factor in saffron is safranal, which comprises about 60% of the volatile components of the saffron (17). The investigations demonstrate that saffron and its active constituents like safranal have anti-tumor, antioxidant and antigenotoxic effects (15-18).
Organic sunscreens are generally aromatic compounds conjugated with a carbonyl group (26). In our previous study, because of the advantages of saffron besides having many aromatic and flavonoid compounds such as kaempherol and quercetin, the SPFs of the lotions containing ground saffron were evaluated and established (27). In this study, according to the aromatic conjugated with a carbonyl group structure of safranal and its antioxidant activity besides its UV absorption spectrum, the possibility of using this component as a sunscreen, was investigated.
Many factors are involved in the delivery of the drugs and cosmetics into the skin from topically applied formulations. Liposomes are preferable in sunscreen formulations. They exhibit unique features by offering easy delivery, no interference with vision, stabilizing the drug, excellent reservoirs for drug loading and water resistance properties (28-30). Several factors; such as, physicochemical properties of the drug and other ingredients, lamellarity, lipid composition, charge, size, vehicle, mode of application and total lipid concentrations have been proven to influence drug deposition into the skin layers. The other advantage of a liposome-based drug product is that fewer drugs need to be administered. Thus, the probability of systemic absorption and adverse drug reactions is reduced (28-32).
Another appropriate effect of a sunscreen product is producing a moisturizing effect. Corneometers have gained worldwide acceptance as an efficient instrument to measure the water content in the stratum corneum (SC) (33).
The aim of this research was to characterize the liposomes containing safranl (Lip-Safranal) and determine and compare the SPF values of these liposomal formulations by two in vitro methods and evaluate the moisturizing effects of the formulations on the skin of human volunteers using Corneometer.
Materials and Methods
Reagents and chemicals
Homosalate, cholesterol and vitamin E, were purchased from Merck (). Lanolin, white petrolatum, stearic acid, propylparaben (PP), methylparaben (MP), disodium EDTA, propylene glycol, triethanolamine, N-[2-hydroxyethyl] piperazine-N_-[2-ethanesulfonic acid] (HEPES) and safranal were purchased from Sigma (USA). Soya phosphatidylcholine (Soya PC) was obtained from the Avanti Polar Lipids (). All solvents used in this study were high performance liquid chromatography (HPLC) grade. All chemicals were of the purest grade available.
Preparation of the homosalate reference as the standard sunscreen
A standard sunscreen formulation was needed for ensuring reproducible results in SPF determinations. This standard was prepared according to the FDA and Australian standards. According to FDA, the SPF of this standard preparation is 4.47±1.279 (21,27). Homosalate (8%), lanolin (5%), white petrolatum (2.5%), stearic acid (4%) and propylparaben (0.05%) were melted at 77 - 82°C as the oil phase. Methylparaben (0.1%), disodium EDTA (0.05%), propylene glycol (5%), triethanolamine (1%) and water up to 100% were heated as the aqueous phase with constant stirring. The aqueous phase was added to oil phase, and the mixture was stirred until it cooled down to room temperature (34-36).
Preparation of liposomes containing safranal (Lip-Safranal)
Lip-Safranal (0.25, 0.5, 1, 2, 4 and 8%) was prepared by fusion method (28). The lipid components consisted of Soya PC (15%), cholesterol (2%), vitamin E (0.3%), propylene glycol (7%), MP (0.1%) and PP (0.02%) were melted at about 75°C (melted lipid). When melted lipid cooled down to 50˚C then Oleic acid (1%) and safranal were added and mixed completely. HEPES buffer (10 mM, pH 6.5) and triethanolamine (0.5%) up to 100% were heated separately at 75˚C and was added to the previously heated melted lipid and stirred vigorously until it cooled down to room temperature. The final products were then homogenized with a homogenizer (Ultra-Turrax IKA T10; IKA Werke GmbH & Co. KG, ) for 3 min at 11,500 rpm, 2 min at 14,500 rpm, 1 min at 20,500 rpm and 1 min at 30,000 rpm. The same procedure was used to prepare control empty liposomes, except for omitting the safranal.
Characterization of liposomes
The average particle size and charge of the liposomes were measured in triplicate by the use of dynamic light scattering (ZetaSizer Nano-ZS; Malvern Instruments Ltd., ).. Liposomal preparations were characterized 12 hr after preparation. For particle size measurements, liposomal suspensions were properly diluted with HEPES buffer in order to avoid multiscattering phenomena. For surface charge determination, liposomal dispersions suitably diluted with MOPS buffer were dropped into the Zetamaster electrophoretic cell and the Z potential was determined by electrophoretic mobility measurement (37,38).
Liposomes encapsulation efficiency was determined indirectly, separating the non-entrapped drug from drug-loaded liposomes by dialysis experiments. According to a previously developed method (38), dispersion of 100 mg of drug-loaded liposomes to 1 ml HEPES buffer was prepared and placed into a dialysis bag of cellulose acetate (Spectra/Por®, MW cut-off 12000, Spectrum, Canada) immersed in a closed vessel containing 45 ml of HEPES buffer at 20 ◦C, and magnetically stirred at 30 rpm. Samples, withdrawn at time intervals, were replaced with equal volumes of fresh solvent and spectrometrically analyzed (UV 1601 Shimadzu) (37). The maximum absorption of the safranal was obtained as 310 nm. The percentage of encapsulation efficiency (EE %) was calculated according to the following equation:
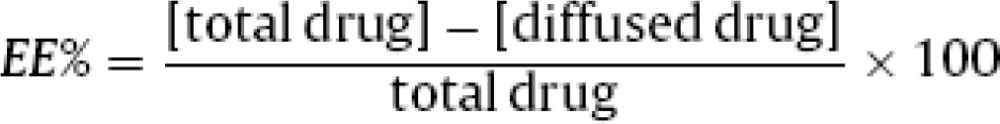 |
Each result was the mean of at least three separate experiments.
SPF determination of the formulations by diluted solution transmittance method
All samples (1 g) were weighed, transferred to a 100 ml volumetric flask, diluted to volume with ethanol, mixed for 5 min, and then filtered through Whatman filters. A 5 ml sample was transferred to a 25 ml volumetric flask and diluted to volume with ethanol. The absorption values were obtained in the range of 290 to 320 nm (every 5 nm) and three determinations were made at each point. Then, Mansur equation was used to determine the SPF values of the formulations. The introduced equation is as follows:
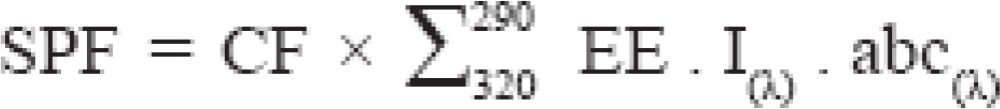 |
In this equation, CF= 10 (correction factor), EE (λ) = erythemogenic effect of radiation at wavelength λ, I(λ) = intensity of solar light at wavelength λ, and abs(λ)=absorbance of sample at wavelength λ. The values for the term “EE × I” are constants, which were determined by Sayre et al and are shown in Table 1(39,40).
Table 1.
The values of EE(λ). I(λ) for conclusion of the SPF values in transmittance method
| λ (nm) | EE (λ). I(λ) |
|---|---|
| 290 | 0.0150 |
| 295 | 0.0817 |
| 300 | 0.2874 |
| 305 | 0.3278 |
| 310 | 0.1864 |
| 315 | 0.0839 |
| 320 | 0.0180 |
SPF determination of the formulations by transpore tape method
The principle of this method is to measure the spectral transmittance of UVR through a sample of a surgical tape which is called transpore tape with and without the sunscreen applied. This substrate was introduced first by Diffey and Robson (41,42). A piece of transpore tape was placed over the quartz cell and then 2 mg/cm2 of sunscreen was applied by spotting the sunscreen at several sites over the entire application area. A gloved finger was used to achieve as uniform thickness as possible with a circular light rubbing motion. After 15 min the transmission was measured by UV spectrophotometer. The data of transmittances were set to five nm intervals from 290 nm to 400 nm. The SPF was predicted from the following equation;
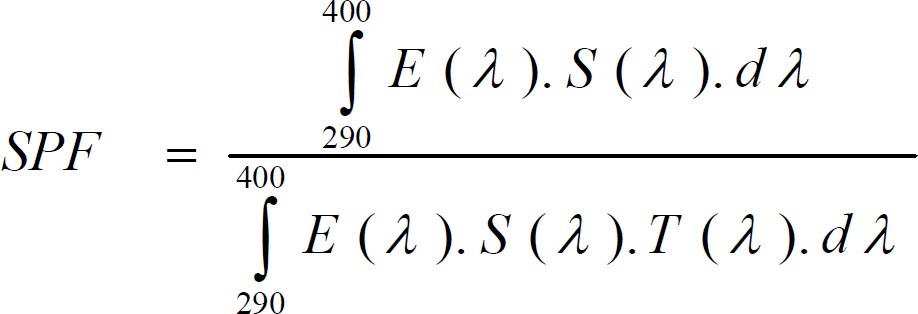 |
In this equation: E (λ): Relative erythemal spectral effectiveness; S (λ): Solar spectral irradiance (Wm-2nm-1); T (λ): Spectral transmittance of the sample (as measured on the UV-1000S)
The concentrations of the safranal liposomes were selected to obtain minimal sunburn protection (Table 2).
Table 2.
Sunscreen potency assessment by the FDA, based on sun protection factor (SPF)
| Sunburn protection | SPF |
|---|---|
| Minimal | 2-12 |
| Moderate | 12-30 |
| High | ≥ 30 |
Moisture content measurement of the skin
The moisture content of the skin was measured by Corneometer (Courage & Khazaka, ). In practice, the technique is used to measure the difference in the hydration state of SC before and after application of cosmetic or other skin treatments. The test was carried out on six volunteers with normal skin, aged between 20 and 35 at room temperature. Before the measurements, they were given time to adapt to the room conditions without covering the measuring sites with clothes. On the day of examination, the skin was not washed, and nothing was applied to the skin surface. The volunteers were instructed not to apply any preparation to the site to be examined one week before the investigation. In all subjects, the tested sites of the skin were free of eczematous involvement. All measured values were expressed as the median of three recordings. The measurements were carried out on exactly the same sites. The testing site of the skin was the middle of the forearm (33, 43-46). The moisture content of the skin was measured without any application of the products and after 0.5, 1, 3 and 5 hr after application of the Lip–Safranal 1 and 4%.
Cell diffusion study
Jacketed Franz cells with a receiver volume of 25 ml were used, and every experiment was conducted in triplicate at 37 °C. HEPES buffer of pH 6.5 was used as the receiver medium. A suitable size of full-thickness skin of a BALB/c mouse was cut and mounted in the Franz cell, with the SC side facing upward. The mouse was properly shaved with electric clippers on the day before the experiment. The membranes were initially left in the Franz cells for 30 min in order to facilitate hydration. Subsequently, 1 g of the liposomal formulation was deposited onto each membrane surface. A 5 ml aliquot was withdrawn from each receiver solution at 1, 2, 4, 8 and 24 hr intervals and replaced with the same volume of HEPES buffer. Aliquots of the collected samples were analyzed for their safranal by the spectrophotometric method. The derived concentration values were corrected by using the equation:
where Mt (n) is the current cumulative mass of drug transported across the skin at the time t, n is the number (times) of sampling, Cn is the current concentration in the receiver medium, Cm is the summed total of the previously measured concentrations, Vr is the volume of the receiver medium, and Vs corresponds to the volume of the sample removed for analysis. For the determination of the amount of liposome retained in the skin, at the end of the experiment, the amount of the formulation remaining on the surface of the membrane was collected and assayed for safranal (47-49).
Statistical analysis
One-way ANOVA was used to assess the significance of the differences between groups. In case of significant F value multiple comparison Tukey-Kramer tests were used to compare the means of different treatment groups. Results with P< 0.05 were considered to be statistically significant.
Results
Characterization of the liposomes
In this study, six concentrations of Lip-Safranal were prepared. Mean diameters of Liposomes determined by PSA has been shown in Table 3. The differences between the sizes were not significant (P> 0.05) but the zeta potential of 0.25%, was significantly higher than 2% and 4% (P< 0.01) and 8% (P< 0.001). Liposomes exhibited a slight increase in their size after 1, 2 and three- month storage at 4 ºC but this was not statistically significant (P> 0.05).
Table 3.
Mean diameter and zeta potential of different Lip-Safranal concentrations (± SD, n= 3)
| Safranal concentrations in liposomes (w/v) | Empty liposomes | 0.25% | 0.5% | 1% | 2% | 4% | 8% |
|---|---|---|---|---|---|---|---|
| Mean diameter (nm) | 102.3±2.63 | 104.5±7.69 | 110.1±2.20 | 118.57±20.93 | 128.6±49.40 | 135.9±21.45 | 90.2±31.80 |
| Zeta potential (mV) | -49.3±3.20 | -52.3±3.60 | -47.3±1.50 | -46.5±3.50 | -37.4±4.40 | -38.4±5.10 | -34.8±2.30 |
| Polydispersity Index | 0.215± 0.11 | 0.304±0.12 | 0.255±0.06 | 0.336±0.03 | 0.109±0.01 | 0.134±0.05 | 0.253±0.07 |
Liposomes with low encapsulation efficiency were achieved. The encapsulation efficiency for Lip-Safranal 1% was 1.46±0.2% and the percentage releases of 71.03±5.11%, 80.67±9.55% and 84.06±11.42% were obtained after 12, 24 and 36 hr respectively (Table 4).
Table 4.
The percentage release of Lip-Safranal concentrations (±SD, n= 3)
| Time | 12 hr | 24 hr | 36 hr |
|---|---|---|---|
| Amount of safranal (µg) | 355.13±25.54 | 48.21±22.23 | 16.98±9.34 |
| Accumulation amount of safranal (µg) | 355.13±25.54 | 403.34±47.77 | 420.32±57.11 |
| Percentage release of safranal | 71.03±5.11 | 80.67±9.55 | 84.06±11.42 |
Statistical analysis showed that encapsulation efficiency was independent of liposome size and the differences between groups were not significant (P> 0.05).
Cell diffusion study
Studies of the in vitro penetration of the formulations across mouse skin were carried out with diffusion cells. The percentage of
safranal penetrated and retained in the skin was determined for the formulations for up to 24 hr. The proportion of Lip-Safranal 1% that penetrated the skin was 8.06±0.48 % and the proportion of safranal in the liposomes that was retained on the skin was 0.47± 0.42 %.
SPF determination of the formulations by diluted solution transmittance method and transpore tape
Six concentrations of Lip-Safranal were evaluated by UV spectrophotometry using Mansur equation and transpore tape methods (39, 41,42). The SPF values of the 8% homosalate reference and the Lip-Safranal 0.25, 0.5, 1, 2, 4 and 8% were measured. Figures 1, 2 show the SPFs of liposomal and homosalate reference formulations by these two in vitro methods. There was no significant difference between the values obtained for SPF of homosalate reference by two in vitro methods and in vivo studies with P> 0.05 (6,35).
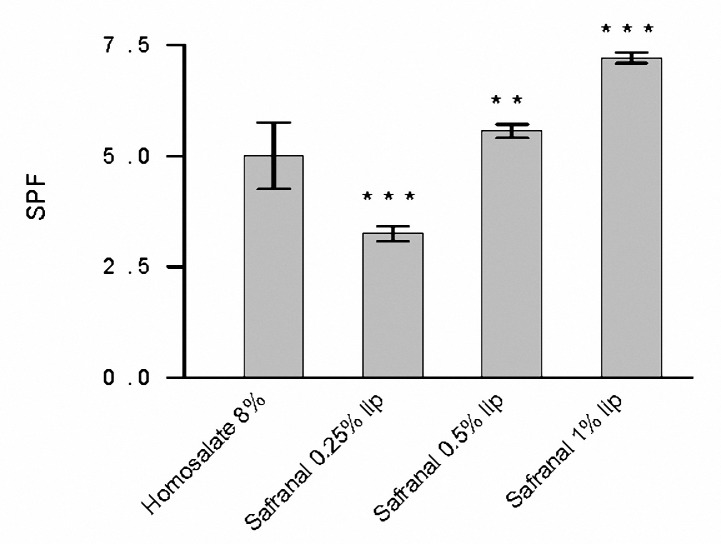
Figure 1.
The SPF values of Lip-Safranal (0.25, 0.5 and 1%) and homosalate reference determined by diluted solution transmittance method. Values are mean±SD, n= 3; **P< 0.01, ***P< 0.001.
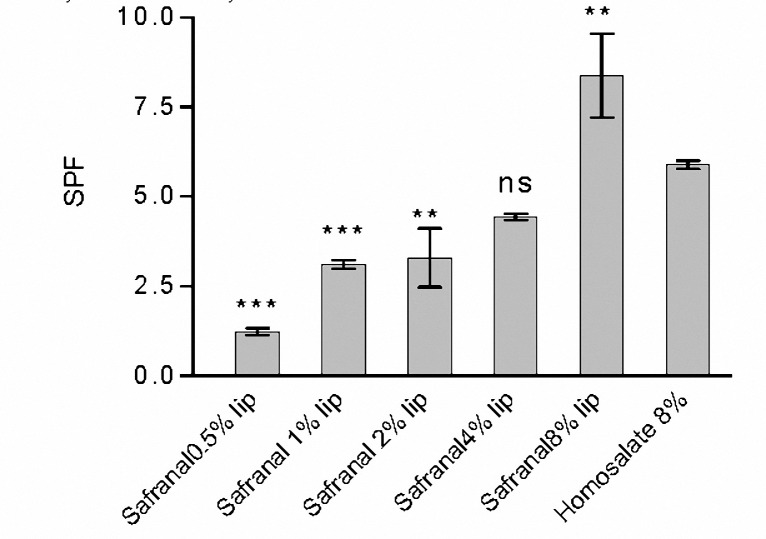
Figure 2.
The SPF values of Lip-Safranal (0.5, 1, 2, 4 and 8%) and homosalate reference determined by transpore tape method. Values are the mean±SD, n= 3; **P< 0.01, ***P< 0.001.
According to the diluted solution transmittance method, the SPF values of Lip-Safranal 0.5 and 1% were significantly higher than 8% homosalate reference (P< 0.05, Figure 1). The SPF of Lip-Safranal 2, 4 and 8% were not accurately obtained as the absorptions was higher than 1. These results show that in very low concentrations, safranal can act as a better antisolar agent compared to homosalate.
There was no significant difference in the SPF values of Lip-safranal 4% and 8% homosalate reference by transpore tape method. However, the SPF of Lip-safranal 8% was significantly higher than 8% homosalate reference (P< 0.05). The SPF of the empty liposomes was obtained as 0.98±0.003.
All of the formulations were stable except for the Lip-Safranal 8% which was returned into two phases after 2 weeks.
These results show that in equal concentration, safranal can act as a better antisolar agent compared to homosalate. The differences in the results obtained from the two in vitro methods will be discussed in the discussion.
Measurement of the moisture content of the SC following application of Lip-Safranal and empty liposomes
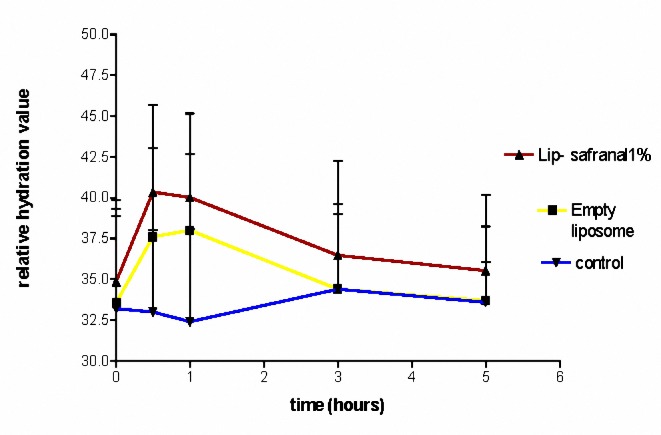
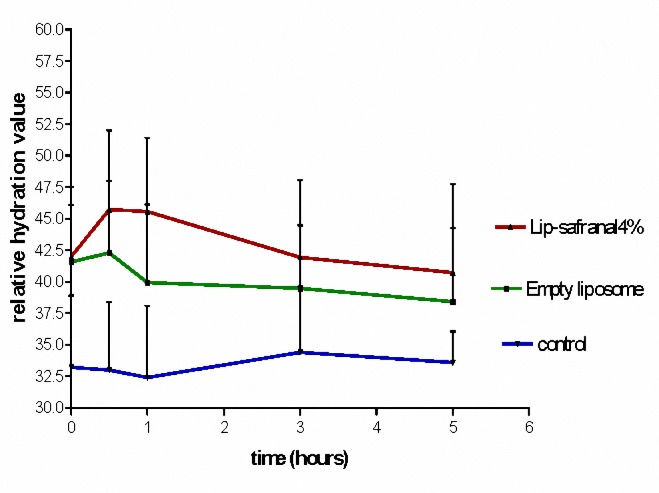
The water contents of Lip-Safranal 1 and 4% and the empty liposomes were measured using Corneometer CM 825. The Corneometer was calibrated to the baseline value for each subject before application of the formulations on the skin. During the first 30 min after application, the water contents were usually higher than normal. Measurement of the water content of skin at this time (fist 30 min) may result in erroneous data (36, 45,46). Therefore, the first measurement was scheduled at 30 minute after application. The water content of skin was measured 0.5, 1, 3 and 5 hr post-application of Lipo-Safranal 1 and 4%, compared to the baseline which was the value before application of the product. All the tested formulations significantly increased the moisture content of the skin compared to control, in all the tested point times (P< 0.01), but there was no significant difference (P> 0.05) between the skin moisture contents after application of Lip-Safranal 1 and 4% and the empty liposomes during 5 hr of measurements (Figure 3, 4). Figure 3 shows the relative hydration values for the readings of the test sites measured for Lip-Safranal 4% and empty liposomes at 0.5, 1, 3, and 5 hr post application in relation to the baseline. The trend of the curves in all the treatment groups was nearly the same. At 30 min application, the highest water content was observed; however, there was no significant difference between Lip-Safranal and empty liposomes. After 1 hr, the moisture contents were decreased in all the formulations. After 5 hr of application, the moisture content of the skin for all of the preparation was almost the same, they reached nearly the same degree of hydration and there was no significant difference among them.
Figure 3.
The relative hydration value of Lip-Safranal 1%, Empty liposome and control (Skin hydration without any application).
Figure 4.
The relative hydration value of Lip-Safranal 4%, Empty liposome and control (Skin hydration without any application).
Discussion
The decrease in intensity of the UVR reaching the skin by sunscreens may reduce the risk of sun-induced skin cancer (50). The efficacy of a sunscreen is usually expressed by the SPF (2, 3, 34-36). Most of the published studies on the determination of SPF have adopted an in vivo method based on experiments on human skin, which is very time-consuming and expensive and have human ethical issues. Therefore, developing an in vitro method which correlates well with in vivo methods is of interest to researchers as an attempt to find a substitute for in vivo methods (1, 2,51). Regarding sun-care experiments, it is also a safety issue, since only positive in vitro responses will direct the future of the in vivo tests (52). Results of several studies indicate that liposomes have been reported as a carrier for active cosmetic ingredients such as humectants (36,53) and sunscreens (30,36).
In this study, the 8% homosalate reference and Lip-Safranal 0.5, 1, 2, 4 and 8 % were prepared. The difference between the sizes was not statistically significant (P> 0.05), however the zeta potential of the preparations has been decreased during the increasing of the safranal concentrations. Minus zeta potential of the empty liposomes is due to the oleic acid in bilayer and when safranal is added the oleic acid in the bilayer would be diluted.
As the safranal is placed in the bilayer of the liposomes, diluting the preparations will produce decreasing the zeta potential of the nanoliposomes. The SPF values of the formulations were determined by two in vitro methods (39, 41,42). The SPF of Lip-Safranal 0.5 and 1% were significantly higher than 8% homosalate reference (P< 0.05) by diluted solution transmittance method. However, the results obtained from transpore tape method indicated that there was no significant difference between the SPF values of Lip-Safranal 4% and that of 8% homosalate reference. These results also indicated that the SPF of Lip-Safranal 8% was significantly higher than 8% homosalate reference (P< 0.05). At first, the SPFs of different concentrations of safranal (0.25, 0.5 and 1%) were determined by diluted solution method as safranal dissolves readily in ethanol. These concentrations were selected to obtain minimal sunburn protection (SPF 2-12) according to FDA standard (Table 2). The SPFs of safranal (2, 4 and 8%) were not obtained by this method as the absorptions were higher than 1. As this situation is totally different from that in which a sunscreen agent is applied directly to the skin, it shows a poor correspondence with some sunscreen’s SPFs especially high ones. Despite this, it is still considered to be useful for a preliminary assessment due to its simplicity (1, 2,6). Since safranal is a volatile component, the transpore tape method was also carried out. The results obtained from the second method are more accurate and reliable, as the transpore tape has uneven topography that distributes the sunscreen in a way similar to human skin and imitates the real situation (15 minutes waiting before SPF determination) (14). After 15 min, some of the safranal will evaporate, and the concentrations will decrease in the formulations. Therefore, we required more safranal concentrations to obtain minimal sunburn protection (SPF 2-12) according to FDA standard (Table 2). Thus safranal with concentrations; 0.5, 1, 2, 4 and 8% were prepared. The proposed UV spectrophotometric methods are simple, rapid and use low cost reagents. They can be performed both during the production process and on the final product (39). These results showed that safranal can act as a better antisolar agent compared to homosalate. High SPF value of Lip-Safranal may be related to the aromatic conjugated with a carbonyl group structure of safranal.
In recent years, natural compounds have gained considerable attention as UV protective agents due to the presumable safe utilization, ecological issues, and minimal side effects besides their antioxidant activity (54,55). Plant extracts, due to presence of a wide range of phenolic acids, flavonoids, and high molecular weight polyphenols, usually cover the full range of UV wavelengths (54, 56,57). Safranal has the aromatic conjugated with a carbonyl group structure and antioxidant activity besides a good UV absorption spectrum. Therefore, the possibility of using the safranal as a sunscreen was investigated in this research.
In a recent review, Abdullaev and Espinosa- focused on the anticancer activity of saffron and its principal ingredients (17).
From the results obtained in our previous study, saffron can be used as a natural UV absorbing agent. The 4% saffron lotion showed an SPF value equivalent to the 8% homosalate lotion reference by an in vitro method. (27).
Topical application of Culcitium reflexum extracts in the form of a gel proved to exert a significant in vivo protection against the UV-induced skin erythema in healthy human volunteers. The flavonoid fraction of Sedum telephium leaf extracts also appears to possess potent protective effects against UV-induced skin erythema in human volunteers (13,54).
One approach to protect human skin against the harmful effects of UVR is to use antioxidants as photo-protective. According to our results safranal could act as a better antisolar agent compared to homosalate, besides it has also antitumor and antioxidant activities (16, 17,58)
Ramon et al. showed that liposomes could be regarded as alternatives to conventional oil/water emulsions in the formulations of lipidic sun filters. When liposomes with a composition and structural organization similar to that of the SC lipids are used the skin penetration is retarded (30). As the intercellular lipids are important in controlling the percutaneous absorption, liposomes may mix with the intercellular lipids and produce a sustained release carrier system that acts as a reservoir for sunscreen; therefore, the sunscreen remains longer on the outermost layers of the skin (29). This property is essential for sunscreen agents because the amount remained inside the SC maybe directly related to its sun protection value (4,59). In the current study, liposomes were selected as a drug delivery system for safranal because of these benefits and water resistance property (6,28). Liposomes in the proper formulations and sizes have been shown to be able to accumulate in the skin (29, 30, 32,47).
In our study, as the dialysis is a dynamic system, and the safranal evaporates during dialysis and releases to the buffer, the encapsulation efficiency is expected to be more than 1 %, which was discussed above. Therefore, the liposomes were used without purification.
In this study, soybean PC (SPC) was used for liposome preparations. SPC contains polyunsaturated fatty acids like linoleic acid, which are beneficial for healthy skin. Furthermore, formulations prepared by SPC increase the skin humidity (6, 60). In the liposome formulation cholesterol was included to stabilize the lipid bilayer and decrease the leakage of encapsulated drugs and vesicle aggregation (6,61). Vitamin E was used to prevent SPC oxidation, PP and MP were used as microbial preservatives, and HEPES to control the pH of the liposomal formulations to achieve maximum stability (61).
In this research, fusion method was used to prepare the topical safranal liposomes. The fusion method is one of the more suitable methods for the preparation of these liposomes, as it provides homogeneous liposomes. The fusion method is simple, efficient, and reproducible. It is devoid of organic solvents like chloroform; and yields homogeneous liposomes with high encapsulation efficiencies (62).
In our study, liposomes with low encapsulation efficiency were prepared and no crystallization of either formulation was observed during storage. Furthermore, liposomes prepared by this method showed enough viscosity that they could be applied directly on the skin without mixing the liposomal formulation with other bases.
Some studies showed that sunscreen with smaller sizes can have more sun protective effects (63). In this study, topical Lip-Safranal prepared by the fusion method plus homogenization provided liposomes of submicron sizes (Table 2). Analysis of the particle size distribution showed that the average size of most of the population of Lip-Safranal was less than 150 nm (according to the average size by the number;Table 2). Furthermore, the results of the Franz diffusion cell studies across mouse skin showed low percentage of penetration and retention in the skin when the formulations were used (Figure 1), which cannot prove that these vesicles possess the high penetration ability as the safranal is very volatile.
In this research, the water contents of the skin were measured by Corneometer CM 825 0.5, 1, 3 and 5 hr post-application of the Lip-Safranal 1 and 4% and empty liposomes as well as the control without application (Figures 3, 4). Various methods have been summarized by Fluhr et al (64) for measuring the hydration state of the SC (stratum corneum). Common techniques for evaluating moisturizer efficacy are as follows: visual techniques (photography, video microscopy, expert visual grading and subject self-assessment), skin hydration measurement (corneometer), skin barrier function (transepidermal water loss measurement) and skin elasticity studies. Among these tests Corneometer has been used more widely (33).
In our study, there was no significant difference in skin moisture contents between 5 hr after application of Lip-Safranal 1 and 4% and the empty liposomes. For each formulation, there was a significant increase in moisture content after 30 minute. After 1 hr, the moisture contents were decreased in all of the formulations. All the increases in water content after application of the formulations were significantly more than that of the control. The shapes of the curves in all samples were nearly the same.
All the tested formulations significantly increased the moisture content of the skin compared to control, but there was no significant difference in skin moisture content between the groups applying the Lip-Safranal or the emty liposomes. This indicates that the increase in water content is due to the liposome and safranal does not have any remarkable moisturizing effect. The liposomes due to their lipophilic structure and their similarity to SC lipids can improve the moisture content. Liposomes provide their own water content and share the water with the skin. The problems of loss of water migrating from the underlying tissues can be resolved by using the liposomes.
Application of humectants to the skin alone is unsatisfactory, since they are not substantive to the skin; they are water soluble and are readily rinsed off (65).
In a patent filled by Unilever Brothers, they used humectants entrapping liposomes in cosmetic creams. These humectants were glycerin, urea and sodium pyroglutamate. Their results showed that the humectants entrapped liposomes absorb great quantities of water. Some moisturizers are designed to promote water retention by their hygroscopic nature while others are designed to prevent water loss from the skin surface by providing an occlusive film or by supplying SC-like lipids (66). The lack of any remarkable moisturizing effect in safranal may be due to the lack of occlusive properties of safranal.
Conclusion
The results of this study indicated that safranal can be used as a natural UV-absorbing agent. The SPF of Li-Safranal 8% was significantly higher than 8% homosalate reference. There was no statistically significant difference between the skin moisture contents after application of the liposomes containing safranl or empty liposomes.
Acknowledgment
This study was financially supported by the of , Pharmaceutical Research Centre, and Biotechnology Research Centre, Mashhad University of Medical Sciences, and the Ministry of Industries of . The results described in this paper are part of a PharmD thesis.
References
- 1.Mitsui T. New Cosmetic Science. 2nd ed. Amsterdam: Elsevier; 1998. [Google Scholar]
- 2.Wilkinson JB, Moore RJ. Harry’s Cosmeticology. 7th ed. Singapore: Longman Scientific and Technical; 1996. [Google Scholar]
- 3.Dutra EA, Oliveira DAG, KedorHackmann ERM, Santoro MLRM. Determination of sun protection factor (SPF) of sunscreens by ultraviolet spectrophotometry. Brazi J Pharmaceut Sci. 2004;40:381–385. [Google Scholar]
- 4.Chatelain E, Gabard B, Surber C. Skin penetration and sun protection factor of five UV filters: effect of the vehicle. Skin Pharmacol Appl Skin Phys. 2003;16:28–35. doi: 10.1159/000068291. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez C, Marti-Mestres G, Ramos J, Maillols H. LC analysis of benzophenone-3: II application to determination of ldquoin vitrordquo and ldquoin vivordquo skin penetration from solvents, coarse and submicron emulsions. J Pharmaceut Biomed Anal. 2000;24:155–165. doi: 10.1016/s0731-7085(00)00399-x. [DOI] [PubMed] [Google Scholar]
- 6.Golmohammadzadeh Sh, Jaafari MR, Khalili N. Determination of SPF and moisturzing effects of liposomal and conventional formulations of octyl methoxycinnamate as a sunscream. Iran J Basic Med Sci. 2007;10:99–110. [Google Scholar]
- 7.Kerr AC, Niklasson B, Dawe RS, Escoffier AM, Krasteva M, Sanderson B, et al. A double-blind, randomized assessment of the irritant potential of sunscreen chemical dilutions used in photopatch testing. Contact Dermatitis. 2007;60:203–209. doi: 10.1111/j.1600-0536.2009.01516.x. [DOI] [PubMed] [Google Scholar]
- 8.Tabrizi S, Mortazavi SA, Kamalinejad M. An in vitro evaluation of various Rosa damascena flower extracts as a natural antisolar agent. Int J Cos Sci. 2003;25:259–265. doi: 10.1111/j.1467-2494.2003.00189.x. [DOI] [PubMed] [Google Scholar]
- 9.Urbach F. The historical aspects of sunscreens. J Photochem Photobiol B. 2001;64:99–104. doi: 10.1016/s1011-1344(01)00202-0. [DOI] [PubMed] [Google Scholar]
- 10.Elmets CA, Young C. Sunscreens and photocarcinogenesis: An objective assessment. Photochem Photobiol. 1996;63:435–439. doi: 10.1111/j.1751-1097.1996.tb03065.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang ZY, Agarwal R, Bickers DR, Mukhtar H. Protection against ultraviolet B radiation-induced photocarcinogenesis in hairless mice by green tea polyphenols. Carcinogenesis. 1991;12:1527–1532. doi: 10.1093/carcin/12.8.1527. [DOI] [PubMed] [Google Scholar]
- 12.Rancan F, Rosand S, Boehm K, Fernandez E, Hidalgo ME, Quihot W. Protection against UVB irradiation by natural filters extracted from lichens. J Photochem Photobiol B. 2002;68:133–139. doi: 10.1016/s1011-1344(02)00362-7. [DOI] [PubMed] [Google Scholar]
- 13.Khazaeli P, Mehrabani M. Screening of sun protective activity of the ethyl acetate extracts of some medicinal plants. Iran J Pharmaceut Res. 2008;7:5–9. [Google Scholar]
- 14.Zargari A. Medicinal plants. 2nd. ed. Tehran: Tehran University Press; 1990. [Google Scholar]
- 15.Nair SC. Antitumour activity of saffron (Crocus sativus) Cancer Lett. 1991;57:109–114. doi: 10.1016/0304-3835(91)90203-t. [DOI] [PubMed] [Google Scholar]
- 16.Abdullaev FI. Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L) Exp Biol Med. 2002;227:20–25. doi: 10.1177/153537020222700104. [DOI] [PubMed] [Google Scholar]
- 17.Abdullaev FI, Espinosa-Aguirre JJ. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect Prev. 2004;28:426–432. doi: 10.1016/j.cdp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Hosseinzadeh H, Younesi H. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002;2:1–8. doi: 10.1186/1471-2210-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosseinzadeh H, Khosravan V. Anticonvulsant effects of aqueous and ethanolic extracts of Crocus sativus L. Arch Iran Med. 2002;5:44–50. [Google Scholar]
- 20.Karimi G, Hosseinzadeh H, Khaleghpanah P. Study of antidepressant effect of aqueous and ethanolic extract of Crocus sativus in mice. Iran J Basic Med Sci. 2001;4:11–15. [Google Scholar]
- 21.Hosseinzadeh H, Karimi GH, Niapoor M. Antidepressant effects of Crocus sativus stigma extracts and its constituents, crocin and safranal, in mice. Acta Hort. 2004;650:435–445. [Google Scholar]
- 22.Cummings SR, Tripp MK, Hemmann B. Approaches to the prevention and control of skin cancer. Cancer Metastasis Rev. 1997;16:309–327. doi: 10.1023/a:1005804328268. [DOI] [PubMed] [Google Scholar]
- 23.Sheu MT, Lin CW, Huang MC, Shen CH, Ho HO. Correlation of in vivo and in vitro measurements of sun protection factor. Int J Cos Sci. 2003;11:128–132. [Google Scholar]
- 24.Chatelain E, Gabard B, Surber C. Skin penetration and sun protection factor of five UV filters: Effect of the vehicle, Skin Pharmaol. Apply Skin Physiol. 2003;6:28–35. doi: 10.1159/000068291. [DOI] [PubMed] [Google Scholar]
- 25.Jimenez MM, Pelletier J, Bobin MF, Martini MC. Influenceo f encapsulation the in vitro percutaneouasb sorption of octyl methoxycinnamate. Int J Pharm. 2004;272:45–55. doi: 10.1016/j.ijpharm.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 26.kullavanijaya P, Henry W. Photoprotection. J Am Acad Dermatol. 2005;52:937–958. doi: 10.1016/j.jaad.2004.07.063. [DOI] [PubMed] [Google Scholar]
- 27.Golmohammadzadeh Sh, Jaafari MR. Does Saffron Have Antisolar and Moisturizing Effects? Iran J Pharmaceut Res. 2010;9:133–140. [PMC free article] [PubMed] [Google Scholar]
- 28.Foldvari M. Effect of vehicle on topical liposomal drug delivery: petrolatum bases. J Microencapsul. 1996;13:589–600. doi: 10.3109/02652049609026043. [DOI] [PubMed] [Google Scholar]
- 29.Verma DD, Verma S, Blume G, Fahr A. Particle size of liposomes influences dermal delivery of substances into skin. Int J Pharm. 2003;258:141–151. doi: 10.1016/s0378-5173(03)00183-2. [DOI] [PubMed] [Google Scholar]
- 30.Ramon E, Alonso C, Coderch L, Lopez O, Parra JL, et al. Liposomes as alternative vehicles for sun filter formulations. Drug Deliv. 2005;12:83–88. doi: 10.1080/10717540490446080. [DOI] [PubMed] [Google Scholar]
- 31.Gregoriadis G, Kirby C, Large P, Meehan A, Senior J. Targeting of liposomes: study of influence factors. In: Gregoriadis G, Senior J, editors. Targeting of Drugs. New York: Plenium Press; 1981. pp. 155–184. [Google Scholar]
- 32.Golmohammadzadeh Sh, Jaafari MR, Khalili N. Evaluation of liposomal and conventional formulations of octyl methoxycinnamate on human percutaneous absorption using the stripping method. J Cosmet Sci. 2008;59:385–398. [PubMed] [Google Scholar]
- 33.Rawlings AV, Canestrari DA, Dobkoweski B. Moisturizer technology versus clinical performance. Dermatol Ther. 2004;17:49–56. doi: 10.1111/j.1396-0296.2004.04s1006.x. [DOI] [PubMed] [Google Scholar]
- 34.Sunscreen Products, Evaluation and Classification. AS/NZS. Australian/New Zealand Standard; 1998. pp. 1–32. [Google Scholar]
- 35.Food and Drug Administration. Sunscreen drug products for over the counter human use: final monograph. Federal Register, US. 1999:27666–27693. [PubMed] [Google Scholar]
- 36.Golmohammadzadeh Sh, Jaafari MR, Khalili N. Determination of SPF and moisturzing effects of liposomal and conventional formulations of octyl methoxycinnamate as a sunscream. Iran J Basic Med Sci. 2007;10:99–110. [Google Scholar]
- 37.Maestrelli F, Rabasco AM, Ghelardini C, Mura P. New ldquodrug-in cyclodextrin-in deformable liposomesrdquo formulations to improve the therapeutic efficacy of local anaesthetics. Int J Pharm. 2010;395:222–231. doi: 10.1016/j.ijpharm.2010.05.046. [DOI] [PubMed] [Google Scholar]
- 38.Mura P, Maestrelli F, Gonzalez-Rodrıguez ML, Michelacci I, Rabasco AM. Development, characterization and in vivo evaluation of benzocaine-loaded liposomes. Eur J Pharm Biopharm. 2007;67:86–95. doi: 10.1016/j.ejpb.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 39.Dutra EA, Oliveira DAG, Kedor-Hackmann ERM, Santoro MLRM. Determination of sun protection factor (SPF) of sunscreens by ultraviolet spectrophotometry. Brazi J Pharmaceut Sci. 2004;40:381–385. [Google Scholar]
- 40.Sayre RM. A comparison of in vivo and in vitro testing of sunscreening formulas. Photochem Photobiol. 1979;29:559–566. doi: 10.1111/j.1751-1097.1979.tb07090.x. [DOI] [PubMed] [Google Scholar]
- 41.Diffey BL, Robson J. A new substrate to measure sunscreen protection factors throughout the ultraviolet spectrum. J Cosmet Sci. 1989;40:127–133. [Google Scholar]
- 42.Gomaa YA, El-Khordagui LK, Darwish IA. Chitosan microparticles incorporating a hydrophilic sunscreen agent. Carbohyd Polym. 2010;81:234–242. [Google Scholar]
- 43.Sato PG, Schmidt JB, Honigsmann H. Comparison of epidermal hydration and skin surface lipids in healthy individuals and in patients with atopic dermatitis. J Am Acad Dermatol. 2003;81:352–358. doi: 10.1067/mjd.2003.105. [DOI] [PubMed] [Google Scholar]
- 44.Alanen E, Nuutinen J, Niclen K, Lahtinen T, Monkkonen J. Measurement of hydration in the stratum corneum with the MoistureMeter and comparison with the Corneometer. Skin Res Technol. 2004;10:32–37. doi: 10.1111/j.1600-0846.2004.00050.x. [DOI] [PubMed] [Google Scholar]
- 45.Dykes PJ. What are meters measuring? Int J Cosmet Sci. 2002;24:241–245. doi: 10.1046/j.1467-2494.2002.00146.x. [DOI] [PubMed] [Google Scholar]
- 46.Fluhr JW, Gloor M, Lazzerini S, Kleesz P, Grieshaber R, Berardesca E. Comparative study of five instruments measuring stratum corneum hydration (Corneometer CM 820 and CM 825, Skicon 200, Nova DPM 9003, Dermalab). Part I. In vitro. Skin Res Technol. 1999;5:161–170. [Google Scholar]
- 47.Jaafari MR, Bavarsad N, Samiei A, Soroush D, Ghorbani S, et al. Effect of Topical Liposomes Containing Paromomycin Sulfate in the Course of Leishmania major Infection in Susceptible BALB/c Mice. Antimicrob Agents Chemother. 2009:2259–2265. doi: 10.1128/AAC.01319-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brain KFK, Walters A, Watkinson AC. Methods for studying percutaneous absorption. In: Walters KA, editor. Dermatological and transdermal formulation. New York. NY: Marcel Dekker Inc; 2002. pp. 197–269. [Google Scholar]
- 49.Khan GM, Frum Y, Sarheed O, Eccleston GM, Meidan VM. Assessment of drug permeability distributions in two different model skins. Int J Pharm. 2005;303:81–87. doi: 10.1016/j.ijpharm.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Antoniou C, Kosmadaki MG, Stratigos AG, Katsambas AD. Sunscreens-whatrsquos important to know? J Eur Acad Dermatol Venereol. 2008;22:1110–1119. doi: 10.1111/j.1468-3083.2008.02580.x. [DOI] [PubMed] [Google Scholar]
- 51.Sheu MT, Lin CW, Huang MC, Shen CH, Ho HO. Correlation of in vivo and in vitro measurements of sun protection factor. Int J Cosmet Sci. 2003;11:128–132. [Google Scholar]
- 52.Velasco MVR, Sarruf FD, Salgado-Santos IMN, Haroutiounian-Filho CA, Kaneko TM, Baby AR. Broad spectrum bioactive sunscreens. Int J Pharmaceutic. 2008;363:50–57. doi: 10.1016/j.ijpharm.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 53.Harish M, Patel M, Moghimi M. Liposomes and the skin Permeability barrier. In: Gregoriadis G, Florence AT, Patel HM, editors. Liposomes in Drug Delivery. Switzerland. Switzerland: Harwood Academic Publishers GmbH; 1993. pp. 37–147. [Google Scholar]
- 54.Svobodova A, Psotova J, Walterova D. Natural phenolic in the prevention of UV-induced skin damage, a review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2003;147:137–145. [PubMed] [Google Scholar]
- 55.Rolim A, Maciel CPM, Kaneko TM, Consiglieri VO, Salgado-Santos IMN, Velasco MVR. Validation assay for total flavonoids (as rutin equivalents) from Trichilia catigua Adr. Juss (Meliaceae) (and) Ptychopetalum olacoides Bentham (Olacaceae) commercial extract. J AOAC Int. 2005;88:1015–1019. [PubMed] [Google Scholar]
- 56.Singh UP, Singh DP, Maurya S, Maheshwari R, Singh M, Dubey RS, Singh RB. Investigation on the phenolics of some spices having pharmacotherapeutic properties. J Herb Pharmacother. 2004;4:27–42. [PubMed] [Google Scholar]
- 57.Liu MC, Lin CT, Shau MD, Chen ZS, Chen MT. Studies on natural ultraviolet absorbers. J Food Drug Anal. 1996;4:243–248. [Google Scholar]
- 58.Fernandez JA. Anticancer properties of saffron, Crocus sativus Linn. In: Khan MT, Ather A, editors. Lead Molecules from Natural Products. New York. New York: Elsevier; 2006. pp. 313–330. [Google Scholar]
- 59.Treffel P, Gabard B. Skin penetration and sun protection factor of ultra-violet filters from two vehicles. Pharm Res. 1996;13:770–774. doi: 10.1023/a:1016012019483. [DOI] [PubMed] [Google Scholar]
- 60.Sood A, Venugopalan P, Venkatesan N, Vyas SP. Liposomes in cosmetics and skin care. Ind Drugs. 1995;33:43–49. [Google Scholar]
- 61.Gregoriadis G, Large CKP, Meehan A, Senior J. Targeting of liposomes: study of influence factors. In: Gregoriadis G, Senior J, editors. Targeting of drugs In. New York: Plenum Press; 1981. pp. 155–184. [Google Scholar]
- 62.Foldvari M, inventor. Biphasic multilamellar lipid vesicles. 5,853,755. U.S. patent. 1998
- 63.Wissing SA, Muller RH. Solid lipid nanoparticles as carrier for sunscreens: in vitro release and in vivo skin penetration. J Control Rel. 2002;81:225–233. doi: 10.1016/s0168-3659(02)00056-1. [DOI] [PubMed] [Google Scholar]
- 64.Fluhr JW, Gloor M, Lazzerini S, Kleesz P, Grieshaber R, Berardesca E. Comparative study of five instruments measuring stratum corneum hydration (Corneometer CM 820 and CM 825, Skicon 200, Nova DPM 9003, Dermalab). Part I. In vitro. Skin Res Technol. 1999;5:161–170. [Google Scholar]
- 65.Straianse SJ. Human skin-moisturizing mechanism and natural moisturizers. Cosmet Toiletries. 1978;93:37–41. [Google Scholar]
- 66.Shaku M, Kuroda H, Okura A. Enhancing stratum corneum functions. Cosmet Toiletries. 1997;112:65–76. [Google Scholar]