Abstract
Objective(s)
Production of extended-spectrum beta-lactamases (ESBLs) by enteric bacteria continues to be a major problem in hospitals and community. ESBLs producing bacteria cause many serious infections including urinary tract infections, peritonitis, cholangitis and intra-abdominal abscess. The aim of this study was to determine the prevalence of ESBLs producing Escherichia coli and Klebsiella pneumoniae bacteria isolated from clinical samples of patients attending Imam Reza and Ghaem University Hospitals, Mashhad, Northeast of Iran.
Materials and Methods
During 2009 and 2010, 82 strains of E. coli and 78 strains of K. pneumoniae were isolated from out-patients and hospitalized patients and they were examined by Oxoid combination disk test and PCR methods.
Results
We found that 43.9% of E. coli and 56.1% of K. pneumoniae produced ESBLs. The frequency of SHV and TEM among the ESBLs producing isolates were 14.4% and 20.6%, respectively. Ratios of ESBLs positive isolates from out-patients to hospitalized patients were 24/33.
Conclusion
This study shows that the prevalence of ESBLs producing E. coli and K. pneumoniae is high in both study groups (out-patients and hospitalized patients). Therefore it seems that continuous surveillance is essential to monitor the ESBLs producing microorganisms in hospitals and community.
Key Words: Escherichia coli, Extended spectrum beta-lactamase (ESBL), Klebsiella pneumonia, PCR, Prevalence, SHV gene, TEM gene
Introduction
Beta-lactam antibiotics are the most common drugs of any application in the treatment of bacterial infections (1). The beta-lactam antibiotics have been widely used since 1980 for the treatment of serious infections caused by gram-negative bacteria, but resistance against these antibiotic groups occurred quickly worldwide (2, 3).
Production of beta-lactamase enzymes is the main mechanism of bacterial resistance against various antibiotics of this class (4). More than 200 different types of extended spectrum beta-lactamases (ESBLs) have been reported around the world so far; they were often identified in Enterobacteriaceae family. Klebsiella pneumoniae is the most common bacterial production of ESBLs; similarly, Escherichia coli is the other very important microorganism. ESBLs productions are rarely observed among other bacteria (2, 3).
ESBLs are often plasmid mediated and most of the enzymes are members of TEM and SHV families (5, 6) that have been described in many countries (2, 3,7,8). The TEM was first reported in E. coli isolated from a patient named Temoniera in (9). The name of the other beta-lactamase, SHV, is due to sulf-hydryl variable active site (9).
ESBLs producing bacteria have been reported increasingly worldwide. They cause many clinical diseases including urinary tract infections, peritonitis, cholangitis and intra-abdominal abscess (10-12). Treatment of the infections caused by these organisms is a major challenge for health care facilities and preventive strategies. Since ESBLs are identified most commonly in E. coli and K. pneumoniae, we studied the prevalence of ESBLs producing in these bacteria isolated from out-patients and hospitalized patients at Imam Reza and Ghaem University Hospitals in Mashhad, Northeast of Iran.
Materials and Methods
This study was conducted at Mashhad University of Medical Sciences, Northeast of Mashhad, Iran. Totally, 160 isolates of E. coli and K. pneumoniae bacteria were selected from out-patients and hospitalized patients of Imam Reza and Ghaem University Hospitals during 2009 and 2010. Different clinical samples including urine, blood, wound and abscess aspirates, peritonitis and pulmonary secretions were processed in this study.
Phenotypic confirmatory test (PCT)
For ESBL assay, bacterial suspensions with concentration of 1.5×108 cfu/ml (0.5 McFarland standard) were prepared in nutrient broth. Oxoid combination disk method was used for detection of ESBLs producing organisms. In this method the bacteria were cultured on a Muller-Hinton agar plate, then cefotaxim (30 µg), cefotaxim/clavulanate (10 µg), ceftazidime (30 µg) and ceftazidime/clavulanate (10 µg) disks (Mast, UK) were placed on media in 20-30 mm with other disks. The plates were incubated for 18-24 hrs at 37 °C. ESBLs producing organisms were detected by an at least 5 mm increasing of zone around cefotaxim/clavulanate and ceftazidime/clavulanate at least 5 mm.
DNA extraction, PCR and sequencing
The colonies of ESBLs producing organisms were suspended in TE buffer and their DNA were extracted by simple boiling (13).
The PCR method for detection of SHV and TEM genes was performed as described previously with minor modifications (14-15); briefly, specific primers for the genes (forward primer 5´-TCAGCGAAAAACACCTTG -3´; Reverse primer 5´-CCCGCAGATAAATCACCA -3´ for SHV gene and forward primer 5´-GAGTATTCAACATTTCCGTGTC -3´; Reverse primer 5´-TAATCAGTGAGGCACCTATCTC -3´ for TEM gene) were used for PCR amplification that produced 471 bp and 861 bp PCR products for SHV and TEM genes, respectively. The PCR mixture consisted of 10 pmol of each primers, 1 μl DNA sample (3 μg/μl), 1.5 mM MgCl2, 0.2 mM each dNTP, and 5 u Taq DNA polymerase (Cinagen, Iran) in a total number of 50 μl of PCR reaction. Amplification of TEM and SHV genes was performed by following program: initial denaturation at 94 °C for 2 min and 35 cycles of 1 min at 94 °C, 30 sec at 52 °C and 1 min at 72 °C. Five min at 72 °C was considered for the final extension. Then, PCR products were analyzed by agarose gels electrophoresis. Eight PCR products from different types of the samples were sent for sequencing to Microgene Company, .
Results
Among all the samples, urine samples were the most positive ones (n= 133, 1.83%). In this study, a total number of 160 bacteria isolates (E. coli n=82, K. pneumoniae n= 78) were collected; of these 57 isolates (35.6%) were ESBLs producing organisms (Fig 1, and Table 1). Our results showed that 25 (15.62%) of isolated E. coli and 32 (20%) of isolated K. pneumoniae were ESBLs producing organisms.
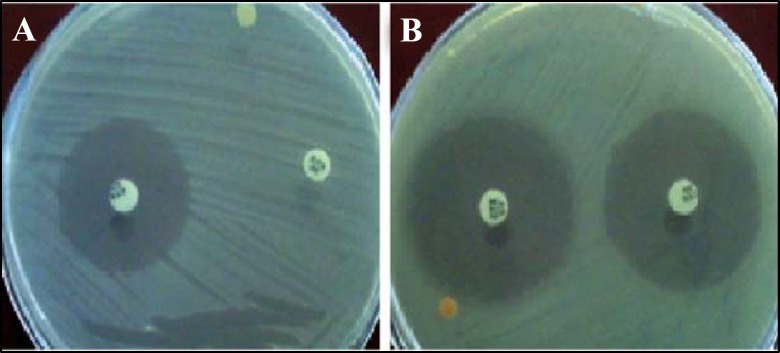
Figure 1.
Oxoid combination disk method results. The ESBLs producing (A) and not producing (B) organisms were differentiated byta increasing of growth inhibition zone only around the cefotaxim/clavulanate (or ceftazidime/clavulanate) for at least 5 mm in ESBLs producing ones
Table 1.
The prevalence of ESBLs producing Escherichia coli and Klebsiella pneumoniae by disk diffusion method
| Bacteria | ESBLs - positive |
ESBLs-negative |
total |
|
||
|---|---|---|---|---|---|---|
| Number | % | Number | % | Number | % | |
| E. coli | 25 | 15.625 | 57 | 35.625 | 82 | 51.25 |
| K. pneumoniae | 32 | 20 | 46 | 28.75 | 78 | 48.75 |
| Total | 57 | 35.625 | 103 | 64.375 | 160 | 100 |
The distribution of ESBLs producing organisms based on out-patients and hospitalized patients are shown in Table 2. The obtained data showed the rate of ESBLs producing organisms in hospitalized patients were higher than out-patients but their differences was not statistically significant (P-value= 0.137).
Table 2.
Prevalence of ESBLs producing bacteria by disk diffusion method in out- patients and hospitalized patients
| Patients | ESBLs- positive |
ESBLs-negative |
Total |
|||
|---|---|---|---|---|---|---|
| Number | % | Number | % | Number | % | |
| Hospitalized patients | 33 | 57.897 | 47 | 45.63 | 80 | 50 |
| Out-patients | 24 | 42.103 | 56 | 54.37 | 80 | 50 |
| Total | 57 | 100 | 103 | 100 | 160 | 100 |
In PCR method the distribution of TEM and SHV genes in isolated ESBLs producing organisms were 33 (20.6%) and 24 (14.4%), respectively (Figure 2, Table 3).
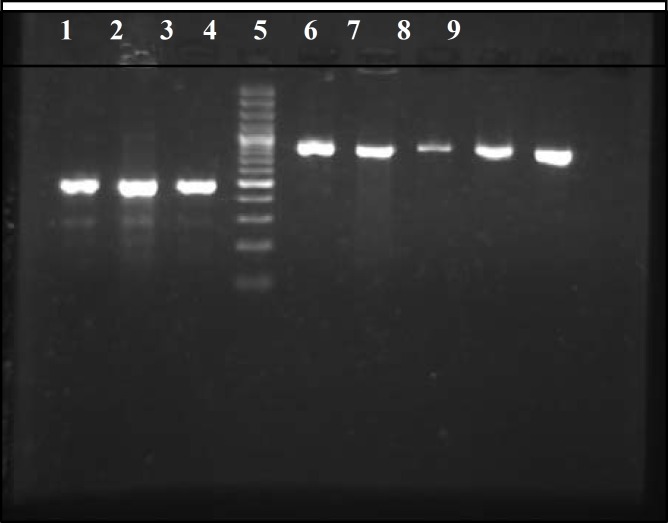
Figure 2.
PCR results for SHV and TEM genes. Lane numbers 1, 2 and 3 are showed a 471 bp fragment of SHV gene. Lane numbers 5-9 are showed a 861 bp fragment of TEM gene and lane 4 is showed the 100 bp DNA size marker
Table 3.
The prevalence of TEM and SHV genes in ESBLs producing organisms in hospitalized patients and out-patients
|
|
Klebsiella pneumoniae
|
Escherichia coli
|
Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TEM positive | SHV positive | TEM and SHV positive | TEM and SHV negative | TEM positive | SHV positive | TEM and SHV positive | TEM and SHV negative | ||
| Hospitalized patients | 5 | 0 | 0 | 9 | 2 | 4 | 11 | 2 | 33 |
| Out-patients | 6 | 0 | 0 | 5 | 3 | 2 | 6 | 2 | 24 |
| Total | 11 | 0 | 0 | 14 | 5 | 6 | 17 | 4 | 57 |
Eight PCR products from different kinds of samples were sequenced and reported as Iranian strains in Gen Bank (Accession Numbers: GU338982, GU338983, GU338984, GU338985, GU338986, GU338979, GU338980 and GU338981). The results obtained from sequencing was compared with the published sequences in Gen Bank and it showed that there was 99-100% (for SHV beta-lactamase gene) and 100% (for TEM-1b beta-lactamase gene) homology between our PCR products and those mentioned in Gen Bank.
Discussion
Resistance to beta-lactam antibiotics of Gram- negative bacteria isolated from clinical samples has been increased worldwide (16). The aim of this study was to evaluate the prevalence of ESBL producing E. coli and K. pneumoniae and to detect the presence of TEM and SHV genes among ESBL producing isolates. Based on the results of this study, the prevalence of ESBL producing E. coli and K. pneumoniae was high (15.62% and 20%, respectively) and a total number of about 68.5% of ESBL producing isolated bacteria were TEM and/or SHV positive. A number of previous studies have showed the high prevalence of ESBLs producing E. coli and K. pneumoniae. In , the prevalence of ESBLs producing E. coli and K. pneumoniae isolates varies in different countries. In , the prevalence of the organisms was reported 46.51% in E. coli and 44.44% in K. pneumoniae isolates (17). The prevalence of the organisms in was in the range of 8.5% to 29.8% in K. pneumoniae and 1.5% to 16.7% in E. coli (18). In a study in , the prevalence of the ESBLs producing organisms in children was reported 27% in E. coli and 64% in K. pneumoniae isolates (19). In Korea, the prevalence of these organisms was in the range of 4.8%-7.5% and 22.5%-22.8% for E. coli and K. pneumoniae, respectively (20). In , the prevalence of the ESBLs producing E. coli and K. pneumoniae was reported 41% in E. coli and 36% in K. pneumoniae isolates (21). In another study in , the prevalence of the ESBLs producing E. coli isolates was reported 56.9% (22).
Reported ESBLs producing rates in E. coli and K. pneumoniae isolates from various parts of Iran varies from 8.9% to 67% in E. coli and 20.3% to 52% in K. pneumoniae isolates (5, 23-27). In our study, the prevalence of ESBLs producing E. coli and K. pneumoniae was 15.6% and 20%, respectively.
In a study that was carried out by Fazly Bazzaz et al in 2007 in Mashhad, the prevalence of ESBLs producing E. coli and K. pneumoniae was reported 57.5% and 61% and generally the prevalence of ESBLs producing organisms were 59.2% by Kirby-Baure disk diffusion method and the phenotypic disk confirmatory test. They also showed that the ratios of ESBLs producing isolates from hospitalized patients to out-patients were 94 to 28 (23). In our study, the prevalence of ESBLs producing organisms was lower in comparison with the reported prevalence by their study (23). Also the ratios of ESBLs producing organisms in hospitalized patients to out-patients were 57.9 to 42.1 that were different from a previous study which suggested that although most of the resistance to antibacterial agents is associated with admission to hospitals, ESBLs producing bacteria may have both community and hospital acquired sources. The ESBLs producing organisms was higher in K. pneumoniae compared to E. coli isolates in both studies.
In another study that was performed by Kalematizadeh in 2008 in , the prevalence of ESBLs K. pneumoniae was 43% (44% in hospitalized and 18% in out- patients) (7). In Isfahan, Dr Rastegar Lari et al showed 51% of isolated E. coli and 70% of isolated K. pneumoniae were ESBLs producing bacteria (28). Their results showed different prevalence of ESBLs producing bacteria compared with our finding. Therefore the pattern of ESBLs producing bacteria varies in different parts of Iran and separate studies of the ESBLs producing bacteria is necessary in various parts to estimate the antibiotic resistance correctly for taking steps for reducing these resistances.
Our molecular study revealed the ESBLs producing organisms contained TEM (20.6%), and SHV (14.4%) genes by PCR. The TEM gene has high frequency compared to SHV gene; a fact which is similar to previous studies (8, 29) but it was different compared to Taşli et al and Ramazanzadeh's results (30, 31). Also, in our study, the SHV gene was not found in ESBLs producing E. coli isolates. Therefore the distribution pattern of TEM and SHV genes in isolated E. coli and K. pneumoniae is also different in various parts of Iran.
In the Rastegar Lari study, ESBLs producing E. coli had 85.6% TEM, 69.2% SHV and 53.8% in both genes that was much higher than in our results (28). In another study in , the ESBLs producing organisms (E. coli and K. pneumoniae) were also positive for SHV (52.7%) and TEM (32.4%) genes (30); and the frequency of SHV gene was higher than TEM gene that was different from our results. Some ESBLs producing bacteria showed negative results in PCR method for SHV and TEM genes based on our study, therefore other beta-lactamases genes may be involved in ESBLs resistance. However, further studies are required for finding the other genes in ESBLs producing E. coli and K. pneumoniae bacteria.
Conclusion
In short, the prevalence of ESBLs producing organisms in Mashhad is high. It seems necessary for clinicians and health care systems to be fully aware of ESBLs producing microorganisms. Also, the ESBLs production monitoring is recommended to avoid treatment failure and suitable infection control in Iran.
Acknowledgment
This study was supported by Mashhad University of Medical Sciences, Mashhad, Iran (grant No. 87277).
References
- 1.Kotra L, Samama J, Mobashery S. Beta-lactamases and resistance to beta-lactam antibiotics. Bacterial resistance to antimicrobials. NewYork: Marcel Decker; 2002. pp. 123–160. [Google Scholar]
- 2.Bradford PA. Extended spectrum beta-lactamases in the 21st century:characterization, epidemiology and the detection of this important resistance threat. Clin Microbiol Rev. 2001;14:933–951. doi: 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim YK, Pai H, Lee HJ, Park SE, Choi EH, Kim J, et al. Bloodstream infections by extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in children: epidemiology and clinical outcome. Antimicrob Agents Chemother . 2002;46:1481–1491. doi: 10.1128/AAC.46.5.1481-1491.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Livermore DM. Beta-lactamases in laboratory and clinical resistance. Clin Microbiol Rev. 1995;8:557–584. doi: 10.1128/cmr.8.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behrooozi A, Rahbar M, Vand YousefiJ. Frequency of extended spectrum beta-lactamase (ESBLs) producing Escherichia coli and Klebseilla pneumoniae isolated from urine in an Iranian 1000-bed tertiary care hospital. Afr J Microbiol Res . 2010;4:881–884. [Google Scholar]
- 6.Ramazanzadeh R, Farhadifar F, Mansouri M. Etiology and antibiotic resistance pattern of community-acquired extended-spectrum beta-lactamas-producing gram negative isolates in Sanandaj. Res J Med Sci. 2010;4:243–247. [Google Scholar]
- 7.Kalematizadeh E. Determination of extended-spectrum beta-lactamases bacteria in Escherichia coli and Klebsiella pneumoniae. Mashhad: Mashhad University of Medical Sciences; 2008. [Google Scholar]
- 8.Herna´ndez JR, Martı´nez-Martı´nez L, Canto´n R, Coque TM, Pascual A. Nationwide study of escherichia coli and Klebsiella pneumoniae producing extended-spectrum -lactamases in Spain. Antimicrob Agents Chemother . 2005;49:2122–2125. doi: 10.1128/AAC.49.5.2122-2125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mansouri M, Ramazanzadeh R. Spread of extended-spectrum beta-lactamase producing Esherichia coli clinical isolates in Sanandaj hospitals. J Biol Sci . 2009;9:362–366. [Google Scholar]
- 10.Gniadkowski M. Evolution and epidemiology of extended-spectrum beta-lactamases (ESBLs) and ESBL-producing microorganisms. Clin Microbiol Infect . 2001;7:597–608. doi: 10.1046/j.1198-743x.2001.00330.x. [DOI] [PubMed] [Google Scholar]
- 11.Schiappa DA, Hayden MK, Matushek MG, Hashemi FN, Sullivan J, Smith KY, et al. Ceftazidime-resistant Klebsiella pneumoniae and Escherichia coli bloodstream infection: a case-control and molecular epidemiologic investigation. J Infect Dis. 1996;174:529–536. doi: 10.1093/infdis/174.3.529. [DOI] [PubMed] [Google Scholar]
- 12.Alcantar-Curiel D, Tinoco JC, Gayosso C, Carlos A, Daza C, Perez-Prado MC, et al. Nosocomial bacteremia and urinary tract infections caused by extended-spectrum beta -lactamase-producing Klebsiella pneumoniae with plasmids carrying both SHV-5 and TLA-1 genes. Clin Infect Dis . 2004;38:1067–1074. doi: 10.1086/382354. [DOI] [PubMed] [Google Scholar]
- 13.Sambrook J, Russell DW. Molecular cloning. 3rd ed. New York (NY): Cold Spring Harbor; 2001. [Google Scholar]
- 14.M'Zali FH, Gascoyne-Binzi DM, Heritage J, Hawkey PM. Detection of mutations conferring extended-spectrum activity on SHV beta-lactamases using polymerase chain reaction single strand conformational polymorphism (PCR-SSCP) J Antimicrob Chemother. 1996;37:797–802. doi: 10.1093/jac/37.4.797. [DOI] [PubMed] [Google Scholar]
- 15.Shahcheragh iF, Nasiri S, Noveiri H. The Survey of Genes Encoding Beta-Lactamases, in Escherichia coli resistant to beta-lactam and non-beta-lactam antibiotics. Iran J Basic Med Sci. 2010;13:230–237. [Google Scholar]
- 16.Goossens H. MYSTIC (Meropenem Yearly Susceptibility Test Information Collection) results from Europe: comparison of antibiotic susceptibilities between countries and centre types. J Antimicrob Chemother . 2000;46:39–52. [PubMed] [Google Scholar]
- 17.Varaiya A, Dogra J, Kulkarni M, Bhalekar P. Extended spectrum beta lactamase (ESBL) producing Escherichia coli and Klebsiella pneumoniae n diabetic foot infection. Indian J Med Microbiol. 2008;26:281–282. doi: 10.4103/0255-0857.42056. [DOI] [PubMed] [Google Scholar]
- 18.Yu WL, Chuang YC, Walther-Rasmussen J. Extendedspectrum β-lactamases in Taiwan: epidemiology, detection, treatment and infection control. J Microbiol Immunol Infect . 2006;39:264–277. [PubMed] [Google Scholar]
- 19.Chaikittisuk N, Munsrichoom A. Extended-spectrum β-Lactamase-producing escherichia coli and Klebsiella pneumoniae in children at Queen Sirikit National Institute of Child Health. J Infect Dis Antimicrob Agents . 2007;24:107–115. [Google Scholar]
- 20.Pai H. The characteristics of extended-spectrum β- lactamases in Korean isolates of Enterobactericeae. Yonsei Med J . 1998;39:514–519. doi: 10.3349/ymj.1998.39.6.514. [DOI] [PubMed] [Google Scholar]
- 21.Jabeen K, Zafar A, Hasan R. Frequency and sensitivity pattern of extended-spectrum β-lactamase producing isolates in a tertiary care hospital laboratory of Pakistan. J Pak Med Assoc. 2005;55:436–439. [PubMed] [Google Scholar]
- 22.Ullah F, Malik SA, Ahmed J. Antibiotic susceptibility pattern and ESBLS prevallence in nosocomial Escherichia coli from urinary tract infections in Pakistan. Afr J Biotechnol. 2009;8:3921–3926. [Google Scholar]
- 23.Fazly BazzazBS, Naderinasab M, Mohamadpoor AH, Farshadzadeh Z, Ahmadi S, Yousefi F. The prevalence of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae among clinical isolates from a general hospital in Iran. Acta Microbiol Immunol Hung. 2009;56:89–99. doi: 10.1556/AMicr.56.2009.1.7. [DOI] [PubMed] [Google Scholar]
- 24.Aminzadeh Z, Sadat KM, Sha'bani M. Bacteriuria by extendedspectrum Beta-lactamase-producing E. coli and Klebsiella pneumoniae: isolates in a governmental hospital in South of Tehran, Iran. Iran J Kidney Dis . 2008;2:197–200. [PubMed] [Google Scholar]
- 25.Feizabadi MM, Etemadi G, Yadegarinia D, Rahmati M, Shabanpoor S, Bokaei S. Antibiotic-resistance patterns and frequency of extended-spectrum beta-lactamase-producing isolates of Klebsiella pneumoniae in Tehran. Med Sci Monit . 2006;12:BR362–365. [PubMed] [Google Scholar]
- 26.Mehrgan H, Rahbar M. Prevalence of extended-spectrum betalactamase- producing Escherichia coli in a tertiary care hospital in Tehran, Iran. Int J Antimicrob Agents . 2008;31:1471–1451. doi: 10.1016/j.ijantimicag.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Bell JM, Chitsaz M, Turnidge JD, Barton M, Walters LJ, Jones RN. Prevalence and significance of a negative extended-spectrum-lactamase (ESBL) confirmation test result after a positive ESBL screening test result for isolates of Escherichia coli and Klebsiella pneumoniae: Results from the SENTRY Asia-Pacific surveillance program. J Clin Microbiol . 2007;45:1478–1482. doi: 10.1128/JCM.02470-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masjedian JaziF, Valehi F, Talebi A, Rastegar LariA. Molecular characterization of resistance to Extended - Spectrum antibiotics in Escherichia coli and Klebsiella pneumoniae. Iran J Med Microbiol. 2007;2:27–34. (In Persian) [Google Scholar]
- 29.Shahcheragh F, Nasiri S, Noveiri H. Detection of extended-spectrum β-lactamases (ESBLs) in Escherichia coli. Iran J Clin Infect Dis . 2009;4:63–70. [Google Scholar]
- 30.Taşli H, Bahar IH. Molecular characterization of TEM- and SHV-derived extended-spectrum beta-lactamases in hospital-based Enterobacteriaceae in Turkey. Jpn J Infect Dis . 2005;58:162–167. [PubMed] [Google Scholar]
- 31.Ramazanzadeh R. Prevalence and characterization of extended-spectrum beta-lactamase production in clinical isolates of Klebsiella spp. Afr J Microbiol Res . 2010;4:1359–1362. [Google Scholar]