Abstract
Objective(s)
Varicocele is associated with impaired testicular function and male infertility, but the molecular mechanisms by which fertility is affected have not been satisfactorily explained. The aim of our study was to investigate whether or not the polymerase gamma (POLG) polymorphism is associated with Iranian varicocele patients.
Materials and Methods
We determined the POLG CAG repeat length in DNA samples extracted from 40 varicocele patients and 30 control subjects by PCR-denature polyacrylamide gel electrophoresis and sequencing.
Results
The distribution of the CAG repeat length in varicocele patients showed no notable difference from that in control subjects, but we found a significant statistical inverse correlation between 10/10 and 10/#10 genotypes and varicocele grade.
Conclusion
These findings indicate that POLG CAG repeats may affects the varicocele grade, but the mechanisms remain to be elucidated.
Key Words: CAG repeats length, Infertility, Mitochondria, POLG gene, Varicocele
Introduction
The varicocele is the most common cause of male infertility worldwide. Varicoceles have been found in 15% of the normal male population and in up to 40% of patients with male infertility (1, 2). In approximately 70% of patients with secondary infertility, a varicocele is an underlying cause (3). The theory behind the reason why varicocele results in impaired spermatogenesis includes oxygen deprivation, poor venous drainage that leads to impaired drainage of gonadotoxins from the testis, and increased oxidants within the semen (4). Allamaneni and colleagues reported a positive correlation between seminal reactive oxygen species (ROS) or ‘‘oxidant’’ levels and varicocele grade (5). They demonstrated that higher seminal ROS levels are seen in men with grade II and III varicoceles compared with men with grade I varicoceles. The mitochondrial oxidative stress is thought to be involved in the pathogenesis of this disease (5, 6). Talebi et al showed that the varicocele samples contain a higher proportion of spermatozoa with abnormal DNA and immature chromatin than those from fertile men (7). Understanding the pathophysiology, treatments, and outcomes of a varicocele and varicocele repair has evolved significantly over the several past decades.
The nuclear catalytic subunit of polymerase gamma (POLG) is the only polymerase in humans that is known to be located in mitochondria and has been proposed as the replicase of mitochondrial DNA (8). In its coding region lies a short polyglutamine tract, which is absent in POLG of mouse or Drosophila (9, 10). Microsatellite repeats have been shown to evolve in length during the course of primate evolution and show considerable variation between individuals. Alterations in the CAG repeat region have been found to be associated with idiopathic sporadic Parkinson (11) and Friedreich ataxia (12).
Many repeat length alleles different from 10 (ranging from 6–15) have been found and are considered mutants (13). Ten copies of CAG repeat were found to be uniformly high (0.88) in different ethnic groups (14), hence are considered as the common allele whereas the mutant alleles (other than 10 ⁄ 10 CAG repeats) were found to be associated with oligospermia male infertility (15). The authors hypothesized that the presence of mutated alleles would lead to a suboptimal mtDNA polymerase resulting in the accumulation of mutations in the mtDNA that would cause impaired energy metabolism of the spermatogenic cells and finally bring about a disturbance of sperm production and/or differentiation. The aim of the present study was to assess whether the POLG polymorphism are associated with Iranian varicocele-associated infertility patients.
Materials and Methods
Patients
The study group included forty Iranian infertile male patients with varicocele. The varicocele diagnosis was made, by the same urologist, for the patients in standing position and via scrotal palpation in a temperature controlled room (23 °C). Semen analysis was performed according to WHO criteria (16). Thirty healthy donors with proven fertility who had a successful pregnancy within the last 12 months and normal spermogram at the time of study were selected as control group. All of the patients and control group were informed of the aims of the study and gave their informed consents to the genetic analysis. The institutional review board at Yazd University of Medical Sciences approved this prospective study.
The amplification of the POLG gene and Sequencing
DNA was isolated from peripheral blood samples using a DNA extraction kit (Sinaclone, Tehran, Iran). The CAG-repeat region of the POLG gene was amplified by PCR with two primers (5'-GGTCCCTGCACCAACCATGA-3' and 5'-CTTGCCCGAAGATTTGCTCGT-3') that was published previously (17). PCR of each sample was set in a 0.5 ml tube using 100 mg of total DNA, 10 pmol of each primer, 200 mmol of dNTPs, 1X PCR buffer containing 2.5 mmol MgCl2 and 1 U Taq DNA polymerase (Roche Diagnostics, Mannheim, Germany). Cycling condition used for the amplification was as follows: initial denaturation at 94 °C for 5 min followed by 30 cycles of denaturation for 1 min at 94 °C, annealing for 1 min at 60 °C and extension for 1.5 min at 72 °C (final extension for 10 min).
PCR products were resolved on 1-mm thick, 8% polyacrylamide gels by electrophoresis under denaturing conditions. Gels were stained with silver to reveal the POLG CAG repeat length (Figure 1). Any DNA fragments showing differences in banding patterns between the control and patient were sequenced to identify the exact mutations. The nucleotide sequence of the amplicon was directly determined by automated sequencing 3700 ABI machine (Macrogene Seoul, South Korea).
Figure 1.
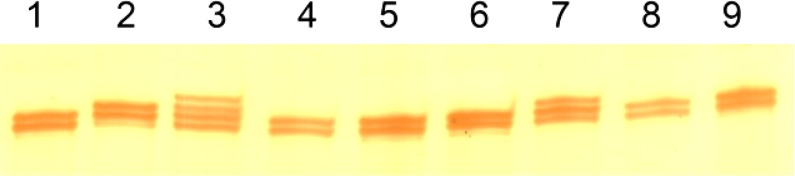
Genotyping of POLG CAG repeat by 8% denature polyacrylamide gel in patients. Lane 1, 4-6, 8 and 9: 10/10; lane 2 and 7: 10/11; lane 3: 10/12.
Statistical analysis
The t-test was applied to determine whether the frequencies of different genotypes in varicocele patients and control groups were significantly different. Values of P< 0.05 were regarded as statistically significant. Data were expressed in mean±SD. The statistical analysis was performed using the GraphPad Prism software (GraphPad Software, Inc. USA).
Results
A total number of 70 individuals (40 varicocele patients and 30 fertile controls) were examined. Forty patients had: (a) Grade I (n=15); (b) Grade II (n=13); (c) Grade III (n=12). The mean age of infertile men with varicocele and normal controls was (Mean±SD) 31.58±6.16, 31.26±5.31 respectively (P=0.8204).
Sperm parameters including sperm count, rapid, slow, nonprogressive motility and immotile and sperm morphology in three groups are listed in Table 1. Sperm count, rapid motility and the rate of normal sperm morphology was significantly lower in the varicocele groups than in the fertile group (P =0.0003, P =0.0001, P =0.0001 respectively), but slow, nonprogressive and immotile motility between varicocele and fertile group was notable different (P= 0.1972, P= 0.1151, The frequency of the POLG gene CAG repeats in the two study populations are given in Table 2. The predominant allele in control subjects had 10 consecutive CAG repeats. This allele was found at similar frequency in control subjects (77.5%) and varicocele patients (83.8%) (P= 0.6425). The observed homozygosity values for the prevalent allele are close to equilibrium predictions. Other alleles of 9, 11 and 12 trinucleotide repeats were detected, but no larger, expanded repeats were found in the control subjects and varicocele patients.
Table 1.
Distribution of semen quality in patients.
| Varicocele | Control (n=30) | P-valuea | ||||
|---|---|---|---|---|---|---|
| Variables | Grade I (n=15) | Grade II (n=13) | Grade III (n=12) | Overall (n=40) | ||
| Age | 34.50±5.82 | 30.55±5.03 | 26.63±5.99 | 31.58±6.16 | 31.26±5.31 | 0.8204 |
| Count (mil.ml-1) | 62.17±45.77 | 57.00±31.76 | 53.21±27.23 | 57.46±38.57 | 98.76±53.03 | 0.0003 |
| Rapid motility (%) | 17.51±9.41 | 13.48±7.94 | 12.78±5.21 | 14.59±7.57 | 23.24±10.05 | 0.0001 |
| Slow motility (%) | 36.17±9.90 | 40.64±11.78 | 32.53±10.20 | 36.44±10.93 | 33.45±7.16 | 0.1972 |
| Nonprogressive motility (%) | 11.41±5.40 | 12.92±3.11 | 13.10±3.67 | 12.47±3.99 | 10.98±3.69 | 0.1151 |
| Immotile motility (%) | 34.91±17.75 | 32.8±18.86 | 41.59±10.34 | 36.43±15.84 | 32.43±9.35 | 0.2228 |
| Normal morphology (%) | 28.08±12.98 | 18.09±12.07 | 23.68±7.35 | 23.63±11.66 | 44.52±14.46 | 0.0001 |
a: Difference between varicocele (n=40) and control (n=30) group
Table 2.
POLG genotype of varicocele patients and controls
| Allele (number of CAG repeats) | Num. of alleles in varicocele patients |
Number of alleles in controls | P- valuea | ||||
|---|---|---|---|---|---|---|---|
| Grade I | Grade II | Grade III | Total | ||||
| 8 | 0 | 0 | 0 | 0 (0%) | 0 (0%) | - | |
| 9 | 1 | 3 | 1 | 5 (6.25%) | 3 (4.8%) | 0.7322 | |
| 10 | 29 | 21 | 14 | 62 (77.5%) | 54 (83.8%) | 0.6425 | |
| 11 | 2 | 2 | 7 | 11 (13.75%) | 4 (13%) | 0.2047 | |
| 12 | 0 | 0 | 2 | 2 (2.5%) | 0 (0%) | 0.2156 | |
| Genotype | Number of varicocele patients | Number of controls | |||||
| Grade I | Grade II | Grade III | Total | ||||
| 10/10 Homozygous | 12 | 8 | 3 | 23 (57.5%) | 21 (70%) | 0.6107 | |
| 10 /≠10 Heterozygous | 3 | 5 | 8 | 16 (40%) | 9 (30%) | 0.5497 | |
| ≠10/≠10 Homozygous + Heterozygous | 0 | 0 | 1 | 1 (2.5%) | 0 (0%) | 0.3890 | |
| Num. of samples | 15 | 13 | 12 | 40 | 30 | ||
a: Difference between varicocele and control group
Discussion
Depletion and rearrangements of mtDNA, including those caused by mutations in other regions of the POLG gene, are often manifested as serious diseases in humans, e.g. progressive external ophtalmoplegia (18). The molecular mechanism of this impairment remains to be elucidated.
Varicocele is the most common reversible cause of infertility. While the exact pathophysiologic mechanism underlying the Varicocele is the most common reversible cause of infertility. While the exact pathophysiologic mechanism underlying the hazardous effects of a varicocele on spermatogenesis and male fertility is not completely understood yet; however, most scientific evidence supports that both venous reflux and testicular temperature elevation seem to play an important role in the development of varicocele induced testicular dysfunction (19, 20).
Numerous studies have indicated an association between different polymorphisms, mutations or deletions in the mitochondrial genome and sperm dysfunction (21). To explore the influence of genetic factors, which associate with varicocele, we investigated the possible role of CAG repeats in POLG gene in these patients. Mutation in the POLG gene might introduce mutation in the mtDNA during replication, which eventually could affect the motility of the spermatozoa (-). POLG gene comprised variable number of CAG repeats, of which 10 copies of CAG repeats were considered as a common allele (14).
The distribution of the CAG repeat length in varicocele patients showed no notable difference from that in control subjects (Table 2). Krausz et al, Aknin-Seifer et al and Rani et al also fail to find any association between CAG repeat variation and male infertility among Italian, French and Indian populations, respectively (13, 24, 25).
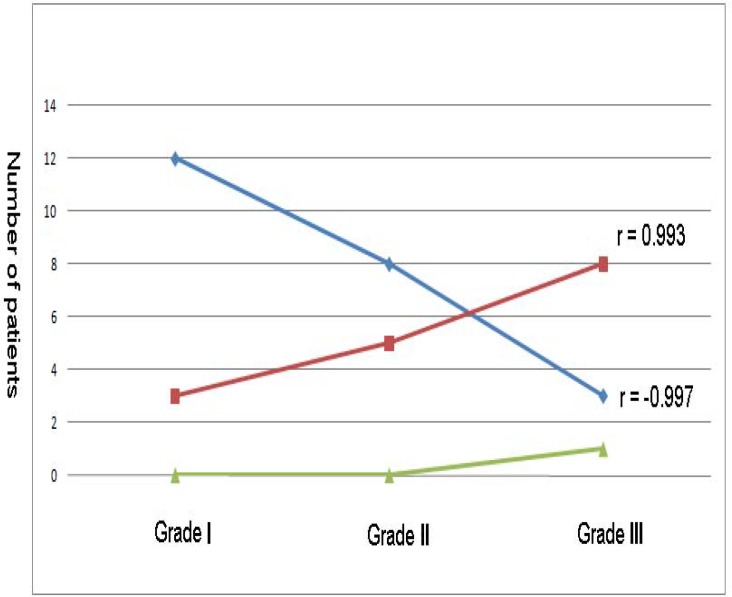
Although our results show that somatic instability of the POLG CAG repeat is not a main factor in pathogenesis of varicocele, but we found a statistically significant correlation between 10/10 genotype with varicocele grade (r= 0.993) and a statistically significant inverse correlation between 10/#10 genotype with varicocele grade (r= -0.997) (Figure 2). These findings suggested that the frequency of 10/#10 genotype increased in grade III and the frequency of 10/10 genotype increased in grade I.
Figure 2.
The Correlation between POLG genotype and Varicocele grade.
Conclusion
Thus, although we did not find any association between the POLG gene polymorphism and Iranian varicocele patients, but we suggest that POLG CAG repeat extensions is a contributing genetic risk factor that affects varicocele grade. The possible mechanisms remain elusive and require further studies.
Acknowledgment
This work was supported by Yazd University. We thank all the patients for providing blood samples for the scientific research as well as the Research and Clinical Center for Infertility, Shahid Sadughi University of Medical Sciences, Yazd, Iran. The authors declare that they have no conflict of interests.
References
- 1.Zini A, Blumenfeld A, Libman J, Willis J. Beneficial effect of microsurgical varicocelectomy on human sperm DNA integrity. Hum Reprod. 2005;20:1018–1021. doi: 10.1093/humrep/deh701. [DOI] [PubMed] [Google Scholar]
- 2.Moro E, Marin P, Rossi A, Garolla A, Ferlin A. Y chromosome microdeletions in infertile men with varicocele. Mol Cell Endocrinol. 2000;161:67–71. doi: 10.1016/s0303-7207(99)00226-9. [DOI] [PubMed] [Google Scholar]
- 3.Jarow JP, Coburn M, Sigman M. Incidence of varicoceles in men with primary and secondary infertility. Urology. 1996;47:73–76. doi: 10.1016/s0090-4295(99)80385-9. [DOI] [PubMed] [Google Scholar]
- 4.Mieusset R, Bujan L. Testicular heating and its possible contributions to male infertility: a review. Int J Androl . 1995;18:169–184. doi: 10.1111/j.1365-2605.1995.tb00408.x. [DOI] [PubMed] [Google Scholar]
- 5.Allamaneni SS, Naughton CK, Sharma RK, Thomas AJ, Jr AgarwalA. Increased seminal reactive oxygen species levels in patients with varicoceles correlate with varicocele grade but not with testis size. Fertil Steril . 2004;82:1684–1686. doi: 10.1016/j.fertnstert.2004.04.071. [DOI] [PubMed] [Google Scholar]
- 6.Kumar R, Venkatesh S, Kumar M, Tanwar M, Shasmsi MB, Gupta NP, et al. Oxidative stress and sperm mitochondrial DNA mutation in idiopathic oligoasthenozoospermic men. Indian J Biochem Biophys. 2009;46:172–177. [PubMed] [Google Scholar]
- 7.Talebi AR, Moein MR, Tabibnejad N, Ghasemzadeh J. Effect of varicocele on chromatin condensation and DNA integrity of ejaculated spermatozoa using cytochemical tests. Andrologia . 2008;40:245–251. doi: 10.1111/j.1439-0272.2008.00852.x. [DOI] [PubMed] [Google Scholar]
- 8.Bertazzoni U, Scovassi AI, Brun GM. Chick-embryo DNA polymerase gamma Identity of gamma-polymerases purified from nuclei and mitochondria. Eur J Biochem. 1977;81:237–248. doi: 10.1111/j.1432-1033.1977.tb11945.x. [DOI] [PubMed] [Google Scholar]
- 9.Zullo SJ, Butler L, Zahorchak RJ, Macville M, Wilkes C, Merril CR. Localization by fluorescence in situ hybridization (FISH) of human mitochondrial polymerase gamma (POLG) to human chromosome band 15q24-->q26, and of mouse mitochondrial polymerase gamma (Polg) to mouse chromosome band 7E, with confirmation by direct sequence analysis of bacterial artificial chromosomes (BACs) Cytogenet Cell Genet. 1997;78:281–284. doi: 10.1159/000134672. [DOI] [PubMed] [Google Scholar]
- 10.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature . 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 11.Luoma PT, Eerola J, Ahola S, Hakonen AH, Hellstrom O, Kivisto KT, et al. Mitochondrial DNA polymerase gamma variants in idiopathic sporadic Parkinson disease. Neurology. 2007;69:1152–1159. doi: 10.1212/01.wnl.0000276955.23735.eb. [DOI] [PubMed] [Google Scholar]
- 12.Heidari MM, Houshmand M, Hosseinkhani S, Nafissi S, Scheiber-Mojdehkar B, Khatami M. Association between trinucleotide CAG repeats of the DNA polymerase gene (POLG) with age of onset of Iranian Friedreich's ataxia patients. Neurol Sci. 2008;29:489–493. doi: 10.1007/s10072-008-1026-y. [DOI] [PubMed] [Google Scholar]
- 13.Krausz C, Guarducci E, Becherini L, degl'Innocenti S, Gerace L, Balercia G, et al. The clinical significance of the POLG gene polymorphism in male infertility. J Clin Endocr Metab. 2004;89:4292–4297. doi: 10.1210/jc.2004-0008. [DOI] [PubMed] [Google Scholar]
- 14.Rovio A, Tiranti V, Bednarz AL, Suomalainen A, Spelbrink JN, Lecrenier N, et al. Analysis of the trinucleotide CAG repeat from the human mitochondrial DNA polymerase gene in healthy and diseased individuals. Eur J Hum Genet. 1999;7:140–146. doi: 10.1038/sj.ejhg.5200244. [DOI] [PubMed] [Google Scholar]
- 15.Rovio AT, Marchington DR, Donat S, Schuppe HC, Abel J, Fritsche E, et al. Mutations at the mitochondrial DNA polymerase (POLG) locus associated with male infertility. Nat Genet. 2001;29:261–262. doi: 10.1038/ng759. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm–Cervical Mucus Interaction. 4th ed. New York: Cambridge University Press; 1999. [Google Scholar]
- 17.Ropp PA, Copeland WC. Cloning and characterization of the human mitochondrial DNA polymerase, DNA polymerase gamma. Genomics. 1996;36:449–458. doi: 10.1006/geno.1996.0490. [DOI] [PubMed] [Google Scholar]
- 18.Van GoethemG, Dermaut B, Lofgren A, Martin JJ, Van BroeckhovenC. Mutation of POLG is associated with progressive external ophthalmoplegia characterized by mtDNA deletions. Nat Genet. 2001;28:211–212. doi: 10.1038/90034. [DOI] [PubMed] [Google Scholar]
- 19.Report on varicocele and infertility. Fertil Steril. 2008;90:S247–249. doi: 10.1016/j.fertnstert.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 20.Richens J. Main presentations of sexually transmitted infections in men. Br Med J. 2004;328:1251–1253. doi: 10.1136/bmj.328.7450.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.St JohnJC, Jokhi RP, Barratt CL. Men with oligoasthenoteratozoospermia harbour higher numbers of multiple mitochondrial DNA deletions in their spermatozoa, but individual deletions are not indicative of overall aetiology. Mol Hum Reprod. 2001;7:103–111. doi: 10.1093/molehr/7.1.103. [DOI] [PubMed] [Google Scholar]
- 22.Lamantea E, Tiranti V, Bordoni A, Toscano A, Bono F, Servidei S, et al. Mutations of mitochondrial DNA polymerase gammaA are a frequent cause of autosomal dominant or recessive progressive external ophthalmoplegia. Ann Neurol . 2002;52:211–219. doi: 10.1002/ana.10278. [DOI] [PubMed] [Google Scholar]
- 23.Del BoR, Bordoni A, Sciacco M, Di FonzoA, Galbiati S, Crimi M, et al. Remarkable infidelity of polymerase gammaA associated with mutations in POLG1 exonuclease domain. Neurology . 2003;61:903–908. doi: 10.1212/01.wnl.0000092303.13864.be. [DOI] [PubMed] [Google Scholar]
- 24.Selvi RaniD, Vanniarajan A, Gupta NJ, Chakravarty B, Singh L, Thangaraj K. A novel missense mutation C11994T in the mitochondrial ND4 gene as a cause of low sperm motility in the Indian subcontinent. Fertil Steril . 2006;86:1783–1785. doi: 10.1016/j.fertnstert.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 25.Aknin-Seifer IE, Touraine RL, Lejeune H, Jimenez C, Chouteau J, Siffroi JP, et al. Is the CAG repeat of mitochondrial DNA polymerase gamma (POLG) associated with male infertility? A multi-centre French study. Hum Reprod. 2005;20:736–740. doi: 10.1093/humrep/deh666. [DOI] [PubMed] [Google Scholar]