Abstract
Objective(s):Alzheimer’s disease (AD) is a complex disease with multifactorial etiology. Inflammation has been proven to have an important role in the pathogenesis of AD. Both CCR2 and CCR5 genes expression increase in AD patients comparing to control subjects. CCR5 gene encodes a protein which is a member of the beta chemokine receptors family of integral membrane proteins. CCR5-Δ32 is a genetic variant of CCR5 and is characterized by the presence of a 32-bp deletion in the coding region of the gene, which leads to the expression of a nonfunctional receptor, and the CCR2-64I has a change of valine to isoleucine at codon 64, in the first transmembrane domain. It has been proved that both genes have important roles in different stages of inflammation.
Materials and Methods:The frequencies of CCR5∆32 and CCR2-64I variations were determined in 156 AD patients and 161 control subjects using polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) methods, and the results were compared among AD and healthy controls.
Results :Statistical analysis showed no significant difference in the distributions of CCR5∆32 and CCR2-64I between the AD patients and healthy controls (P> 0.05). Stratifying the samples by gender, genetic background and presence of ApoEε4 allele showed no significant effect on the distributions of CCR5∆32 and CCR2-64I (P> 0.05).
Conclusion:Our study did not show an association between CCR5∆32 and CCR2-64I variations and AD in the Iranian population. Further confirmatory studies with bigger number of samples are recommended.
Key Words: Alzheimer’s disease, Genetic Association study, CCR2, CCR5, Inflammation, Iranian population
Introduction
As the most common neurodegenerative disorder, Alzheimer’s disease (AD) currently affects 20 to 30 million people worldwide (1). The molecular and cellular mechanisms responsible for the etiology and pathogenesis of AD have not been fully defined (2). However, experimental studies suggest that inflammation plays a fundamental function in the pathogenesis of AD (3); and several community-based studies have linked anti-inflammatory interventions to a lowered risk of developing AD (4).
Microglias are the mononuclear phagocytes of the brain and their numbers increase in the AD brain, which are clustered in and around Amyloid Beta (Aβ) deposits (5). Evidence for the presence of microglia in senile plaques derives from immunohistochemical studies that examined the brains of AD patients (6). Electron microscopy studies have shown that those microglias are closely opposed to Aβ (7). In vitro studies have shown that Aβ directly activates microglia, leading to production of reactive oxygen and nitrogen species, tumor necrosis factor (TNF-α), IL-1 (8, 9) and complement proteins (10). Interaction of microglia with Aβ also leads to secretion of chemokines, such as monocyte chemotactic protein-1 (MCP-1, also known as CCL2).
Chemokines are a family of proinflammatory cytokines that can stimulate the target-cell-specific directional migration and recruitment of leukocytes to sites of inflammation via interaction with a family of chemokine receptors (11). They and their receptors constitute a large set of proteins, and two subfamilies, CXC and CC chemokines depending on whether they express a CC or CXC amino acid motif in their N-termini, and their receptors, have been identified (12), which coordinate cellular responses to inflammation insult or injury (13). There are growing evidences that chemokines and their receptors are unregulated in AD brain (14) CCR2 and CCR5 are two types of CC receptors, which predominantly express on monocytes surfaces (15). These receptors can bind and signal to different CC chemokines including MCP-1 (CCL2) and RANTES (16, 17).
Hetero-dimeric CCR2 and CCR5 interaction may be implicated in the in vivo processes that hamper leukocyte rolling on blood vessels and induce leukocyte parking in tissues during inflammatory responses (18). Indeed, abolishing such accumulation, as occurs in mice deficient in the chemokine receptor CCR2 leads to development of early visible Aβ deposits, specifically around blood vessels, and has been associated with increased mortality in these mice (19). CCL2 is a potent monocyte chemoattractant. Binding of CCL2 to its receptor, CCR2 also stimulates production of reactive oxygen species (20). CCL2 and CCR2 seem to have important functions in the recruitment of mononuclear cells into tissues in both acute and chronic inflammation, and targeted disruption of the CCR2 gene cause decreasing recruitment of monocytes into the peritoneum in a model of acute inflammation (21). Thus, CCR2 controls the recruitment and/or infiltration of mononuclear phagocytes into the brain and CCL2–CCR2 interactions seem to play a key part in recruitment and/or activation of microglia to sites of Aβ deposition in AD. CCR2 deficiency leads to lower microglia accumulation and higher brain β-amyloid levels, indicating that early microglia accumulation promotes Aβ clearance (22). In addition to CCL2, other chemokines and their receptors have been shown to be expressed in Aβ-stimulated monocytes and microglia in AD brain (14). Previous immunohistochemical studies of AD brains have shown that the chemokine receptor CCR5 is present on microglia of both control and AD brains, with increased expression on reactive microglia associated with amyloid deposits in AD suggesting that CCR5 might play a function in the regulation of the brain immune response in AD (23).
Recent study provides clear evidence that peripheral T cells of AD patients overexpressed MIP-1α, which binds to CCR5 on brain endothelial cells to promote T cells transendothelial migration across the blood–brain barrier (24). In addition, CCR5 is a necessary membrane co-receptor for the binding and entry of human immunodeficiency virus (HIV) into target cells (25). Both CCR2 and CCR5 genes are characterized by polymorphisms in their sequence. A single nucleotide polymorphism (SNP) in the CCR2 gene causes a conservative change of a valine with an isoleucine at codon 64 (CCR2-64I), in the first transmembrane domain of the receptor (26) and a 32-bp deletion (CCR5Δ32) in the coding region which leads to the expression of a nonfunctional and truncated receptor (27). Previous researches, have reported that these two variations may be involved in AD progression (28- 31).
Because inflammation may play an important role in progression of Alzheimer’s disease, and CCR2 and CCR5 have a primary function in recruitment of leukocytes to inflammatory sites, we hypothesized that these variations might influence the risk of developing AD in our population.
Materials and Methods
This case control study was conducted in the Genetics Research Centre, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran Inthisstudy,totally156Alzheimer’s patients and 161 healthy controls from Iranian population in several elderly care centres in Tehran city were recruited. The Alzheimer’s disease in patients were diagnosed and confirmed by psychiatrist according to criteria introduced in the DSM-IV and control subjects were selected by the assessment of their medical histories and physical conditions. The participants older than 65 years old were included. An agreement was made prior to their entering the study. The main inclusion criterion for the case group was the diagnosis of AD by DSM-IV criteria; in control group, had the participants have any serious neurologic or psychological disorder, they were excluded from the study. The participants or their families were asked about some personal and baseline information, also ethnicity, job, educational level and gender was considered as co variable (Table 1).
Table 1.
Comparison of mean age, gender, job, education level and genetic background between Alzheimer,s disease (AD) cases and control subjects
| AD patients (n=156) | Control subjects (n=161) | P value | ||
|---|---|---|---|---|
| Age | 78.55 ± 7.80a | 77.14 ± 6.95 | 0.091 | |
| Sex (M/F)b | 63/91 | 63/99 | 0.714 | |
| Job | Housewife | 55.8% | 56.2% | 0.938 |
| Own business | 23.4% | 21.0% | ||
| Worker | 9.2% | 8.6% | ||
| Farmer | 3.2% | 3.1% | ||
| Employee | 8.4% | 11.1% | ||
| Education level |
Illiterate | 41.6% | 43.2% | 0.427 |
| Primary school | 29.2% | 29.6% | ||
| Secondary school | 16.2% | 12.3% | ||
| Diploma | 11.1% | 9.3% | ||
| Academic | 1.9% | 5.6% | ||
| Genetic background | Fars | 61.0% | 63.6% | 0.490 |
| Turk | 25.3% | 25.3% | ||
| Kurd | 3.9% | 1.8% | ||
| Lor | 0.7% | 2.5% | ||
| Gilak& Mazani | 9.1% | 6.8% | ||
a Mean ± SD; b Male/Female
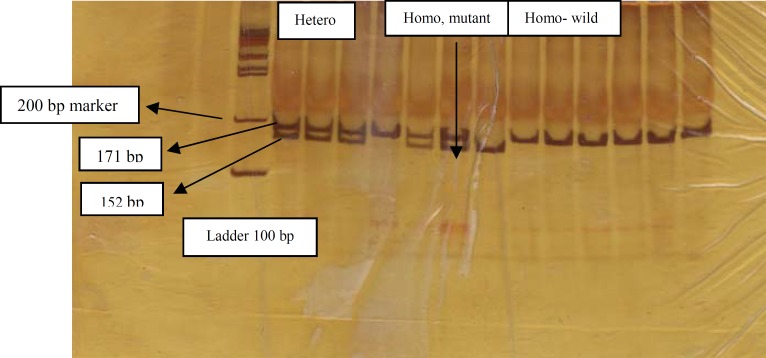
Five ml of peripheral blood samples were collected in tubes containing 200 µl of 0.5 M EDTA; genomic DNA was extracted from peripheral blood using the salting out method, and two pairs of primers were used for amplifying and analyzing the mentioned variations, which are presented in Table 2. The total volume of 25 μl containing 30 ng genomic DNA, 10 pmol of each primer, 1 μl dNTP mix (Fermentas, Life Science), 2.5 μl 10× buffer and 0.5 U of Taq DNA polymerase (Fermentas Life Science, Lithuania) with 1.5 mM MgCl2 were mixed in the 0.5 ml Eppendorf microtube for amplification of target sequences. PCRs were performed for 33 cycles, and 95 ˚C pre-denaturation for 4 min followed by denaturation at 94 ˚C for 45 sec, annealing at (60 ˚C for CCR5 and 58 ˚C for CCR2) for 30 sec, extension at 72 ˚C for 40 sec and final extension at 72 ˚C for 5 min. CCR5Δ32 genotyping was determined by PCR and 8% polyacrylamid gel electrophoresis (PAGE). PCR for amplifying 220 bp wild type and 188 bp variant type products was carried out (Figure A). For CCR2-64I genotyping, PCR-RFLP and 8% polyacrylamid gel electrophoresis (PAGE) were used and BsaBI restriction enzyme was performed for 171 bp PCR product digestion. PCR product digestion created 152- and 19-bp fragments for variant type (CCR2-V64I) and no digestion, therefore 171 bp intact fragment for wild type (Figure B). We obtained informed consent from participants or their families.
Figure A.
PCR amplification of the human CCR5 gene. No mutant homozygote variant was found in this study
Figure B.
PCR amplification and restriction digestion of CCR2 by BsaBI
Table 2.
Primer sequences and PCR product sizes
| PCR primers | PCR product sizes |
|---|---|
|
CCR5Δ32 Variation F: TCT CCC AGG AAT CAT CTT TAC C R: AGC CCT GTG CCT CTT CTT C |
Δ32 allele : 188 bp w.t allele: 220 bp |
|
CCR2-G/A (V64I) F: TTT GTG GGC AAC ATG ATG G R: GCA CAT TGC ATT CCC AAA G |
CCR2 : 171 bp |
The data was analyzed using SPSS ver 11.5 (SPSS, Chicago, Ill, USA). Logistic regression analysis was performed to assess the effect of mutant genotype or allele in study groups and related odds ratio (OR) and 95% confidence interval (CI) reported. P-values less than 0.05 were considered as significant.
The results were merged with the result of previous study for APOE polymorphisms to analyze if the interaction effects of APOE with CCR5 or CCR2 are statistically significant.
Results
The distributions of CCR5 and CCR2 genotype and allele frequencies of each group are summarized in Table 3. There were no statistical differences in CCR5 and CCR2 genotypes and allele frequencies in AD compared to healthy controls (P> 0.05). Also there were no significant differences between male and female in both AD patients and health controls, when stratified by gender (P> 0.05). No ∆32/∆32 genotype was detected among controls and Alzheimer's patients.
Table 3.
Genotype and allele frequencies of SNPs in the human CCR2 and CCR5 genes in Alzheimer,s disease (AD) patients and controls
| AD patients N=156 |
Controls N=161 |
P-value | Odds ratio | |
|---|---|---|---|---|
| CCR5 w.t./w.t. | 149 (95.5%) | 153 (95%) | Reference group | |
| CCR5 w.t./Δ32 | 7 (4.5%) | 8 (5%) | 0.95 | 1.1 (0.39-3.15) |
| w.t. allele | 305 (97.8%) | 314 (97.5%) | 0.95 | 1.1 (0.4-3.1) |
| Δ32 allele | 7 (2.2%) | 8 (2.5%) | ||
| CCR2 w.t./w.t. | 133 (85.2%) | 131(81.4%) | Reference group | |
| CCR2 w.t./ 64I | 21 (13.5%) | 28 (17.4%) | 0.41 | 1.35 (0.73-2.5) |
| CCR2 64I/64I | 2 (1.3%) | 2 (1.2%) | 0.62 | 0.98 (0.14-7.1) |
| w.t allele | 287 (92%) | 290 (90%) | 0.48 | 1.27 (0.73-2.2) |
| 64I allele | 25 (8%) | 32 (10%) |
When we stratified the CCR5 and CCR2 results with ApoEε4 allele for synergic effects, by logistic regression, we could not find any significant differences between combined genotypes for risk of AD (P>0.05) (Table4).
Table 4.
Allele frequencies of CCR5 Δ32 and CCR2-64I variations in cases and controls based on APOE ε4 allele
| APOE ε4 | CCR5Δ32 | AD patients | Controls | P-value |
|---|---|---|---|---|
| - | - | 115 (73.7%) | 146 (90.7%) | 0.85 |
| - | + | 6 (3.8%) | 8 (5%) | |
| + | - | 35 (22.4%) | 7 (4.3%) | 0.50 |
| + | + | 1(0.6%) | 0 | |
| ApoE ε4 | CCR2-64I | AD patients | Controls | |
| - | - | 102 (65.5%) | 129 (80.1%) | 0.91 |
| - | + | 18 (11.5%) | 25 (15.5%) | |
| + | - | 30 (19.2%) | 6 (3.7%) | 0.69 |
| + | + | 6 (3.8%) | 1(0.6%) |
Discussion
In our study performed for the investigation of CCR5 ∆32 and CCR2-64I variations association with the risk of late onset AD, the sample size consisted of 156 Alzheimer’s patients and 161 control subjects. After genotypes and alleles comparison analyses between the two groups (patients and controls) using χ2 test, no association between CCR5 ∆32 genotypes and AD was found [OR= 1.1 (95% CI= 0.39-3.15)]. In the next step analysis was performed for CCR5 genotypes and alleles between females and males; that has the same result and no difference in genotypes distribution between Alzheimer’s patients and control subjects by gender stratification was identified (P= 0.274 and P= 0.280 for males and females, respectively).
In contrast to other organs, the brain does not show a classical immune response, so it is believed to be immune privileged (32).
Chronic inflammation is assumed to have an important role to disease progression through the production of inflammatory mediators (33). Neuroinflammation is also involved in the pathogenesis of many neurodegenerative disorders (14) and markers of neuroinflammation are prominent in numerous CNS disorders including Parkinson’s disease (34), multiple sclerosis (35) and AD.
There are many evidences indicating the presence of inflammatory reactions during the Alzheimer’s pathogenesis (14). CCR2 and CCR5 are chemokine receptors expressed on microglia that mediate accumulation of leukocytes at sites of inflammation. It was suggested that both CCR2 and CCR5 expression were increased in AD patients compared to controls; but two studies from Italy (28, 31) and two studies from Spain (29, 30) showed no statistically significant differences between AD and control groups. Mohaddes Ardebili showed significant association for CCR2 but no association for CCR5 in West Northern part of Iran (Eastern Azerbaijan) (36). These chemokine receptors were more known to act as co-receptors for HIV entry into the leukocytes, as CCR5∆32 and CCR2-V64I have shown to create high resistance against AIDS progression. The well-known polymorphism CCR5∆32 that results in a truncated protein is not distributed equally among the world’s population and the north European Caucasians have the highest frequency compared to other parts of the world. As Gharagozloo showed that the frequency of this polymorphism is low in our country (25), we can speculate that finding a higher accumulation of this polymorphism either in AD patients or normal controls can make it either a risk or protective factor amon our population, respectively. Since Hetero-dimeric CCR2 and CCR5 interaction may be implicated in the in vivo processes that hinder leukocyte rolling on blood vessels and induce leukocyte parking in tissues during inflammatory responses. It was decided to determine whether these polymorphisms have any association with AD onset in our population or not.
Considering the fact that Iranian population may have a different variations compared to the European population, it should be mentioned that therefore sequencing the genes may result in finding new population specific variations. These new variants may have significant correlations with LOAD comparing to control group. Sample size was low in this study, and further confirmatory studies with bigger number of samples are suggested as well.
Conclusions
Our study failed to show an association between CCR5∆32 and CCR2-64I variations and Alzheimer's disease in the Iranian population.
Acknowledgment
We express our sincere thanks and gratitude to all Alzheimer's and control patients or their families for their kind participation in this study. We also thank Iran Alzheimer Association for their sincere collaborations.
References
- 1.Selkoe DJ. Defining molecular targets to prevent Alzheimer’s disease. Arch Neurol. 2005;62:192–195. doi: 10.1001/archneur.62.2.192. [DOI] [PubMed] [Google Scholar]
- 2.Kamer AR, Craig RG, Dasanayake AP, Brys M, Glodzik-Sobanska L, Leon MJ. Inflammation and Alzheimer's disease: Possible role of periodontal diseases. Alzheimer Dement. 2008;4:242–250. doi: 10.1016/j.jalz.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 3.McGeer PL, McGeer EG. NSAIDs and Alzheimer’s disease: epidemiological, animal model and clinical studies. Neurobiol Aging. 2007:639–647. doi: 10.1016/j.neurobiolaging.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer’s disease progression: a randomized controlled trial. JAMA. 2003;289:2819–2826. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- 5.Heneka MT, O’Banion MK. Inflammatory processes in Alzheimer’s disease. J. Neuroimmunol. 2007;184:69–91. doi: 10.1016/j.jneuroim.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 6.McGeer PL, Itagaki S, Tago H, McGeer EG. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci Lett. 1987;79:195–200. doi: 10.1016/0304-3940(87)90696-3. [DOI] [PubMed] [Google Scholar]
- 7.Perlmutter LS, Barron E, Chui HC. Morphologic association between microglia and senile plaque amyloid in Alzheimer’s disease. Neurosci Lett . 1990;119:32–36. doi: 10.1016/0304-3940(90)90748-x. [DOI] [PubMed] [Google Scholar]
- 8.Coraci IS, Husemann J, Berman JW, Hulette C, Dufour JH, Campanella JK, et al. CD36, a Class B scavenger receptor, is expressed on microglia in Alzheimer’s disease brains and can mediate production of reactive oxygen species in response to b-amyloid fibrils. Am J Pathol . 2002;160:101–112. doi: 10.1016/s0002-9440(10)64354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El KhouryJB, Moore KJ, Means TK, Leung J, Terada K, Toft M, et al. CD36 mediates the innate host response to b-amyloid. J Exp Med . 2003;197:1657–1666. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haga S, Ikeda K, Sato M, Ishii T. Synthetic Alzheimer amyloid beta/A4 peptides enhance production of complement C3 component by cultured microglial cells. Brain Res . 1993;601:88–94. doi: 10.1016/0006-8993(93)91698-r. [DOI] [PubMed] [Google Scholar]
- 11.Moser B, Loetscher M, Piali L, Loetscher P. Lymphocyte responses to chemokines. Int Rev Immunol. 1998;16:323–344. doi: 10.3109/08830189809043000. [DOI] [PubMed] [Google Scholar]
- 12.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 13.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 14.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, et al. Inflammation and Alzheimer's disease. Neurobiol Aging . 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fantuzzi L, Borghi P, Ciolli V, Pavlakis G, Belardelli F, Gessani S. Loss Of CCR2 expression and functional response to monocyte chemotactic protein (MCP-1) during the differentiation of human monocytes: role of secreted MCP-1 in the regulation of the chemotactic response. Blood. 1999;94:875–883. [PubMed] [Google Scholar]
- 16.Premack BA, Schall TJ. Chemokine receptors: gateways to inflammation and infection. 1996;Nat Med:1174–1178. doi: 10.1038/nm1196-1174. [DOI] [PubMed] [Google Scholar]
- 17.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 18.Mellado M, Rodriguez-Frade JM, Vila-Coro AJ, Fernández S, Martín deAnaA, Jones DR, et al. Chemokine receptor homo- or heterodimerization activates distinct signaling pathways. EMBO J. 2001;20:2497–507. doi: 10.1093/emboj/20.10.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El KhouryJ, Toft M, Hickman SE, Means TK, Terada K. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med . 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- 20.Zachariae CO, Anderson AO, Thompson HL, Appella E, Mantovani A, Oppenheim JJ, et al. Properties of monocyte chemotactic and activating factor (MCAF) purified from a human fibrosarcoma cell line. J Exp Med. 1990;171:2177–2182. doi: 10.1084/jem.171.6.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viola A, Luster AD. Chemokines and their receptors: drug targets in immunity and inflammation. Annu Rev Pharmacol Toxicol . 2008;48:171–197. doi: 10.1146/annurev.pharmtox.48.121806.154841. [DOI] [PubMed] [Google Scholar]
- 22.Khoury JE, Luster AD. Mechanisms of microglia accumulation in Alzheimer’s disease: therapeutic implications. Trends in Pharmacological Sciences. 2008;29:626–632. doi: 10.1016/j.tips.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Xia MQ, Qin SX, Wu LJ, Mackay CR, Hyman BT. Immunohistochemical study of the -chemokine receptors CCR3 and CCR5 and their ligands in normal and Alzheimer’s disease brains. Am J Pathol . 1998;153:31–37. doi: 10.1016/s0002-9440(10)65542-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Man SM, Ma YR, Shang DS, Zhao WD, Li B, Guo DW, et al. Peripheral T cells overexpress MIP-1α to enhance its transendothelial migration in Alzheimer’s disease. Neurobiol Aging. 2007;28:485–496. doi: 10.1016/j.neurobiolaging.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Gharagozloo M, Doroudchi M, Farjadian S, Pezeshki AM, Ghaderi A. The frequency of CCR5Delta32 and CCR2-64I in Southern Iranian normal population. Immunol Lett. 2005;96:277–281. doi: 10.1016/j.imlet.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Smith MW, Dean M, Carrington M, Winkler C, Huttley GA, Lomb DA, et al. Contrasting genetic influence of CCR2 and CCR5 variants on HIV-1 infection and disease progression. Science. 1997;277:959–965. doi: 10.1126/science.277.5328.959. [DOI] [PubMed] [Google Scholar]
- 27.Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R, et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;367:77–86. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 28.Galimberti D, Fenoglio C, Lovati C, Gatti A, Guidi I, Venturelli E, et al. CCR2-64I polymorphism and CCR5Delta32 deletion in patients with Alzheimer's disease. J Neurol Sci. 2004;225:79–83. doi: 10.1016/j.jns.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Huerta C, Alvarez V, Mata IF, Coto E, Ribacoba R, Martínez C, et al. Chemokines (RANTES and MCP-1) and chemokine-receptors (CCR2 and CCR5) gene polymorphisms in Alzheimer's and Parkinson's disease. Neurosci Lett. 2004;370:151–154. doi: 10.1016/j.neulet.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Combarros O, Infante J, Llorca J, Peña N, Fernández-Viadero C, Berciano J. The chemokine receptor CCR5-Δ32 gene mutation is not protective against Alzheimer’s disease. Neurosci Lett. 2004;366:312–314. doi: 10.1016/j.neulet.2004.05.058. [DOI] [PubMed] [Google Scholar]
- 31.Balistreri CR, Grimaldi MP, Vasto S, Listi F, Chiappelli M, Licastro F, et al. Association between the polymorphism of CCR5 and Alzheimer's disease: results of a study performed on male and female patients from Northern Italy. Ann N Y Acad Sci. 2006;1089:454–461. doi: 10.1196/annals.1386.012. [DOI] [PubMed] [Google Scholar]
- 32.Lampson LA. Molecular bases of the immune response to neural antigens. Trends Neuroscience. 1987;10:211–216. [Google Scholar]
- 33.Etminan M, Gill S, Samii A. Effect of non-steroidal anti-inflammatory drugs on risk of Alzheimer's disease: Systematic review and meta-analysis of observational studies. Bri Med J. 2003;327:128. doi: 10.1136/bmj.327.7407.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirsch EC, Hunot S, Damier P, Faucheux B. Glial cells and inflammation in Parkinson’s disease: A role in neurodegeneration. Ann Neurol. 1998;44:115–120. doi: 10.1002/ana.410440717. [DOI] [PubMed] [Google Scholar]
- 35.Sorenson TL, Tani M, Jensen J, Pierce V, Lucchinetti C, Folcik VA, et al. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J Clin Invest. 1999;103:801–815. doi: 10.1172/JCI5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohaddes ArdebiliSM, Rezazadeh M, Gharesouran J, Yeghaneh T, Farhoudi M, Ayromlou H, et al. Association of CCR2 gene but not CCR5 gene polymorphisms with Alzheimer’s disease. J Sci Islam Republic Iran. 2011;22:111–116. [Google Scholar]