Abstract
Objective(s)
In this study we investigated the expression of GABAA receptor subunits during brain development. These receptors may change in the embryonic chick forebrain.
Materials and Methodes
The expression levels of four types of GABAA receptor gamma subunits (γ1, γ2, γ3 and γ4) were quantified in the embryonic chick forebrain at 32 hr, 3, 7, 14, and 20 days of incubation and day one after hatching. The expression level of mRNA in the forebrain of embryonic chicken was measured using real-time RT-PCR.
Results
The expression level of each subunit increased gradually with development and reached a plateau on 20th day of embryonic development. A reduction was observed on day one after hatching in all gamma subunits.
Conclusion
This may explain the different physiological and pharmacological function of GABA receptor gamma subunits before and after hatching.
Key Words: Developmental expression, Embryonic forebrain, GABAA Receptor subunit, Real-time RT-PCR
Introduction
Gamma-aminobutyric-acid (GABA) is a major inhibitory neurotransmitter in the centeral nervous system (1, 2) that intervenes most of its effects through GABAA receptors. GABA receptors are classified into two major groups: ionotropic GABA type-A receptors that form ion channels (GABAARs) and metabotropic GABA type-B (GABABRs) which are G protein-coupled receptors (3). The GABAAR is a member of the cysteine-loop family of ligand-gated ion channels. Receptors of this class comprise five subunits arranged symmetrically around a central ion-conducting pore. Each subunit consists of four α-helical transmembrane domains and a large extracellular amino-terminal domain that harbors the ligand binding sites and the signature cysteine-loop (4).
The channel formed by these receptors, is a pentameric assembly resulting from five of at least 21 subunits, grouped in the eight classes: alpha (α1-6), beta (β1-4), gamma (γ1-4), delta, pi, epsilon, theta, and rho (ρ1-3) (2, 3) which permits an immense number of putative receptor isoforms.
The subunit combination of a GABAARs determines not only their natural functions but also the specific effects of allosterical modulators of benzodiazepines, barbiturates, steroids, general anaesthetics, some convulsants, polyvalent cations, and ethanol (5-9).
GABAARs may have different physiological and pharmacological functions based on their expression in different stages of the chicken life (10-12). Since quantitative changes in GABAA receptor gamma subunits during embryonic development are not well-known, the aim of present study was to demonstrate the presence of these subunits expression in chicken forebrain.
Materials and Methods
Animals
Fertile eggs of Ross breed obtained from a comercial parent farm were used in all experiments. Efforts were made to minimize animal suffering and the number of animals used. All experiments were approved by the Animal Ethics Committee for the University of Isfahan, Isfahan, Iran.
Preparation and dissections
The fertilized eggs were incubated in a standard incubator (Gujiran, Iran) at standard conditions. The embryo were collected at the specified times (32 hr, 3, 7, 14, 20, and 21 days) and the forebrain were dissected under a binocular microscope (Olympus). At the early stages of development, whole brain was dissected.
Tissue preparation
At specified times (32 hr, 3, 7, 14, 20, and 21 days after incubation), the whole brain at 33 hr after hatching and forebrain of the animals were rapidly removed from the fertelized eggs (n= 12 for each time point) and placed into ice-cold RLT (Qiagen). The tissue segments were stored at -70 °C.
RNA extraction and RT
Total cellular RNA was isolated from frozen tissues using the RNeasy Plus Mini Kit (Qiagen) for isolation. The extracted RNA was dissolved in diethyl pyrocarbonate (DEPC) treated water. The purity and integrity of the extracted RNA was evaluated by optical density measurements (260/280 nm ratios) and by visual observation of samples on agarose gel electrophoresis. Both methods indicated integrity of the extracted RNA with little or no protein contamination. Complementary DNA synthesis reactions were performed using 1 µg DNase (Fermentas) which treated total RNA from each sample and cDNA Synthesis Kit (Fermentas) with random hexamer (Fermentas) priming in a 20 µl reaction according to the manufacturer’s instructions.
Quantitative RT-PCR
Real-time PCR was performed in the Chromo4 Detection System (BioRad, USA). Briefly, 20 ng of cDNA and gene specific primers were added to SYBR Green Master Kit (Takara, Tokyo, Japan) and subjected to PCR amplification (1 cycle at 95 °C for 30 sec, and 45 cycles at94 °C for 5 sec, 55 °C for 20 sec, and 72 °C for 34 sec). All PCR reactions were run in duplicate. The amplified transcripts were quantified using the comparative Ct method (http://www.pebiodocs.com/pebiodocs/04303859.pdf.).
Gene-specific primers were designed using Beacon Designer 7.5 software. The primers used for real-time PCR were:
Gbrγ1: 5'- AGGACATCTCTGGGTAAT -3'/5'- CAAGGTGCCATATTCCATCAAG -3'.
Gbrγ2: 5'- TGGAATGATGGCAGAGTGTTG -3'/5'- GTAGTCTTCACCACCTCAGTTG -3'.
Gbrγ3: 5'- TTGCTGCTCTGATGGAGTATG -3'/5'- ACTGTAGTTGGTGGTCTCATATC -3'.
Gbrγ4: 5'-CAGAGATAGAGGAGGATGAAGATG-3'/5'- GAAGAGCAGGAAGGCAGTG -3'
β-tubulin: 5'- ATTGTGATGGACTCTGGT -3'/5'- TCGGCTGTGGTGGTGAAG -3'.
Expression levels were normalized to that of β –tubulin. Relative expression data was quantified using 2-(Ct Control-Ct sample) where Ct is the cycle threshold. Relative standard curves were generated by plotting the threshold value (Ct) versus the log of the amount of total cDNA added to the reaction and used to check the efficiency of primers. Calculation of Ct, standard curve preparation and quantification of mRNA in the samples were performed by software provided with the Chromo4 (Opticon 3). All target genes were normalized to the β -tubulin housekeeping gene.
Statistical analysis
To determine significance, all data were subjected to statistical analysis using a computer program (GraphPad Prism). One-way ANOVA was used, as indicated in the figure legends, followed by a post-hoc test (Tukey) of differences between specific time points. All data are presented as the mean±SEM A level of P< 0.05 was considered significant.
Results
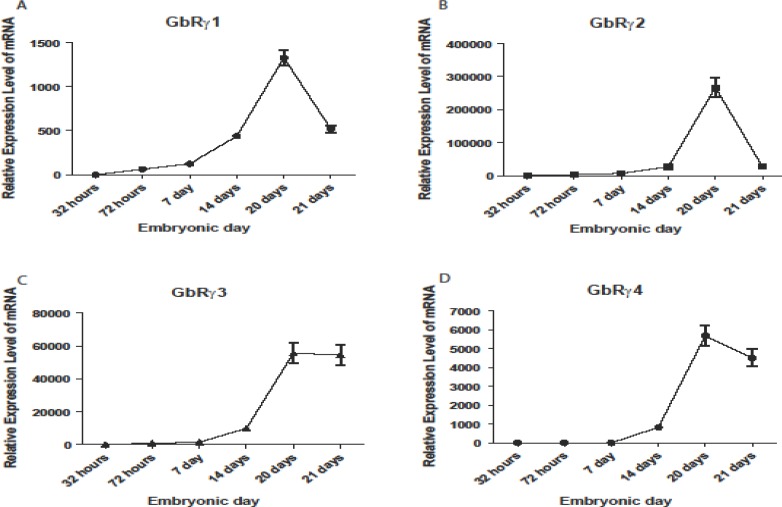
GABAA receptor gamma subunit genes showed significant alterations in chicken embryonic development. Figure 1 shows the temporal changes in mRNA expression of these genes. In each case, the data were expressed as a ratio to that expressed in 32 hr after hatching.
Temporal alterations in GABA A gamma 1 expression during development
GABAA gamma 1 expression was altered during embryonic development (Figure 1, panels A). One-way ANOVA indicated significant enhancment in gene expression during development. GABAA gamma 1 mRNA was increased by 62.73±3.16 fold at 72 hr and 124.47±6.32, 438.01±22.65, and 1328.51±82.53 fold on 7, 14, and 20 days during development, respectively, then decreased by day one after hatching (518.85±44.22). Therefore, the pattern of GABAA gamma 1 mRNA increase resembled a wave travelling during development.
Figure 1.
Quantitative RT-PCR analysis of GABAA receptor gamma 1 (A), gamma 2 (B), gamma 3 (C), and gamma 4 (D) mRNA expression during chick embryonic development. Expression of the investigated genes simultaneously elevated on day 20. Expression pattern of all of these genes was different. Error bars show mean±SD
Temporal alterations in GABA A gamma 2 expression during development
Different pattern was seen for the expression of GABAA gamma 2 (Figure 1, panels B). GABAA gamma 2 mRNA was significantly (P< 0.05) increased in all time points, and decreased in day one after hatching. The peak in enhancment was similar to the GABAA gamma 1.
Temporal alterations in GABA A gamma 3 expression during development
GABAA gamma 3 mRNA (Figure 1, panels C) was higher than 32 hr on all days. There was a profound increase on 3 days followed by an increase on day 7, 14, and 20. The greatest alterations were seen on 20th day again. The mRNA expression level of GABAA gamma 3 remained stable after hatching.
Temporal alterations in GABA A gamma 4 expression during development
GABAA gamma 4 mRNA began to increase on day 14 (824.85±86.51) during development, and then continued to increase to 5680.22±542.02 fold on 20th day, and reduced to 4513.52±447.85 fold on day one after hatching. Enhancement on 20th day was significant (P< 0.00) (Figure 1, panel D).
Discussion
Although GABAergic neurons and also a high amount of GABA in embryonic neurons and CNS of newborns have been found, the GABA may have different role in embryonic stages and mature ages (13). Regarding this, physiological and pharmacological features of GABA receptors may be changed during brain development. Northern and western blot analysis proved that age dependence occurs within α1, α2, α3, α5 and β1 subunits but the details in different brain zones have not been displayed. It has been reported that the expression of each GABA subunit receptor gene changes during the initial development (14).
Rodriguez et al used "in situ hybridization" technique to analyze the site expression of four mRNAs, α1, α2, β2 and γ2 GABA receptor subunits, in the vision of developing chicken (9).
All subunits that had already been expressed during the E10 level in most of the vision layers such as α1, α2, and β3 undergo remarkable changes from E10 to E20 level though γ1 undergoes just a few changes in some levels (9). The results of this experiment show that chicken brain have a low amount of γ1 subunit expression in the first days after birthand also a very low expression of γ4 in ED18. The results of this study demonstrate that neurons in different layers, show different expression paterns; influenced by many cellular treatments such as reproduction, after mitotic neuronic migration, cell programmed death, and differentiation, hense understanding this pattern becomes more complicated (9).
In the present study, the expression level of γ2, γ3 and γ4 started to increase on day 14 of embryonic develeopment and reached to its maximum through developmental embryonic stages.
Enomoto and colleagues measured the developmental changes of the expression of seven types of GABA receptor subunits (α1, β2, β3, β4, γ1, γ2 and γ4) in brainstem of chicken embryo during 2-20 days of incubation and also immediately after emerging from egg. The expression stage had been determined using RT-PCR. The electrophoresis patterns of γ4. Γ1, β3 and α1 RT-PCR products show that γ4 had a later arrival. The expression of α1, β3 and , γ1 has started during E2 to E6 levels but the expression of γ4 started in E8 and the expression increases during the development and arrives to a uniform step in E14. In brainstem between E4 to E7 just an isolated γ2 band was discovered although after E8 another band with a higher molecular weight appeared. In brain-stem, the γ2s or the smaler part, appeared in E4. The γ21, the larger part, at first appeard in E9 and got to a uniform step in E15. The absence of γ4 and γ21 in embriyonic stage shows the pharmacological difference of this subunits during development (11).
To date, however, very few investigations about the GABA receptors expression quantity in chicken embryo have been reported. In the present investigation, the quantitative expression of γ receptor subunits was done for the first time.
Since the GABA receptors play a very important role in the nerveus system, functions of these receptors during the developmental phases are very important. Therefore, by knowing the expression changes of these receptores we can understand not only their physiological role but also their pharmacological features.
In accordance to the report by Emoto and colleagues, γ1 started to increase at E4 stage and got to its maximum in E20 stage and decreased after emerging from egg. Moreover, the expression of γ2, γ3, γ3, and γ4 increased in E14 and reached to the maximum at the last stage.
Conclusions
All together, these data showed a spatiotemporal expression pattern for GABAA receptor gamma subunits in forebrain that can explain the diversity of GABAA receptor properties.
Acknowledgment
This study was supported by grant from the University of Isfahan, Isfahan, Iran, (Grant No. 860818).
References
- 1.Schousboe A, Waagepetersen HS. Encyclopedia of neuroscience. Oxford: Academic Press; 2009. Gamma-aminobutyric acid (GABA) pp. 511–515. [Google Scholar]
- 2.Snyder SH, Ferris CD. Novel neurotransmitters and their neuropsychiatric relevance. Am J Psychiatr . 2000;157:1738. doi: 10.1176/appi.ajp.157.11.1738. [DOI] [PubMed] [Google Scholar]
- 3.Macdonald RL, OlsenR . GABA receptor channels. Physiology and Pathology of Chloride Transporters and Channels in the Nervous System: From Molecules to Diseases. 2009. p. 257. [Google Scholar]
- 4.Davies M, Bateson AN, Dunn SMJ. Molecular biology of the GABA A receptor: functional domains implicated by mutational analysis. Front Biosci. 1996;1:214–233. doi: 10.2741/a127. [DOI] [PubMed] [Google Scholar]
- 5.Sarto-Jackson I, Sieghart W. Assembly of GABAA receptors (Review) Mol Mem Biol. 2008;25:302–310. doi: 10.1080/09687680801914516. [DOI] [PubMed] [Google Scholar]
- 6.Florey E. GABA: history and perspectives. Can J Physiol Pharmacol. 1991;69:1049. doi: 10.1139/y91-156. [DOI] [PubMed] [Google Scholar]
- 7.Olsen RW, Sieghart W. GABAA receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Möhler H, Fritschy JM. GABAB receptors make it to the top--as dimers. Trends Pharmacol Sci. 1999;20:87. doi: 10.1016/s0165-6147(99)01323-1. [DOI] [PubMed] [Google Scholar]
- 9.Rodríguez GilDJ, Vacotto M, Rapacioli M, Scicolone G, Flores V, Fiszer dePlazas. Development and localisation of GABAA receptor 1, 2, 2 and 2 subunit mRNA in the chick optic tectum. J Neurosci Res . 2005;81:469–480. doi: 10.1002/jnr.20579. [DOI] [PubMed] [Google Scholar]
- 10.Burd GD, Rubel EW. Development of GABA immunoreactivity in brainstem auditory nuclei of the chick: ontogeny of gradients in terminal staining. J Comp Neurol. 1989;284:504–518. doi: 10.1002/cne.902840403. [DOI] [PubMed] [Google Scholar]
- 11.Enomoto K, Kataoka H, Hirota A. Semiquantitative Analysis of the erpression of GABA-A receptor subunits in the developing embryonic Chick Brain Stem. Japan J Physiol. 2001;51:53–61. doi: 10.2170/jjphysiol.51.53. [DOI] [PubMed] [Google Scholar]
- 12.Mehta AK, Ticku MK. An update on GABAA receptors. Brain Res Rev. 1999;29:196–217. doi: 10.1016/s0165-0173(98)00052-6. [DOI] [PubMed] [Google Scholar]
- 13.Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci . 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 14.Laurie D, Wisden W, Seeburg P. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]