Abstract
Objective(s)
The brief interruption of cerebral blood flow causes permanent brain damage and behavioral dysfunction. The hippocampus is highly vulnerable to ischemic insults, particularly the CA1 pyramidal cell layer. There is no effective pharmacological strategy for improving brain tissue damage induced by cerebral ischemia. Previous studies reported that pentoxifylline (PTX) has a neuroprotective effect on brain trauma. The possible neuroprotector effects of PTX on behavioral deficit were studied in male Wistar rats subjected to a model of transient global brain ischemia.
Materials and Methods
Animals (n= 32) were assigned to control, sham-operated, vehicle, and PTX- treated (200 mg/kg IP) groups. PTX administered at 1hr before and 3 hr after ischemia. Global cerebral ischemia was induced by bilateral common carotid artery occlusion, followed by reperfusion.
Results
Morris Water maze testing revealed that PTX administration in cerebral ischemia significantly improved hippocampal-dependent memory and cognitive spatial abilities after reperfusion as compared to sham-operated and vehicle-treated animals. After the behavioral test, the rats were sacrificed and brain sections were stained with Nissl staining. There were no significant differences between number of pyramidal cells in both control and PTX groups.
Conclusion
Our study demonstrated that pentoxifylline had a protective effect on rats with transient global ischemia and could reduce cognitive impairment.
Key Words: Neuroprotective, Pentoxifylline, Spatial Memory, Water Maze
Introduction
Stroke is a major cause of death in the developed countries (1- 3). Stroke causes immense human suffering leaving the patient usually grossly disabled. Therefore, it is viewed as a leading cause for the loss of quality-adjusted life-years (4). Stroke has been considered as untreatable and even today there is no effective drug therapy to help stroke patients.
The ischemia models have enabled the effective study of pathological mechanisms of stroke and within the last decades the complex molecular mechanisms leading to cell death following cerebral ischemia have been partly elucidated (5-8). This has lead to attempts to find ways to interfere with these mechanisms.
The attempts to help stroke patients have predominantly been concentrated on prevention of acute cell death. It has been shown that oxidative stress has an important role in the pathophysiology of stroke and brain ischemia–reperfusion can produce the excessive amount of both ROS and/or RNS Which can lead to cellular damage and promote cell death (9). The hippocampus is most vulnerable to the neurodegenerative effects of ischemia in humans (10-12) and animals (13, 14). Behavioral and cognitive disturbances, particularly within the learning and memory domains, are the most visible symptoms of cerebral Ischemia.
Indeed, more than one hundred agents have been proved to be neuroprotective in experimental models (15). Unfortunately, despite these promising prospects in the prevention of neurodegeneration and cell death, the drugs that have been evaluated clinically have failed, usually because of an unsuitable time-window, lack of efficacy or the presence of unwanted side effects (16, 17).
Pentoxifylline (PTX) is a methylxanthine derivative and a nonspecific type 4 phosphodiesterase inhibitor, clinically used in the treatment of lower extremity claudication. The mechanisms underlying its beneficial effects seem to be related to alterations in cellular functions and to the improvement of microcirculatory perfusion in both peripheral and cerebral vascular beds (18, 19). PTX is termed a hemorrheologic modifier for its effects decreasing the deformability of red blood cells. In vitro as well as in vivo experiments indicated an additional therapeutic potential for PTX as an anti-inflammatory and immunomodulator (20-22).
The PTX anti-inflammatory properties include the inhibition of TNF-alpha production (23) that seems to be due to reduced TNF protein levels by the inhibition of TNF mRNA transcription (24). TNF-alpha is expressed in the ischemic brain (25), and is known to rapidly upregulate in the brain after injury (26). This last study demonstrated that the exogenous TNF-alpha exacerbates focal ischemic injury, and the blockade of the endogenous TNF-alpha is neuroprotective. Furthermore, TNFalpha inhibition may represent a novel pharmacological strategy for the treatment of ischemic stroke.
Previous studies reported that PTX has a neuroprotective effect in experimental models of global as well as focal cerebral ischemia. Thus, PTX treatment has been shown to improve recovery of the cerebral electrical function in dogs, after transient cerebral global ischemia, by a mechanism that does not involve improvement of cerebral blood flow or global oxygen consumption (27). Furthermore, the pretreatment with PTX decreased the incidence and severity of hypoxic-ischemic injury in immature rat brain, by attenuating the expression of IL-1beta and TNF-alpha genes (28). PTX also afforded neuroprotection in a rat model of cerebral ischemia, such as occlusion of the ipsilateral common carotid and middle cerebral arteries (29). The objectives of the present work were to investigate the possible neuroprotective effects of pentoxifylline on a model of global transient ischemia, by evaluating the animal's locomotor activity and cognitive functions (acquisition and learning processes, and spatial memory).
Materials and Methods
Animals & chemicals
Adult male Wistar rats 12-13-weeks-old weighing (250-300 g) from Pharmacology Department of Tehran University of Medical Sciences were used in all experiments. The rats were housed under a 12 hr. Light/dark cycle. Animals were allowed free access to food and water. All of them were housed in animal house for at least 5 days prior to experiments.
All procedures used in the study were approved by the ethics committee for the use of experimental animals at Tehran University of Medical Sciences.
All chemicals were purchased from Sigma except PTX powder that was gifted kindly by the Amin Pharmaceutical Co (Esfehan-Iran).
Experimental groups and drugs
Animals (n=32) were divided randomly into 4 groups as described below:
1- Control group: rats only anesthetized by pentobarbital sodium (40 mg / kg)
2- Ischemia group: After anesthesia by Pentobarbital sodium, common carotid arteries on both sides occluded for 20 min followed by reperfusion.
3- Experimental Group: After anesthesia and ischemia for 20 min followed by reperfusion , 200 mg/kg PTX was injected intraperitoneally (IP) at the beginning of reperfusion phase.
4- Vehicle Group: After anesthesia and ischemia for 20 min followed by reperfusion, 0.5 ml was injected (IP) at the beginning of reperfusion phase.
Animals were sacrified after 4 days. All hippocampi were removed for histological assessment (Nissl method).
Surgical procedure
To induce transient cerebral ischemia, rats were anesthetised with sodium pentobarbital anesthesia (40 mg/kg, IP). A rectal temperature probe was inserted and body temperature was monitored and maintained at 37 °C using heating lamps. Both common carotid arteries were exposed, freed from its carotid sheet, then vagus nerves were carefully separated. Both common carotid arteries were occluded for 20 min using Aneurism micro clips.
During ischemia the animals were monitored for body temperature, loss of righting reflex and unresponsiveness to gentle touch.
Subsequently, the carotid arteries were released and inspected for immediate reperfusion. Recirculation of blood flow was established by releasing the clips and restoration of blood flow in the carotid arteries was confirmed by observation. Animals were returned to their home cage after the surgery and kept separately for 4 days (96 hr). Then, the rats were killed by decapacitation after perfusion intracardiacally. Brains were rapidly, removed and put in the fixator for more than 3 days.
Histopathology
A period of 4 days after ischemia, rats were anesthetized intraperitoneally with pentobarbital-Na (40 mg/kg) and transcardic perfusion was performed with heparin (10 U/ml) in 0.9% saline, followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH=7.4). Their brain were removed and post –fixed in the same fixator for more than 3 days. Paraffin-embedded coronal sections were cut for Nissl staining method.
Nissl staining
For Nissl staining, 10 µm-thick sections were mounted directly onto gelatin-coated glass slides and air-dried. The slides were stained with 1.0% cresyl violet, dehydrated, and cover slipped with Entellan. The number of the CA1 pyramidal cells of hippocampus in stained sections (3 sections of the hippocampus of each rat between the levels of 2/3 and 5 mm posterior to bregma fortune) were counted at x400 magnification of light microscope by blindly investigation. Only cells with evident nucleus and nucleolus were included. Images were taken at x400 magnification with a microscope (Olympus AX-70) and analyzed by image tool 2 software.
Morris water maze
To assess spatial learning, the Morris water maze task was used and performed as previously described (30). Briefly, a hidden clear plastic platform (18 cm diameter) was placed 47 cm away from the wall of the water maze (170 cm diameter, 45-cm deep) and 2 cm below the water surface. The platform remained in the same location for all sessions and trials during-pretraining and testing.
The water maze was divided into four quadrants and the starting quadrant was randomized daily, with all rats using the same daily order. Rats were released into the maze head-up and facing the wall of the maze. If an animal failed to find the platform in 60 sec, it was then placed on the platform for 20 sec. Each session consisted of four trials, and data from these four trials were averaged to form the daily score. Rats were allowed to rest for a minimum of 30 sec between trials. All animals were pre-trained for 4 consecutive days in the week preceding 2 vessels occlusion. The escape latency, velocity and distance were recorded in all trials. The behavioral data set was analyzed.
Statistical analysis
The significant difference was determined by a one-way ANOVA, followed by the Tukey's multiple comparison test. Statistical significance was defined as a P value≤ 0.05.
Results
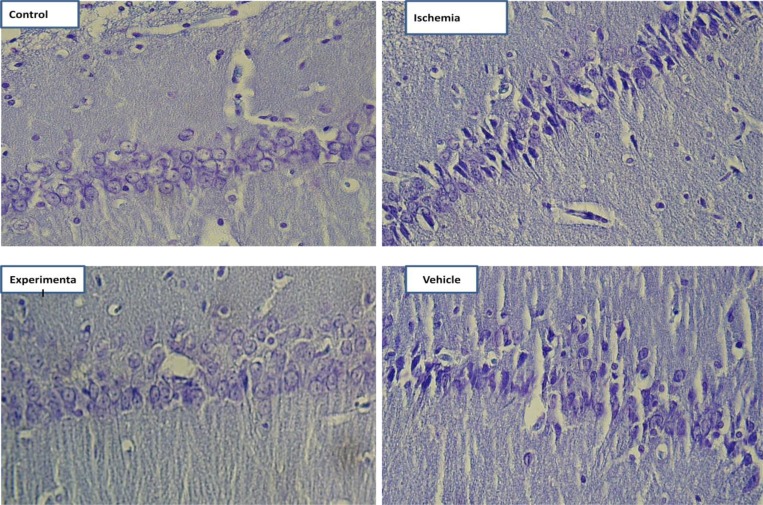
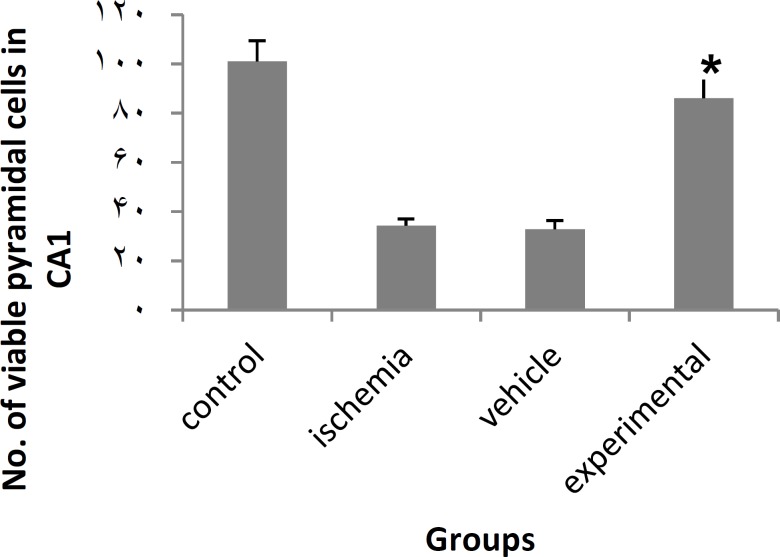
Data from cell count (Nissl staining) showed that 20 min of bilateral common carotid occlusion caused marked CA1 cell loss (Figure 1, 2). There was no statistically significant difference in number of viable pyramidal cells between control and experimental groups (Pvalue= 0.161).
Figure 1.
Photomicrographs of coronal sections of CA1 region of hippocampus. ((Nissl staining, Scale bar= 30µm)
Figure 2.
Effect of PTX on number of CA1 pyramidal cells in ischemia-induced memory deficit model.
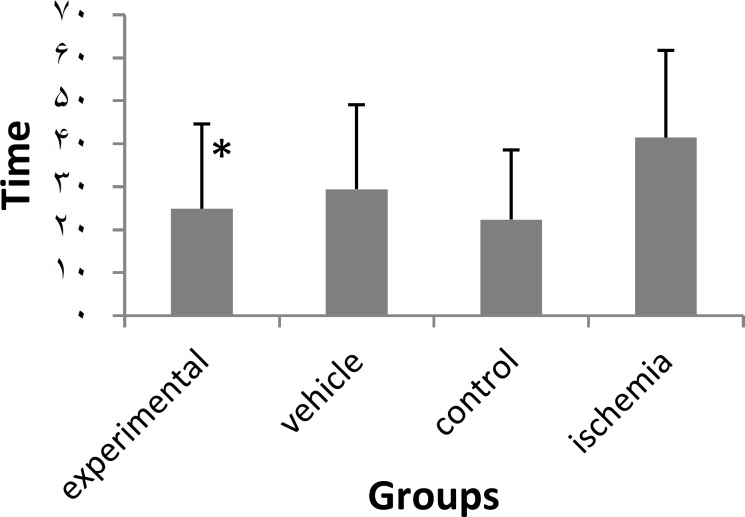
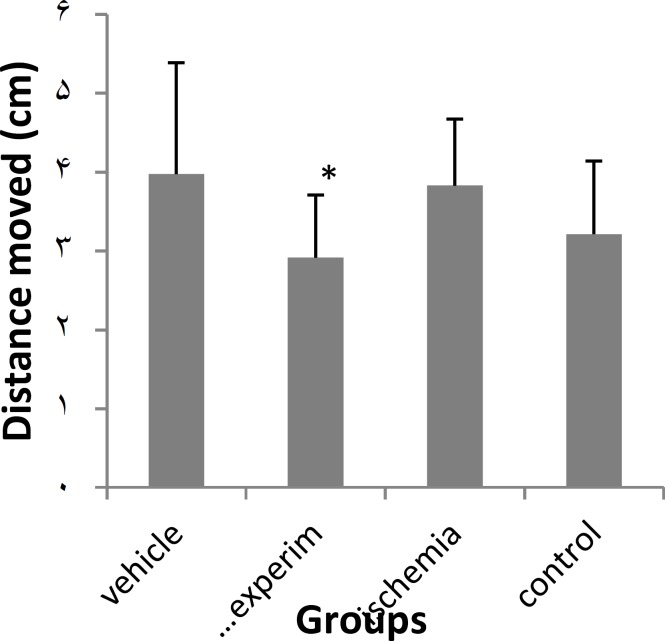
In the water maze task , used to evaluate spatial memory expressed as latency time (s) and distance moved (cm) to find the hidden platform, the experimental group (PTX) significantly improved the spatial memory and effects were significant different from those of sham-operated and vehicle groups but there was no significant difference between the experimental and control groups (Figures 3, 4).
Figure 3.
Effect of PTX on latency time in ischemia-induced memory deficit model.
*P≤ 0.05, statistically different from ischemia and vehicle groups. Data are mean±SD
Figure 4.
Effect of PTX on distance moved in ischemia-induced memory deficit model.
*P≤ 0.05, statistically different from ischemia and vehicle groups. Data are mean±SD
Discussion
In this study pyramidal cells of CA1 region were damaged and spatial memory deficit was seen in rats which were subjected to 20 min bilateral common carotid occlusion. Transient global cerebral ischemia is a clinical outcome of cardiac arrest and other situations that depletes the oxygen in brain during a short period which can lead to the loss of CA1 neurons of the hippocampus (31-33). Degeneration of the CA1 pyramidal neurons is associated with severe impairments of hippocampal functions, such as spatial learning and memory (34).
Although the mechanism of ischemia/reperfusion (IR) remains unclear, it seems that reactive oxygen species (ROS) are one of the most important factors that induce neuronal death in IR insult. It is well believed that IR is accompanied by the excessive generation of ROS, which may either directly damage the cellular macromolecule to cause cellular signaling pathways or gene regulation to induce apoptosis (35).
Due to the oxidative mechanism of ischemia-induced cell death and injury, there is increasing interest in focusing on neuroprotective agents that may ameliorate the damage of ROS (36).
Anti-inflammatory properties of PTX include the inhibition of TNF-alpha production which is expressed in brain after ischemia (23).
PTX is known to inhibit TNF-alpha, PAF and phosphodiesterase inhibition has been proposed as an effective strategy to decrease the severity of neonatal hypoxic-ischemic brain injury (37). Besides, a report indicated that the PTX treatment dose dependently prevents the occurrence of spontaneous brain damage, by reducing inflammatory events (38) what might be the case in the present study.
PTX can block cytokine expression (39) so it reduces the activation of NF-Kappa B and the production of TNF-alpha (40-42). These anti-inflammatory related events, associated with a possible action of PTX on elevating intracellular cAMP and reduction of oxidative stress (43-49), could be responsible for the neuroprotection afforded by this drug, leading to a decrease in neurologic deficits and an improvement in dopaminergic neurotransmission. Our data agree with another study suggesting that PTX reduces cerebral injury and preserves neurologic functions in transient global ischemia, in rats (50).
In the present study deficits in the ischemia and vehicle groups were observed in spatial memory but much better results were demonstrated in the water maze task in the experimental group. These results suggest that PTX has neuroprotective effect which is in agree with the findings of Bruno et al (51) but despite of that research, the effective dose of PTX in our study is 200 mg/kg.
Acknowledgment
This work was supported by a grant from the Cellular and Molecular Research Centre, Tehran University of Medical Sciences, Tehran, Iran. Also we would like to thank Amin Pharmaceutical Company (Esfahan-Iran) for the gift of pentoxifylline.
References
- 1.Diener HC. Empfehlungen für die Einrichtung von Schlaganfallspezial-stationen (Stroke units) Act Neurol. 1996;23:171–175. [Google Scholar]
- 2.Nagahiro S, Uno M, Sato K, Goto S, Morioka M, Ushio Y. Pathophysiology and treatment of cerebral ischemia. J Med Invest. 1998;45:57–70. [PubMed] [Google Scholar]
- 3.Wolf PA, Kannel WB, D’Agostino RB. Epidemiology of Stroke. In: Ginsberg MD, Bogousslavsky J, editors. Cerebrovascular disease: pathophysiology, diagnosis, and management. Massachusetts: Blackwell Science, Malden; 1998. pp. 834–849. [Google Scholar]
- 4.Broderick J, Brott T, Kothari R, Miller R, Khoury J, Pancioli A, et al. The greater cincinnati/northern kentucky stroke study: preliminary first ever and total incidence rates of stroke among blacks. Stroke. 1998;29:415–451. doi: 10.1161/01.str.29.2.415. [DOI] [PubMed] [Google Scholar]
- 5.Dietrich WD. Neurobiology of stroke. Int Rev Neurobiol. 1998;42:55–101. doi: 10.1016/s0074-7742(08)60608-x. [DOI] [PubMed] [Google Scholar]
- 6.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 7.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 8.Snider BJ, Gottron FJ, Choi DW. Apoptosis and necrosis in cerebrovascular disease. Ann N Y Acad Sci. 1999;893:243–253. doi: 10.1111/j.1749-6632.1999.tb07829.x. [DOI] [PubMed] [Google Scholar]
- 9.Li RC, Guo SZ, Lee SK, Gozal D. Neuroglobin protects neurons against oxidative stress in global ischemia. J Cereb Blood Flow Metab. 2010;30:1874–1882. doi: 10.1038/jcbfm.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cummings JL, Tomiyasu U, Read S, Benson DF. Amnesia with hippocampal lesions after cardiopulmonary arrest. Neurology. 1984;34:679–681. doi: 10.1212/wnl.34.5.679. [DOI] [PubMed] [Google Scholar]
- 11.Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the Hippocampus. J Neurosci. 1986;6:2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petito CK, Feldmann E, Pulsinelli WA, Plum F. Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology. 1987;37:1281–1286. doi: 10.1212/wnl.37.8.1281. [DOI] [PubMed] [Google Scholar]
- 13.Pulsinelli WA, Brierley JB. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 1979;10:267–272. doi: 10.1161/01.str.10.3.267. [DOI] [PubMed] [Google Scholar]
- 14.Pulsinelli WA, Buchan AM. The four-vessel occlusion rat model method for complete occlusion of vertebral arteries and control of collateral circulation. Stroke. 1988;19:913–914. doi: 10.1161/01.str.19.7.913. [DOI] [PubMed] [Google Scholar]
- 15.Hachinski V. Relevance of stroke models of stroke. Arch Neurol. 1996;53:1308. doi: 10.1001/archneur.1996.00550120120027. [DOI] [PubMed] [Google Scholar]
- 16.Grotta J. The current status of neuronal protective therapy: why have all neuronal protective drugs worked in animals but none so far in stroke patients. Cerebrovasc Dis. 1994;4:115–120. [Google Scholar]
- 17.The European Ad Hoc Consensus G: Neuroprotection as initial therapy in acute stroke. Third Report of an Ad Hoc Consensus Group Meeting. Cerebrovasc Dis. 1998;8:59–72. doi: 10.1159/000015817. [DOI] [PubMed] [Google Scholar]
- 18.Seiffge D. Pentoxifylline: its influence on interaction of blood cells with the vessel wall. Atherosclerosis. 1997;131:27–28. doi: 10.1016/s0021-9150(97)06121-2. [DOI] [PubMed] [Google Scholar]
- 19.Windmeier C, Gressner AM. Pharmacological aspects of pentoxifylline with emphasis on its inhibitory actions on hepatic fibrogenesis. Gen Pharmacol. 1997;29:181–196. doi: 10.1016/s0306-3623(96)00314-x. [DOI] [PubMed] [Google Scholar]
- 20.Teixeira MM, Gristwood RW, Cooper N, Hellewell PG. Phosphodiesterase (PDE)4 inhibitors: anti-inflammatory drugs for the future. Trends Pharmacol Sci . 1997;18:164–170. doi: 10.1016/s0165-6147(97)01049-3. [DOI] [PubMed] [Google Scholar]
- 21.Laurat E, Poirier B, Tupin E, Caligiuri G, Hansson GK, Bariety J, et al. In vivo downregulation of T helper cell 1 immune responses reduces atherogenesis in apolipoprotein E-knockout mice. Circulation. 2001;194:197–202. doi: 10.1161/01.cir.104.2.197. [DOI] [PubMed] [Google Scholar]
- 22.Haddad JJ, Land SC, Tarnow-Mordi WO, Zembala M, Kowalczyk D, Lauterbach R. Immunopharmacological potential of selective phosphodiesterase inhibition. I. Differential regulation of lipopolysaccharide-mediated proinflammatory cytokine (interleukin-6 and tumor necrosis factor-alpha) biosynthesis in alveolar epithelial cells. J Pharmacol Exp Ther. 2002;300:559–566. doi: 10.1124/jpet.300.2.559. [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez M, Diez TejedorE, Alonso deLeciñanaM, Fuentes B, Carceller F, Roda JM. Thrombolysis and neuroprotection in cerebral ischemia. Cerebrovasc Dis. 2006;2:118–126. doi: 10.1159/000091711. [DOI] [PubMed] [Google Scholar]
- 24.Lin SL, Chiang WC, Chen YM, Lai CF, Tsai TJ, Hsieh BS. The renoprotective potential of pentoxifylline in chronic kidney disease. J Chin Med Assoc. 2004;68:99–105. doi: 10.1016/S1726-4901(09)70228-X. [DOI] [PubMed] [Google Scholar]
- 25.Navashiro H, Martin D, Hallenbeck JM. Inhibition of tumor necrosis factor and amelioration of brain infarction in mice. J Cereb Blood Flow Metab. 1997;17:229–232. doi: 10.1097/00004647-199702000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, et al. Tumor necrosis factoralpha. A mediator of focal ischemic brain injury. Stroke. 1997;28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- 27.Toung TJ, Kirsch JR, Maruki Y, Traystman RJ. Effects of pentoxifylline on cerebral blood flow, metabolism, and evoked response after total cerebral ischemia in dogs. Crit Care Med. 1994;22:273–281. doi: 10.1097/00003246-199402000-00019. [DOI] [PubMed] [Google Scholar]
- 28.Kim KB, Jeon GH, Kim YR, Lee JH, Lee KH, Eun BL, et al. The effect of pentoxifylline on IL-1 beta and TNF-alpha mRNA gene expression in hypoxic-ischemic brain injury of immature rat. J Korean Child Neurol Soc. 1999;7:181–187. [Google Scholar]
- 29.Evans SM, Pinto PereiraLM, Addae JI. Neuroprotection by caffeine and pentoxifylline during cerebral ischaemia. West Indian Med J. 1999;48:23–25. [PubMed] [Google Scholar]
- 30.Xu D, Bureau Y, McIntyre DC, Nicholson DW, Liston P, Zhu Y, et al. Attenuation of ischemia-induced cellular and behavioral deficits by X chromosome-linked inhibitor of apoptosis protein overexpression in the rat hippocampus. J Neurosci. 1999;19:5026–5033. doi: 10.1523/JNEUROSCI.19-12-05026.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanabe M, Watanabe T, Ishibashi M, Hirano N, Tabuchi S, Takigawa H. Hippocampal ischemia in a patient who experienced transient global amnesia after undergoing cerebral angiography. Case illustration. J Neurosurg. 1999;91:347. doi: 10.3171/jns.1999.91.2.0347. [DOI] [PubMed] [Google Scholar]
- 32.Colbourne F, Li H, Buchan AM, Clemens JA. Continuing postischemic neuronal death in CA1: influence of ischemia duration and cytoprotective doses of NBQX and SNX-111 in rats. Stroke. 1999;30:662–668. doi: 10.1161/01.str.30.3.662. [DOI] [PubMed] [Google Scholar]
- 33.Bendel O, Bueters T, von EulerM, Ögren SO, Sandin J, von EulerG. Reappearance of hippocampal CA1 neurons after ischemia is associated with recovery of learning and memory. J Cereb Blood Flow Metab. 2005;25:1586–1595. doi: 10.1038/sj.jcbfm.9600153. [DOI] [PubMed] [Google Scholar]
- 34.von EulerM, Bendel O, Bueters T, Sandin J, von EulerG. Profound but transient deficits in learning and memory after global ischemia using novel water maze test. Behav Brain Res. 2006;166:204–210. doi: 10.1016/j.bbr.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 35.Sugawara T, Chan PH. Reactive oxygen radicals and pathogenesis of neuronal death after cerebral ischemia. Antioxid Redox Signal. 2003;5:597–607. doi: 10.1089/152308603770310266. [DOI] [PubMed] [Google Scholar]
- 36.Zhang YB, Kan MY, Yanga ZH, Dingc WL, Yid J, Chena HZ, et al. Neuroprotective effects of N-stearoyltyrosine on transient global cerebral ischemia in gerbils. Brain Res. 2009;1287:55–64. doi: 10.1016/j.brainres.2009.06.070. [DOI] [PubMed] [Google Scholar]
- 37.Eun BL, Liu XH, Barks JD. Pentoxifylline attenuates hypoxic-ischemic brain injury in immature rats. Pediatr Res. 2000;47:73–78. doi: 10.1203/00006450-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 38.Banfi C, Sironi L, De SimoniG, Gelosa P, Barcella S, Perego C, et al. Pentoxifylline prevents spontaneous brain ischemia in stroke-prone rats. J Pharmacol Exp Ther. 2004;310:890–895. doi: 10.1124/jpet.104.067090. [DOI] [PubMed] [Google Scholar]
- 39.Sliwa K, Woodiwiss A, Candy G, Badenhorst D, Libhaber C, Norton G, et al. Effects of pentoxifylline on cytokine profiles and left ventricular performance in patients with decompensated congestive heart failure secondary to idiopathic dilated cardiomyopathy. Am J Cardiol. 2002;90:1118–1122. doi: 10.1016/s0002-9149(02)02779-0. [DOI] [PubMed] [Google Scholar]
- 40.Ji Q, Zhang L, Jia H, Xu J. Pentoxifylline inhibits endotoxin-induced NF-Kappa B activation and associated production of proinflammatory cytokines. Ann Clin Lab Sci. 2004;34:427–436. [PubMed] [Google Scholar]
- 41.Zhang M, Xu YJ, Saini HK, Turan B, Liu PP, Dhalla NS. Pentoxifylline attenuates cardiac dysfunction and reduces TNF-alpha level in ischemic-reperfused heart. Am J Physiol Heart Circ Physiol . 2005;289:H832–839. doi: 10.1152/ajpheart.00178.2005. [DOI] [PubMed] [Google Scholar]
- 42.Zhang M, Xu YJ, Saini HK, Turan B, Liu PP, Dhalla NS. TNF-alpha as a potential mediator of cardiac dysfunction due to intracellular Ca2+-overload. Biochem Biophys Res Commun. 2005;327:57–63. doi: 10.1016/j.bbrc.2004.11.131. [DOI] [PubMed] [Google Scholar]
- 43.Davila-Esqueda ME, Martinez-Morales F. Pentoxifylline diminishes the oxidative damage to renal tissue induced by streptozotocin in the rat. Exp Diabesity Res. 2004;5:245–251. doi: 10.1080/154386090897974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davila-Esqueda ME, Vertiz-Hernandez AA, Martinez-Morales F. Comparative analysis of the renoprotective effects of pentoxifylline and vitamin E on streptozotocin-induced diabetes mellitus. Ren Fail. 2005;27:115–122. [PubMed] [Google Scholar]
- 45.Radfar M, Larijani B, Hadjibabaie M, Rajabipour B, Mojtahedi A, Abdollahi M. Effects of pentoxifylline on oxidative stress and levels of EGF and NO in blood of diabetic type-2 patients: a randomized, double-blind placebo-controlled clinical trial. Biomed Pharmacother . 2005;59:302–306. doi: 10.1016/j.biopha.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 46.Myers SI, Horton JW, Hernandez R, Walker PB, Vaughan WG. Pentoxifylline protects splanchnic prostacyclin synthesis during mesenteric ischemia/reperfusion. Prostaglandins. 1994;47:137–150. doi: 10.1016/0090-6980(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 47.Schermuly RT, Roehl A, Weissmann N, Ghofrani HA, Leuchte H, Grimminger F, et al. Combination of nonspecific PDE inhibitors with inhaled prostacyclin in experimental pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1361–L1368. doi: 10.1152/ajplung.2001.281.6.L1361. [DOI] [PubMed] [Google Scholar]
- 48.Strieter RM, Remick DG, Ward PA, Spengler RN, Lynch JP, 3rd LarrickJ, et al. Cellular and molecular regulation of tumor necrosis factor-alpha production by pentoxifylline. Biochem Biophys Res Commun. 1988;155:1230–1236. doi: 10.1016/s0006-291x(88)81271-3. [DOI] [PubMed] [Google Scholar]
- 49.Guggilam A, Haque M, Kerut EK, McIlwain E, Lucchesi P, Seghal I, et al. TNF-alpha blockade decreases oxidative stress in the paraventricular nucleus and attenuates sympathoexcitation in heart failure rats. Am J Physiol Heart Circ Physiol. 2007;293:H599–609. doi: 10.1152/ajpheart.00286.2007. [DOI] [PubMed] [Google Scholar]
- 50.Sirin BH, Yilik L, Coskun E, Ortac R, Sirin H. Pentoxifylline reduces injury of the brain in transient ischemia. Acta Cardiol. 1998;53:89–95. [PubMed] [Google Scholar]
- 51.Bruno RdeB, Marques TF, Batista TM, Lima JC, de ArrudaKG, Lima PF, et al. Pentoxifylline treatment improves neurological and neurochemical deficits in rats subjected to transient brain ischemia. Brain Res. 2009;1260:55–64. doi: 10.1016/j.brainres.2008.12.064. [DOI] [PubMed] [Google Scholar]