Abstract
Background
Methadone disposition and pharmacodynamics are highly susceptible to interactions with antiretroviral drugs. Methadone clearance and drug interactions have been attributed to cytochrome P4503A4 (CYP3A4), but actual mechanisms are unknown. Drug interactions can be both clinically and mechanistically informative. This investigation assessed effects of the protease inhibitor indinavir on methadone pharmacokinetics and pharmacodynamics, hepatic and intestinal CYP3A4/5 activity (using alfentanil), and intestinal transporter activity (using fexofenadine).
Methods
Twelve healthy volunteers underwent a sequential crossover. On three consecutive days they received oral alfentanil plus fexofenadine, intravenous alfentanil, and intravenous plus oral (deuterium-labeled) methadone. This was repeated after 2 weeks of indinavir. Plasma and urine analytes were measured by mass spectrometry. Opioid effects were measured by miosis.
Results
Indinavir significantly inhibited hepatic and first-pass CYP3A activity. Intravenous alfentanil systemic clearance and hepatic extraction were reduced to 40-50% of control, apparent oral clearance to 30% of control, and intestinal extraction decreased by half, indicating 50% and 70% inhibition of hepatic and first-pass CYP3A activity. Indinavir increased fexofenadine area under the plasma concentration-time curve 3-fold, suggesting significant P-glycoprotein inhibition. Indinavir had no significant effects on methadone plasma concentrations, methadone N-demethylation, systemic or apparent oral clearance, renal clearance, hepatic extraction or clearance, or bioavailability. Methadone plasma concentration-effect relationships were unaffected by indinavir.
Conclusions
Despite significant inhibition of hepatic and intestinal CYP3A activity, indinavir had no effect on methadone N-demethylation and clearance, suggesting little or no role for CYP3A in clinical disposition of single-dose methadone. Inhibition of gastrointestinal transporter activity had no influence of methadone bioavailability.
Introduction
Methadone is a utilitarian opioid, owing to effectiveness in treating numerous pain conditions and opiate addiction, administration by numerous routes, and long duration of effect. Methadone is cost-effective for treating acute, chronic, neuropathic, and cancer pain, in adults and children, in first- or second-line therapy, and can be administered orally, intravenously, nasally, and rectally.1-4 It is particularly useful perioperatively.4 Methadone use grew substantially over the last decade, primarily if not exclusively for pain, becoming a “darling drug of the pain management community”.5 Methadone prescriptions increased 13-fold 1997-2006,*,† and 7-fold (population-adjusted) 1997-2004.6 Tragically, however, during the same period, population-adjusted accidental methadone-related deaths increased 1800%, fatalities increased 390%, methadone was the drug with the greatest increase in fatalities, and the sixth most frequently suspected drug in death and serious nonfatal outcomes.6,‡ The decade-long increase in methadone toxicity persists.
Methadone use is confounded by considerable inter- and intra-individual variability in pharmacokinetics, including metabolism, clearance, and drug interactions susceptibility, which can cause toxicity, inadequate analgesia, or withdrawal.7-9 Toxicity may occur at seemingly therapeutic plasma concentrations, suggesting pharmacodynamic variability or drug interactions. Despite decades of investigation, cause(s) of variable methadone disposition remain enigmatic. Much attention focused on identifying cytochrome P450(s) (CYP) responsible for methadone clearance and metabolism to the inactive N-demethylated metabolite 2-ethyl-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP). N-demethylation is considered a determinant of methadone elimination, because clearance and plasma EDDP/methadone area under the curve ratio are correlated.10 For more than a decade, and based on extrapolation of initial in vitro metabolism studies, numerous publications and clinical guidelines attributed clinical methadone disposition to CYP3A4, and warn of CYP3A4-mediated drug interactions.8,11-17 Newer information, however, consistently shows both CYP2B6 and CYP3A4 having the highest activity towards methadone N-demethylation in vitro, and CYP2B6 but not CYP3A4 metabolizing methadone stereoselectively, mirroring stereoselective methadone clearance.18-22 Nevertheless, it remains disputed which isoform determines clinical methadone disposition. Another potential pharmacokinetic factor is the efflux transporter P-glycoprotein (P-gp), which influences methadone intestinal absorption, brain access, pharmacodynamics, and analgesia in animals.23-26 In humans, P-gp participation in clinical methadone disposition and drug effects remains unknown.
Antiretroviral drugs cause well-known drug interactions, both untoward and therapeutically useful. Such interactions may also provide useful insights into mechanisms of drug disposition. Methadone-antiretroviral drug interactions are well-noted.8,17 Previous mechanistic investigations of methadone-antiretroviral interactions, including ritonavir, ritonavir/indinavir, and nelfinavir, showed no reduction (or an increase) in methadone metabolism and clearance, despite profound inhibition of hepatic and intestinal CYP3A4,27-30 suggesting lack of major CYP3A4 involvement in clinical methadone disposition. However alternative explanations have been offered, such as compensatory increases in methadone renal clearance.17
This comprehensive crossover investigation in healthy volunteers determined (1) indinavir effects on methadone disposition and clinical effects, (2) potential mechanisms, including altered CYP3A and/or P-gp-mediated bioavailability, first-pass, and systemic clearance; (3) indinavir effects on methadone pharmacodynamics; and (4) ability of noninvasive miosis to detect indinavir-CYP3A drug interactions. Intravenous and oral (deuterium-labeled) methadone were administered simultaneously.19,27-30 Clearances of intravenous and oral alfentanil, a non-selective CYP3A4/5 (henceforth referred to as CYP3A) substrate, phenotyped hepatic and first-pass CYP3A activities.31,32 Oral fexofenadine probed P-gp.27-30 Miosis was a surrogate for opioid plasma concentrations, to estimate CYP3A activity noninvasively, and assess methadone pharmacodynamics.
Materials and Methods
Clinical Protocol
The investigation was approved by the University of Washington Institutional Review Board (Seattle, Washington) and subjects provided written informed consent. Inclusion and exclusion criteria were the same as described previously.27-30 The final study population was twelve healthy subjects (six men, six women; 23 ± 4 yr, range 18–31; 76 ± 13 kg, range 62–97).
The protocol was a 2-period sequential crossover (controls first, for logistical considerations) with each subject serving as their own control. Hepatic and first-pass CYP3A, intestinal transporters activity, and methadone disposition were assessed before and after 2 weeks of indinavir. Detailed aspects of the protocol were similar to those described previously,19,27-32 and are summarized here: First-pass CYP3A activity and intestinal P-gp (and other transporters) activity were evaluated using oral alfentanil and fexofenadine as in vivo probes. Subjects received 4 mg intravenous ondansetron followed 30 min later by 43 μg/kg oral alfentanil, and fexofenadine (60 mg) was administered 1 h later. Subjects received a standard breakfast and lunch 3 and 6 h after alfentanil. Venous blood was sampled and plasma stored for later analysis. Coincident with blood sampling, dark-adapted pupil diameter was measured (Pupilscan Model 12A infrared pupillometer, Keeler Instruments, Broomall, PA). The next day, hepatic CYP3A activity was evaluated using intravenous alfentanil (15 μg/kg alfentanil bolus), given 30 min after ondansetron. Subjects received a standard lunch 4 hr after alfentanil, and free access to food and water thereafter. Venous blood was sampled and dark-adapted pupil diameter was measured coincident with blood sampling. The next day, oral deuterated racemic (d5)-methadone HCl (11.0 mg, equivalent to 9.86 mg free base) and intravenous racemic unlabelled (d0)-methadone HCl (6.0 mg, equivalent to 5.4 mg free base, Roxane Laboratories, Columbus, OH) were simultaneously administered, 30 min after ondansetron. Venous blood samples were obtained, dark-adapted pupil diameters were measured, and 24-h urine samples were collected for 96 h. After approximately one month, subjects began taking oral indinavir 800 mg three times daily for 21 days. The above drug administration and sampling protocols were repeated, beginning on day 16 of indinavir.
Sample size was determined using a paired t-test for the outcome variable methadone systemic clearance. A previous study found 22 and 33% interday/intrasubject variability in intravenous and oral methadone clearances, respectively, and to detect a 30% change in clearance, using a paired t-test, with 33% variability, 1–ß = 0.8, α = 0.05, would require 12 subjects.27-30
Plasma alfentanil and fexofenadine were simultaneously quantified using solid-phase extraction and liquid chromatography electrospray mass spectrometry, and plasma and urine methadone and 2-ethyl-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP) enantiomer concentrations were quantified using on-line extraction and electrospray mass spectrometry as described previously.27,29,30 Pharmacokinetic and pharmacodyamic data were analyzed using noncompartmental methods, as described previously.19,27-32 Area under the plasma concentration-time curve (AUC) was determined using the trapezoidal rule. Systemic clearance was (CLIV)=DoseIV,/AUCIV,, apparent oral clearance was (CL/F)=Doseoral/AUCoral,, bioavailability was (Foral)=(AUCoral/Doseoral) × (DoseIV/AUCIV), steady-state volume of distribution was (Vss)=mean residence time × CL, volume of distribution based on the terminal phase was (Vz)=Dose/(AUC × λ) where λ is the terminal elimination rate constant. Hepatic extraction ratio (EH) was determined as (CLIV/Qp), where hepatic plasma flow (Qp) was estimated as the product of hepatic blood flow (25.3 and 25.5 ml/kg in males and females)33 and hematocrit (estimated as 40 and 36 in males and females) and negligible extrahepatic metabolism was assumed. Gastrointestinal extraction ratio was (EG) = 1 - Foral/(FH × Fabs) where the oral dose was assumed to be entirely absorbed and thus Fabs was considered to be unity, and FH=1-EH. Alfentanil effect (miois) disposition curves were analyzed using noncompartmental analysis, analogously to conventional plasma concentration curves, to yield effect parameters (area under the effect curve, AUEC) similar to conventional pharmacokinetic parameters, as described previously.31,32
Statistical analysis
Two-tailed paired t-tests assessed differences between groups for pharmacokinetic and effect parameters. Non-normal data were log transformed for analysis but reported as nontransformed results (arithmetic mean ± SD). Statistical significance was assigned at p < 0.05. Area under the curve ratios (indinavir/control) were evaluated using the geometric mean and 90% confidence intervals, as recommended.§ Confidence intervals excluding 1.0 were considered statistically significant. Relationships between methadone clearance and CYP3A activity were evaluated by Spearman correlation analysis. Data were analyzed using SigmaPlot 11.2 (Systat Software Inc., San Jose, CA).
Modeling and Prediction
Mathematical model
In vitro-in vivo modeling using drug metabolism data was conducted to understand and predict inhibitor effects on alfentanil and methadone disposition. Reversible (competitive or noncompetitive) inhibition of a intravenously administered “victim” drug by a “perpetrator” drug is described by:34,35
| (1) |
where is the plasma AUC ratio of the victim drug in the absence (AUC) and presence (AUC’) of perpetrator, ClIV and Cl’IV are the uninhibited and inhibited clearances, fm is the fraction of drug cleared by the altered metabolic pathway of interest, IH,u is the unbound concentration of the perpetrator in the liver (typically estimated using the unbound plasma concentration), and the unbound inhibitor constant Ki,u is the free drug concentration that produces half-maximum enzyme inhibition. Ki,u is determined directly by measuring the unbound inhibitor concentrations in in vitro microsomal incubations, or by correcting the nominal (added) inhibitor concentrations for non-specific binding to microsomal lipid and protein, using the unbound fraction (fu,mic), calculated as:
| (2) |
where fu,mic is the unbound drug fraction in a microsomal incubation, C is the microsomal protein concentration (mg/ml), and log P/D is the octanol/buffer partition coefficient (for a weak base, pKa>7.4) or distribution coefficient (neutral or weak acid, pKa<7.4)36.
For hepatic clearance of an intravenous drug which is catalyzed exclusively by one process, enzyme or isoform(s) (e.g., CYP3A4/5), and incorporating the influence of the hepatic extraction ratio (EH),37,38 equation 1 becomes:
| (3) |
where fhep is the fraction of systemic clearance that results from hepatic clearance.
For hepatic clearance of an intravenous drug eliminated by multiple enzymatic pathways (p), and/or where each pathway is catalyzed by one or more enzymes or isoforms,37,38 equation 3 becomes:
| (4) |
where fm,n is the fraction of hepatic clearance that is the result of the nth enzyme.
When a victim drug is administered orally, reversible inhibition of intestinal enterocyte and hepatocyte metabolism is described by:34,35,39
| (5) |
When the perpetrator and substrate are not co-administered, the concentration of the perpetrator in enterocytes (IG) can be estimated using the unbound concentration of perpetrator in the systemic circulation.36 When the perpetrator and substrate are administered together, the predicted maximum concentration of perpetrator in the enterocyte (IG) is given by equation 2, where dose, ka, fa, and Qg are the perpetrator dose given orally, the first-order absorption rate constant, the fraction of perpetrator dose that is absorbed into the gut wall, and enterocyte blood flow, respectively.34 Values for ka, fa, and Qg are 0.03 min−1, 1.0, and 248 ml/min, respectively.34
| (6) |
Model Application
Indinavir is a reversible (variably described as noncompetitive, competitive, or mixed) human CYP3A inhibitor at clinically relevant concentrations (unlike other protease inhibitors, which cause irreversible mechanism-based inactivation).40-44 The Ki for indinavir in human liver microsomes has been reported (or calculated from a reported IC50) as 3μM,41 0.17-0.86 μM in six studies,42 and 0.5 μM,43 with an overall average of 0.49 μM in the latter studies. The Ki values reported or calculated for expressed CYP3A4 are 0.24 μM,44 and 0.75 μM.43 Based on protein concentrations provided in these reports, an indinavir octanol/water partition coefficient of 2.7 at pH 7, and equation 2, the average fu,mic is 0.53, and the average calculated indinavir Ki,u is 0.25 and 0.42 μM in human liver microsomes and CYP3A, respectively. Therefore subsequent calculations used 0.34 μM as the indinavir Ki,u.
Alfentanil is a low- to moderate-extraction drug cleared exclusively by hepatic metabolism, with systemic clearance equivalent to hepatic intrinsic clearance, and metabolism is catalyzed exclusively by CYPs 3A4 and 3A5.31,45,46 Based on previous studies,31 EH and FG are 0.3 and 0.6, fm (which is due entirely to CYP3A, and thus equal to fm,CYP3A) is 0.98, and fhep is 0.99.38 Therefore, equation (4) for intravenous dosing reduces to:
| (7) |
For oral alfentanil, equation 5 becomes:
| (8) |
Indinavir plasma concentrations were not measured. To estimate IH,u, it is necessary to note that indinavir is rapidly eliminated (1-2 h half-life), so even at steady-state, pre-dose plasma concentrations are extremely low if not negligible.47,48 Absorption of oral indinavir is slow, with maximum plasma concentrations occurring after 2 h.47,48 Average steady-state total indinavir concentrations over an 8 h dosing interval are 3.6 μM (based on total AUC divided by the doing interval),42,47,48 and, based on 61% protein binding,49 average unbound indinavir concentrations thus calculated as 1.4 μM. Subjects received their morning indinavir dose 3 h after intravenous alfentanil administration. Therefore, plasma indinavir concentrations were likely low or insignificant during the first 4 h of alfentanil elimination, and higher than average during the second 4 h. That supposition was confirmed by the observation that over the 8 h intravenous alfentanil AUC determinations, indinavir inhibition of alfentanil elimination was much greater in the second 4 h of compared with the first 4 h (vide infra). Therefore, over the total 8-h period of alfentanil measurement, average effective unbound indinavir concentrations (Ki,u) were estimated as 0.7 μM. Subjects received their morning indinavir dose 2 h after oral alfentanil, and took their evening dose after the last blood sample (12 h). Analogous to the above calculations, for oral alfentanil dosing, unbound indinavir plasma concentrations based on 8 h AUC should be divided over 12 h, giving an average unbound concentration of 1.0 μM. The calculated maximal enterocyte indinavir concentration after oral indinavir (equation 8) is 158 μM, equivalent to 84 μM when considering fu,mic.
Methadone is a low-extraction drug cleared by a combination of hepatic and renal clearance. For intravenous methadone, hepatic clearance (CLH) of R- and S-methadone averaged 73 and 79% of total clearance (CLIV), therefore fhep is 0.76, and EH averaged 0.09.27,29,30,50 The fraction of total hepatic methadone clearance catalyzed by CYP3A (fm,CYP3A) is unknown. This can, however, be approximated using urine excretion of methadone and EDDP, since the latter reflects CYP3A metabolism. For intravenous methadone, urine R-EDDP averaged 19% of the dose and 41% of the total R-enantiomer recovered, and urine S-EDDP averaged 33% of the dose and 61% of the total S-enantiomer recovered.27,29,30,50 If all of the nonrecovered portion of the dose was non-CYP3A4-mediated metabolism, then fm,CYP3A would be 0.19 and 0.33 for R- and S-methadone, respectively. Conversely, if the non-recovered portion of the dose was also proportionally metabolized by CYP3A4, then fm,CYP3A would be 0.41 and 0.61 for R- and S-methadone, respectively. These represent the lower and upper estimates of fm,CYP3A for R-methadone (0.19-0.41) and S-methadone (0.33-0.61). For the plasma methadone AUC ratio after intravenous dosing, equation 4 becomes:
| (9) |
Methadone undergoes little apparent intestinal metabolism, with FG predicted as 0.97,51 and averaging 0.96 in several investigations.27,29,30,50 Therefore, for the plasma methadone AUC ratio after oral dosing, equations (5) and (9) give:
| (10) |
If, however, only the CYP3A-catalyzed pathways for methadone are considered, and CYP3-dependent parameters such as the plasma EDDP/methadone AUC ratio or urine EDDP formation clearance (Clf) are the outcome, then fm,CYP3A is by definition 1.0, and the relevant equation is 11 for intravenous dosing (equivalent to fm,CYP3A=1 in equation 9), and to equation 12 for oral dosing:
| (11) |
| (12) |
Since indinavir is administered three times daily, and methadone concentrations are measured for 4 days, the average unbound indinavir plasma concentration of 1.4 μM is used in the above calculations. Indinavir was taken 3 h after oral methadone, and, since methadone absorption peaks after about 4 h, this would coincide with peak indinavir concentrations. Therefore, the inhibitor and substrate are effectively coadministered, and equation 6 used to calculate the predicted maximum enterocyte indinavir concentration (84 μM) after oral administration, for application in the above models.
Results
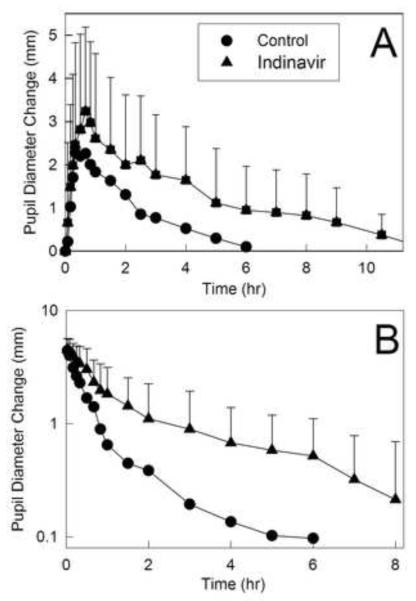
Miosis has been used as a surrogate for alfentanil plasma concentrations and clearance and a noninvasive probe for CYP3A activity,28-32 and pupil data were available before plasma alfentanil concentrations. Alfentanil miosis was thus used for early assessment of indinavir effects on CYP3A (fig. 1 and table 1). Indinavir increased and prolonged alfentanil miosis, and significantly increased the AUEC0–∞ ratio for both intravenous and oral alfentanil. These early results predicted significant inhibition of CYP3A activity.
Figure 1.
Indinavir effects on first-pass and hepatic cytochrome P4503A (CYP3A) activity, assessed using alfentanil as a CYP3A probe. Pupil diameter change from baseline (miosis) was used as a surrogate for alfentanil plasma concentrations. Shown is miosis after (A) 43 μg/kg oral alfentanil and (B) 15 μg/kg intravenous alfentanil. Each data point is the mean ± SD (n=12).
Table 1.
Alfentanil and methadone effect parameters
| Control | Indinavir | |
|---|---|---|
| Intravenous alfentanil | ||
| Maximum miosis (mm) | 4.5 ± 0.9 | 5.0 ± 1.0 |
| AUEC0-∞ (mm•hr) | 3.4 ± 2.6 | 9.2 ± 8.0 * |
| AUEC0-∞ ratio (indinavir/control) | 2.5 (1.5, 4.1) | |
| Effect t1/2 (hr) | 0.6 ± 0.5 | 1.6 ± 1.2 * |
| Oral alfentanil | ||
| Maximum miosis (mm) | 3.0 ± 1.6 | 4.3± 1.7 * |
| AUEC0-∞ (mm•hr) | 7.9 ± 5.1 | 15.3± 10.8 * |
| AUEC0-∞ ratio (indinavir/control) | 2.0 (1.4, 3.2) | |
| Effect t1/2 (hr) | 1.5 ± 1.4 | 2.3 ± 1.6 * |
| Methadone | ||
| Maximum miosis (mm) | 3.6 ± 1.0 | 3.6 ± 1.0 |
| AUEC0-∞ (mm•hr) | 87 ± 66 | 96± 56 |
| AUEC0-∞ ratio (indinavir/control) | 1.2 (0.9, 1.5) | |
| AUEC0-∞ / AUC0-∞ (mm•ml•ng−1) | 0.08 ± 0.05 | 0.09 ± 0.05 |
| AUEC0-∞ / AUC0-∞ ratio (indinavir/control) | 1.1 (0.8, 1.4) |
Subjects received 6.0 mg intravenous and 11.0 mg oral methadone HCl, 15 μg/kg intravenous alfentanil and 43 μg/kg oral alfentanil at all sessions. Results (n=12) are the arithmetic mean ± SD, except area under the curve ratios (indinavir/control) which are the geometric mean (90% CI). AUEC, area under the effect (miosis)-time curve; AUEC0-∞ / AUC0-∞ ratio is the methadone miosis AUEC and the total (intravenous plus oral) plasma R-methadone concentration AUC.
Significantly different from control (p<0.05)
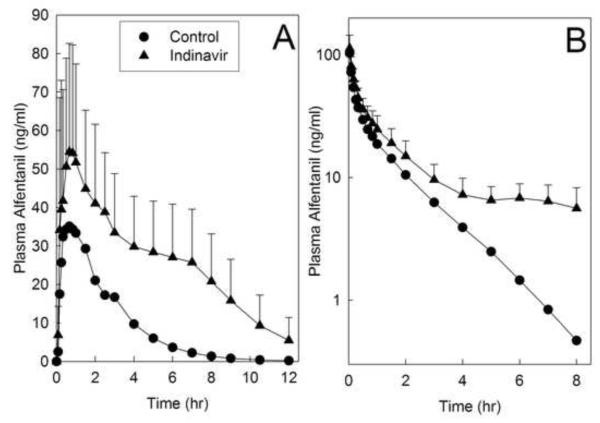
Indinavir effects on alfentanil plasma concentrations are shown in figure 2, and pharmacokinetic parameters provided in table 2. Indinavir significantly inhibited hepatic and first-pass CYP3A activity. The AUC ratio (indinavir/control) for intravenous alfentanil was significantly increased 2-fold by indinavir, and systemic clearance and the hepatic extraction ratio were both reduced to 40-50% of control, indicating 50% inhibition of hepatic CYP3A activity. Indinavir increased the AUC ratio for oral alfentanil more than 3-fold, reduced apparent oral clearance to 30% of control, and increased oral bioavailability, indicating 70% inhibition of first-pass CYP3A activity. The intestinal extraction ratio was decreased by half, indicating significant inhibition by indinavir of intestinal CYP3A activity. It is notable that indinavir inhibition of alfentanil elimination was much greater beginning 3-4 h after alfentanil administration (fig. 2). This was consistent with subjects receiving their morning indinavir dose 3 h after intravenous alfentanil and 2 h after oral alfentanil administration. Because predose plasma indinavir concentrations are very low, even at steady-state, and they peak 2 h after dosing,47,48 plasma indinavir concentrations were likely low or insignificant during the first 4 h of alfentanil elimination, and much higher in the 4-8 h thereafter.
Figure 2.
Indinavir effects on first-pass and hepatic cytochrome P4503A (CYP3A) activity, assessed using alfentanil as a CYP3A probe. Shown are alfentanil concentrations after (A) oral (43 μg/kg) and (B) intravenous (15 μg/kg) administration. Subjects received their morning indinavir dose 3 hr after intravenous alfentanil and 2 hr after oral alfentanil administration. Each data point is the mean ± SD (n=12).
Table 2.
Intravenous and oral alfentanil pharmacokinetic parameters
| Control | Indinavir | |
|---|---|---|
| Intravenous alfentanil | ||
| Cmax (ng/ml) | 103 ± 21 | 113 ± 32 |
| AUC0-∞ (ng •hr •ml−1) | 72 ± 27 | 136 ± 35 * |
| AUC0-∞ ratio (indinavir/control) | 2.0 (1.7, 2.3) | |
| CLIV (ml•kg−1•min−1) | 4.0 ± 1.5 | 2.0 ± 0.8 * |
| Elimination t1/2 (hr) | 1.3 ± 0.2 | 3.7 ± 1.2 * |
| EH | 0.25 ± 0.09 | 0.12 ± 0.02 * |
| Oral alfentanil | ||
| Cmax (ng/ml) | 46 ± 19 | 68 ± 23 * |
| AUC0-∞ (ng •hr •ml−1) | 107 ± 59 | 333± 157 * |
| AUC0-∞ ratio (indinavir/control) | 3.3 (2.6, 4.1) | |
| CL/F (ml•kg−1•min−1) | 9.4 ± 5.9 | 2.8 ± 1.7 * |
| Elimination t1/2 (hr) | 1.3 ± 0.3 | 2.2 ± 0.8 * |
| Foral | 0.51± 0.19 | 0.78 ± 0.23 * |
| EG | 0.35 ± 0.17 | 0.16 ± 0.21 * |
Subjects received 15 μg/kg intravenous alfentanil and 43 μg/kg oral alfentanil. Results are the arithmetic mean ± SD (n=12), except the AUC0-∞ ratio (indinavir/control), which is the geometric mean (90% CI). AUC, area under the plasma concentration-time curve; Cmax, peak plasma concentration; CLIV, systemic clearance of intravenous alfentanil; CL/F, apparent oral clearance of alfentanil; EH, hepatic extraction ratio; EG, intestinal extraction ratio; Foral, bioavailability.
Significantly different from control (p<0.05)
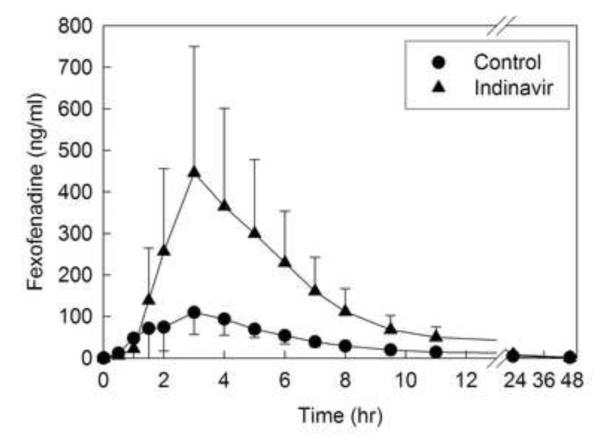
Disposition of oral fexofenadine was used to evaluate the activity of the intestinal efflux pump P-gp, and other gastrointestinal transporters. Indinavir significantly increased fexofenadine peak plasma concentrations and overall AUC 3-fold, with only a small decrease in the systemic elimination rate (fig. 3, table 3).
Figure 3.
Indinavir effects on gastrointestinal transporter activity, assessed using fexofenadine as a transporter probe. Each subject received 60 mg oral fexofenadine on all occasions. Each data point is the mean ± SD (n=12).
Table 3.
Fexofenadine pharmacokinetic parameters
| Control | Indinavir | |
|---|---|---|
| Cmax (ng/ml) | 134 ± 61 | 453± 298* |
| AUC0-∞ (ng •hr •ml−1) | 729 ± 185 | 2413 ± 1344 * |
| AUC0-∞ ratio (indinavir/control) | 2.9 (2.1, 4.0) | |
| CL/F (ml•kg−1•min−1) | 19.6 ± 5.6 | 7.3 ± 3.5 * |
| Elimination t1/2 (hr) | 13.6 ± 2.7 | 10.3 ± 3.6 * |
Results are the arithmetic mean ± SD (n=12), except the AUC0-∞ ratio (indinavir/control), which is the geometric mean (90% CI). AUC, area under the plasma concentration-time curve; Cmax, peak plasma concentration; CL/F, apparent oral clearance of fexofenadine.
Significantly different from control (p<0.05)
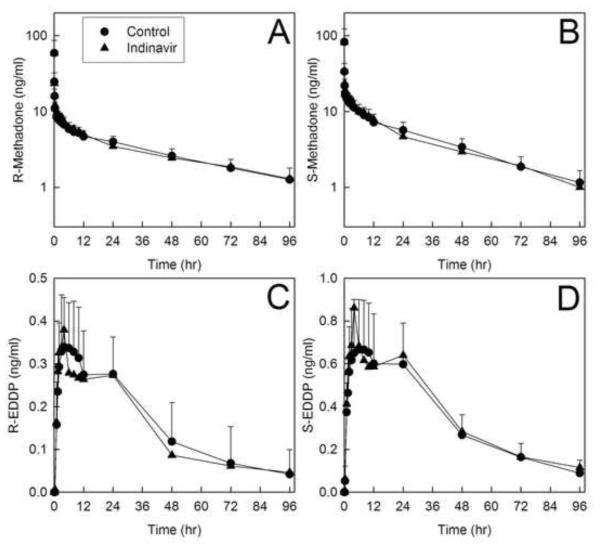
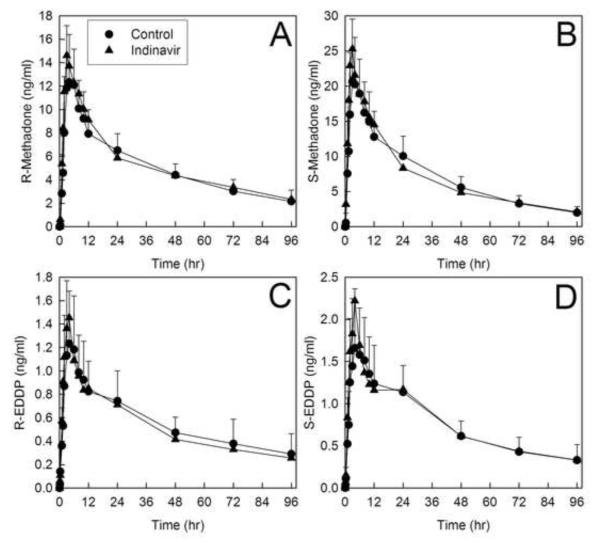
Disposition of both methadone enantiomers after intravenous administration was essentially unaffected by indinavir. Plasma concentrations are provided in figure 4, and pharmacokinetic parameters in tables 4 and 5. Methadone and EDDP plasma concentrations with and without indinavir pretreatment were essentially superimposable. The plasma AUCs and AUC ratios (indinavir/control) for both R- and S-methadone were unchanged, as were the systemic clearance, hepatic clearance, and hepatic extraction. R-methadone half-life was longer after indinavir, although clearance and volume of distribution were not changed. Methadone N-demethylation was similarly essentially unaffected by indinavir, which had no significant effect on the plasma AUC ratio (indinavir/control) for either R- and S-EDDP/methadone, nor on the formation clearances of R- and S-EDDP. A small difference in R-EDDP Cmax was observed. Renal clearance, which accounted for approximately one-third of both methadone enantiomers systemic clearance, was not altered by indinavir. Renal clearance was not significantly affected by indinavir.
Figure 4.
Effect of indinavir on intravenous methadone disposition. Shown are plasma (A) R-methadone, (B) R-EDDP (2-ethyl-1,5-dimethyl-3,3-diphenylpyrrolidine), (C) S-methadone and (D) S-EDDP concentrations. Subjects received 6.0 mg intravenous methadone HCl (5.4 mg free base). Each data point is the mean ± SD (n=12). Some SD are omitted for clarity.
Table 4.
Intravenous and oral methadone pharmacokinetic parameters
| Control | Indinavir | Control | Indinavir | |
|
|
|
|||
| Intravenous methadone | R-methadone |
S-methadone |
||
| Cmax (ng/ml) | 59 ± 28 | 54 ± 29 | 83 ± 39 | 76 ± 40 |
| AUC0-96 (ng •hr •ml−1) | 304 ± 54 | 304 ± 34 | 430 ± 93 | 418 ± 63 |
| AUC0-∞ (ng •hr •ml−1) | 387 ± 106 | 410 ± 77 | 481 ± 110 | 493 ± 113 |
| AUC0-∞ ratio (indinavir/control) |
1.07 (0.99, 1.16) | 1.03 (0.93, 1.13) | ||
| CLIV (ml•kg−1•min−1) | 1.64 ± 0.42 | 1.53 ± 0.43 | 1.33 ± 0.43 | 1.29 ± 0.37 |
| CLH (ml•kg−1•min−1) | 1.16 ± 0.36 | 1.02 ± 0.27 | 1.03 ± 0.38 | 0.96 ± 0.29 |
| Elimination t1/2 (hr) | 44 ± 10 | 54 ± 17 * | 30± 6 | 37± 15 |
| Vss (L/kg) | 5.6 ± 1.1 | 5.9 ± 1.0 | 3.1 ± 1.0 | 3.2± 0.6 |
| EH | 0.07 ± 0.02 | 0.06 ± 0.02 | 0.06 ± 0.02 | 0.06 ± 0.02 |
| R-EDDP |
S-EDDP |
|||
| Cmax (ng/ml) | 0.38 ± 0.12 | 0.40 ± 0.12 | 0.73 ± 0.24 | 0.90 ± 0.35 * |
| AUC0-96 (ng •hr •ml−1) | 16 ± 7 | 15 ± 6 | 33 ± 10 | 35 ± 8 |
| AUC∞ (ng •hr •ml−1) | 22 ± 10 | 24 ± 6 | 38 ± 11 | 42 ± 8 |
| Elimination t1/2 (hr) | 35 ± 12 | 40 ± 11 | 29 ± 6 | 33 ± 13 |
| AUC0-96 (EDDP/methadone) | 0.05 ± 0.02 | 0.05 ± 0.02 | 0.08 ± 0.03 | 0.09 ± 0.03 |
| AUC∞ (EDDP/methadone) | 0.06 ± 0.03 | 0.06 ± 0.02 | 0.08 ± 0.03 | 0.09 ± 0.03 |
| AUC∞ (EDDP/methadone) ratio (indinavir/control) |
1.03 (0.84, 1.27) | 1.10 (0.98, 1.23) | ||
| Oral methadone | R-methadone |
S-methadone |
||
| Cmax (ng/ml) | 15 ± 4 | 17 ± 4 * | 24 ± 7 | 30 ± 10 * |
| Tmax (hr) | 4 ± 1 | 3 ± 2 | 4 ± 2 | 3 ± 2 |
| AUC0-96 (ng •hr •ml−1) | 489 ± 104 | 509 ± 66 | 688 ± 169 | 685 ± 131 |
| AUC0-∞ (ng •hr •ml−1) | 641 ± 204 | 711± 154 | 780 ± 200 | 805 ± 194 |
| AUC0-∞ ratio (indinavir /control) |
1.12 (1.02, 1.23) | 1.04 (0.94, 1.16) | ||
| CL/F (ml•kg−1•min−1) | 1.86 ± 0.57 | 1.67 ± 0.61 | 1.57 ± 0.69 | 1.46 ± 0.46 |
| Elimination t1/2 (hr) | 45 ± 12 | 55 ± 20 * | 30 ± 6 | 35 ± 11 * |
| Vz/F | 6.8 ± 1.6 | 7.2 ± 1.2 | 4.0 ± 1.4 | 4.1 ± 0.7 |
| Foral | 0.91 ± 0.10 | 0.92 ± 0.10 | 0.87 ± 0.10 | 0.90 ± 0.11 |
| R-EDDP |
S-EDDP |
|||
| Cmax (ng/ml) | 1.5 ± 0.6 | 1.7 ± 0.7 | 1.9 ± 0.7 | 2.5 ± 1.0 * |
| AUC0-96 (ng •hr •ml−1) | 54 ± 17 | 52 ± 12 | 73 ± 20 | 76 ± 16 |
| AUC∞ (ng •hr •ml−1) | 83 ± 33 | 77 ± 20 | 101 ± 21 | 100 ± 22 |
| Elimination t1/2 (hr) | 56 ± 27 | 55 ± 29 | 47 ± 15 | 42 ± 10 |
| AUC0-96 (EDDP/methadone) | 0.11 ± 0.04 | 0.10 ± 0.03 | 0.11 ± 0.04 | 0.11 ± 0.03 |
| AUC∞ (EDDP/methadone) | 0.14 ± 0.06 | 0.12 ± 0.05 | 0.14 ± 0.06 | 0.13 ± 0.04 |
| AUC∞ (EDDP/methadone) ratio (indinavir/control) |
0.86 (0.72, 1.03) | 0.94 (0.84, 1.05) | ||
Subjects received 6.0 mg intravenous and 11.0 mg oral methadone HCl at all sessions. Results are the arithmetic mean ± SD (n=12), except area under the concentration-time curve (AUC) ratios (indinavir/control), which are the geometric mean (90% CI). AUC, area under the plasma concentration-time curve; Cmax, peak plasma concentration; CLH, hepatic clearance; CLIV, systemic clearance; CL/F, apparent oral clearance; Cmax, peak plasma concentration; EDDP, 2-ethyl-1,5-dimethyl-3,3-diphenylpyrrolidine; EH, hepatic extraction ratio; Foral, bioavailability; Vss, steady-state volume of distribution; Vz/F, apparent volume of distribution based on the terminal phase.
Significantly different from control (p<0.05)
Table 5.
Methadone and metabolite renal excretion and clearance
| Control | Indinavir | Control | Indinavir | |
|---|---|---|---|---|
|
|
|
|||
| R-methadone |
S-methadone |
|||
| % dose recovered 0-96 hr | ||||
| Intravenous d0-methadone | 29 ± 10 | 33 ± 5 | 23 ± 9 | 26 ± 4 |
| Oral d5-methadone | 24 ± 8 | 29 ± 3 * | 20 ± 7 | 22 ± 3 |
| d0-EDDP | 17 ± 5 | 15 ± 3 | 30 ± 10 | 31 ± 7 |
| d5-EDDP | 18 ± 5 | 18 ± 2 | 30 ± 9 | 32 ± 6 |
| Renal or metabolite formation clearance (ml•kg−1•min−1) |
||||
| Intravenous d0-methadone Clr | 0.48 ± 0.19 | 0.51 ± 0.18 | 0.30 ± 0.12 | 0.33 ± 0.11 |
| Oral d5-methadone Clr | 0.40 ± 0.16 | 0.44 ± 0.14 | 0.25 ± 0.09 | 0.28 ± 0.08 |
| d0-EDDP Clf | 0.27 ± 0.10 | 0.24 ± 0.08 * | 0.39 ± 0.16 | 0.41 ± 0.17 |
| d5-EDDP Clf | 0.29 ± 0.11 | 0.27 ± 0.09 | 0.38 ± 0.13 | 0.42 ± 0.17 |
Results are the mean ± SD (n=12) CLr, renal clearance; CLf, formation clearance; EDDP, 2-ethyl-1,5-dimethyl-3,3-diphenylpyrrolidine.
Significantly different from control (p<050.05)
Indinavir also had no significant influence on the disposition of oral methadone. Plasma concentrations are shown in figure 5, and pharmacokinetic parameters provided in tables 4 and 5. Methadone and EDDP plasma concentrations with and without indinavir pretreatment were essentially superimposable. Methadone enantiomers plasma AUCs and AUC ratios (indinavir/control) were negligibly or not changed. Methadone half-life was longer (15-20%) after indinavir, although apparent oral clearance and volume of distribution were unchanged, as was bioavailability. Methadone N-demethylation was also unchanged by indinavir. Neither the plasma AUC ratios (indinavir/control) for either R- and S-EDDP/methadone, nor the formation clearances of R- and S-EDDP, were different in indinavir-treated subjects.
Figure 5.
Effect of indinavir on oral methadone disposition. Shown are plasma (A) R-methadone, (B) R-EDDP (2-ethyl-1,5-dimethyl-3,3-diphenylpyrrolidine), (C) S-methadone and (D) S-EDDP concentrations. Subjects received 11.0 mg oral methadone HCl (9.9 mg free base). Each data point is the mean ± SD (n=12). Some SD are omitted for clarity.
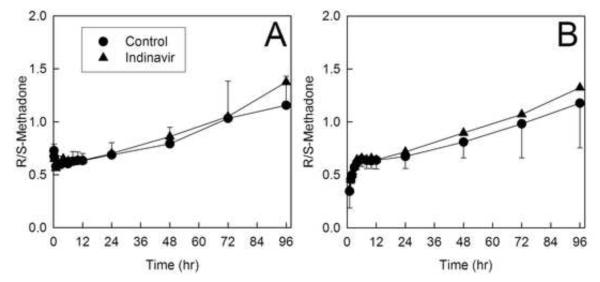
Methadone disposition is stereoselective, as is apparent from figures 4 and 5, and tables 3 and 4. Consequently, there is a well-described time-dependent increase in the plasma methadone R/S ratio (fig. 6). Indinavir, however, had no influence on the plasma R/S ratio, of both intravenous and oral methadone.
Figure 6.
Effect of indinavir on stereoselective methadone elimination. The plasma R/S-methadone concentration ratios for (a) intravenous and (b) oral methadone are shown. Each data point is the mean ± SD (n = 12).
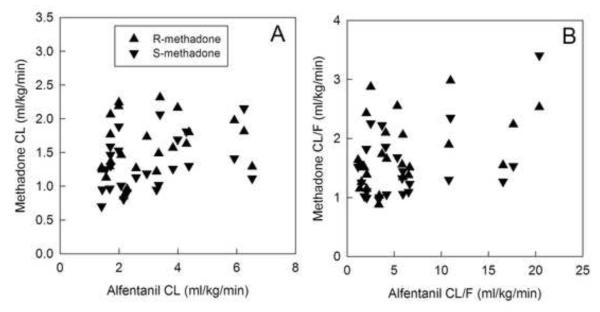
The relationship between methadone clearance and CYP3A activity, measured using alfentanil clearance, was evaluated by linear correlation analysis (fig. 7). For intravenous R- and S-methadone, there was no significant correlation between systemic methadone clearance and hepatic CYP3A activity. Similarly for oral R- and S-methadone, there was also no significant correlation between methadone oral clearance and first-pass CYP3A activity.
Figure 7.
Relationship between methadone enantiomers clearance (CL) and cytochrome P4503A (CYP3A) activity. (A) Intravenous (IV) methadone clearance and hepatic CYP3A activity (IV alfentanil CL). Spearman correlation coefficients were 0.36 and 0.35 for R- and S-methadone, respectively, both p>0.05. (B) Apparent oral methadone CL and first-pass CYP3A activity (oral alfentanil apparent CL). Spearman correlation coefficients were 0.40 and 0.20 for R- and S-methadone, respectively, both p>0.05. Each data point is the result for a single subject. There were no significant correlations between methadone clearance and CYP3A activity.
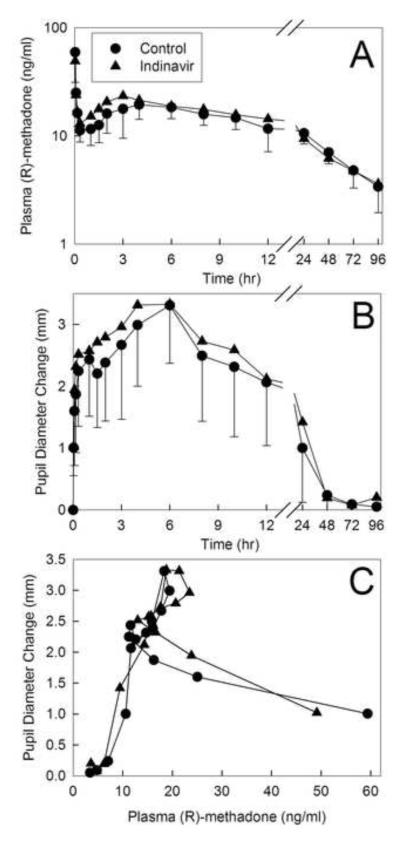
Methadone effects were evaluated using changes in dark-adapted pupil diameter (miosis). Plasma concentrations of total (sum of intravenous d0- and oral d5) R-methadone (the pharmacologically active enantiomer) are shown in figure 8A. Due to the slow absorption of oral methadone there was a second plasma concentration peak at 3 h, after the initial intravenous peak. Total R-methadone plasma concentrations were not different between groups. Miosis in the indinavir-treated subjects was not significantly different from controls (fig. 8B), nor was the AUEC (table 1). The R-methadone plasma concentration-effect relationship (pharmacodynamics) was not significantly affected by indinavir (fig. 8C), nor was the effect/concentration AUEC0–∞/AUC0–∞ ratio (table 1).
Figure 8.
Effect of indinavir on methadone pharmacodynamics. Subjects simultaneously received 11.0 mg oral and 6.0 mg intravenous methadone HCl. Each data point is the mean ± SD (n=12). Some SD are omitted for clarity. (A) Total (intravenous d0 and oral d5) plasma R-methadone concentrations. (B) Dark-adapted pupil diameter change from baseline (miosis). (C) Plasma concentration-effect relationships (miosis vs total R-methadone plasma concentration). Each data point is the mean concentration and mean effect at each time point.
In vitro drug metabolism and inhibition data can be extrapolated to predict the in vivo effects of a “perpetrator” drug on the disposition of a “victim” drug.34,35,39 Models incorporate hepatic interactions for intravenous drugs, and intestinal as well as hepatic drug interactions for an oral drug. Models often predict the parent drug AUC ratio (perpetrator/control) in the absence and presence of perpetrator, but other relative metrics, such as those specifically reflecting metabolism, can also be used. For intravenous and oral alfentanil, assuming CYP3A-dependent clearance and based on in vitro indinavir inhibition of CYP3A, the predicted plasma alfentanil AUC ratios (indinavir/control) were 2.1 and 3.7, respectively. For methadone (where the fraction metabolized by CYP3A was assumed to be 0.19-0.41 and 0.33-0.61 for R- and S-enantiomers, respectively), assuming indinavir inhibition of CYP3A-dependent clearance, the predicted plasma R- and S-methadone AUC ratios were 1.1-1.3 and 1.2-1.6 respectively, for intravenous dosing, and 1.2-1.4 and 1.3-1.6 for oral dosing. For the specific CYP3A-catalyzed N-demethylation of methadone, the predicted (indinavir/control) ratios for plasma EDDP/methadone AUC and EDDP formation clearance were both 2.5 for both enantiomers after intravenous dosing, and both 2.6 for both enantiomers after oral dosing.
Discussion
The first major finding of this investigation was that indinavir significantly inhibited clinical CYP3A activity. Indinavir in vitro is a reversible inhibitor of expressed and human liver microsomal CYP3A isoforms, and, unlike other antiretroviral drugs (ritonavir, saquinavir, amprenavir, lopinavir and nelfinavir), is not a mechanism-based inhibitor.43,44,52 Less information is available regarding indinavir effects on clinical drug disposition, and on CYP3A activity specifically, as indinavir is usually coadministered with ritonavir. Indinavir increased rifampicin and rifabutin AUCs 1.7- and 2-fold, respectively, increased nelfinavir and clarithromycin AUCs, and halved amprenavir clearance.53 While attributed to CYP3A inhibition, actual mechanisms were never identified. The present investigation revealed that indinavir reduced hepatic and first-pass CYP3A activities to 50 and 70% of control, respectively. Alfentanil hepatic and intestinal extraction ratios were both reduced in half, and bioavailability increased. Greater effects of indinavir on oral versus intravenous alfentanil are consistent with intestinal contributions to first-pass metabolism and inhibition.39 Indinavir increased both alfentanil miosis AUEC and plasma AUC ratios, demonstrating the value of alfentanil miosis as a noninvasive approach to assessing CYP3A and drug interactions.28-32 While indinavir was reported not to inhibit CYP3A, using dapsone hydroxylation and cortisol 6ß-hydroxylation as phenotyping probes, it was later recognized that neither were valid CYP3A probes.54 This first demonstration of indinavir inhibiting both hepatic and intestinal CYP3A shows that both liver and intestine can be sites of indinavir drug interactions.
Indinavir effects on CYP3A were quantitatively comparable to those of nelfinavir,29 but less than other antiretrovirals. For example, intravenous and oral alfentanil AUCs were increased, respectively, 4- and 10-fold by ritonavir,28 and 13- and 30-fold by ritonavir/indinavir,30 consistent with the lower CYP3A inhibitory potency of indinavir.43,44,55 Nonetheless, the aim of this investigation was not profound CYP3A inhibition, but rather CYP- and organ-specific effects. Indinavir did not appreciably inhibit CYP1A2, CYP2B6, CYP2C9 or CYP2E1 in human liver microsomes,52,55 or clinically.56
The second major finding of this investigation was that indinavir altered intestinal transporters activity. Indinavir increased fexofenadine Cmax and AUC, with comparatively minimal effect on systemic elimination, suggesting increased systemic absorption, due either to decreased intestinal efflux or increased absorption. Fexofenadine was used as an intestinal P-gp probe based on published recommendations and prior application. 28-30,57 Conceived originally as a selective P-gp probe, fexofenadine is now known to be a multiple transporters substrate, with intestinal absorption influenced by P-gp-mediated efflux and organic anion transporting polypeptide (OATP) 1A2-mediated uptake.58,59 Therefore, increased fexofenadine concentrations could reflect inhibited P-gp-mediated efflux, or enhanced OATP1A2-mediated uptake. In vitro, indinavir is a poor P-gp inhibitor compared with other protease inhibitors.60,61 No information is available on indinavir and the potential for OATP1A2 induction. An alternative explanation for the results is that indinavir altered hepatic fexofenadine uptake, which determines fexofenadine clearance.62 Nevertheless, this hypothesis is inconsistent with the minimal effect of indinavir on fexofenadine elimination rates.
Indinavir effects on fexofenadine can be compared to those of other antiretrovirals. Nelfinavir decreased fexofenadine Cmax suggesting increased intestinal efflux and P-gp activity, although AUC was not altered.29 Combination ritonavir/indinavir was similar to indinavir, causing a 4-fold increase in AUC, even despite enhanced fexofenadine elimination, suggesting impaired intestinal efflux and inhibition of P-gp.30 In contrast, ritonavir alone only increased fexofenadine AUC 1.4-fold.28 This suggests that ritonavir/indinavir effects were attributable primarily to indinavir, and that ritonavir transport effects may differ from indinavir.
The primary purpose of this investigation was to determine indinavir effects on methadone disposition, and attendant mechanism(s). It is the first to evaluate indinavir effects on intravenous methadone disposition, oral and intravenous methadone concurrently, metabolism, and renal excretion. Thus, the third major finding was that indinavir had no effect on intravenous or oral methadone plasma concentrations, systemic or apparent oral clearance, hepatic clearance, hepatic extraction, or bioavailability, or renal clearance, and negligibly increased plasma AUC ratios. Indinavir had no effect on methadone metabolism, as EDDP/methadone plasma AUC ratios, EDDP enantiomer formation clearances, and plasma R/S-methadone ratios were essentially unchanged. A previous investigation of oral methadone also found no indinavir effects on plasma methadone and EDDP concentrations or AUCs.63
A fourth purpose of this investigation was to evaluate relationships between methadone bioavailability and indinavir effects on gastrointestinal drug transporters. Bioavailability was unchanged despite inhibition of intestinal P-gp efflux and/or OATP1A2 uptake activity. Methadone is a P-gp substrate in vitro,25 and the P-gp inhibitor valspodar in rats increased oral methadone bioavailability.26 The present investigation does not support the hypothesis that intestinal P-gp significantly mediates methadone absorption and first-pass extraction, possibly because of high intestinal passive permeability, or involvement of transporters other than those affected by indinavir.
A fifth purpose was to evaluate the role of CYP3A in methadone disposition. Methadone metabolism and clearance were not decreased, despite reduction of hepatic and first-pass CYP3A activities. Additional insights may be gained from modeling and prediction of indinavir effects on clinical alfentanil and methadone disposition. For intravenous and oral alfentanil, the predicted plasma alfentanil AUC ratios based on in vitro indinavir inhibition of CYP3A (2.1 and 3.7, respectively), compare very favorably with observed ratios (2.0 and 3.3), overestimating them by a negligible 5-12%. Indeed, predictions within 2-fold of measured values are considered highly accurate.39,64 Therefore, indinavir effects on alfentanil disposition are consistent with predicted inhibition of hepatic and intestinal CYP3A, validating the clinical prediction models. Predicated on a hypothesis that CYP3A4 also mediates methadone metabolism and clearance, the models similarly predicted that indinavir would also impair methadone elimination. Nevertheless, whereas predicted plasma methadone AUC ratios were 1.1-1.6 and 1.2-1.6 for intravenous and oral methadone, indinavir caused no change in AUC ratios. More importantly, for N-demethylation, whereas predicted EDDP-related ratios were 2.5 and 2.6 for intravenous and oral methadone, respectively, indinavir had no effect on these AUC ratios. While the predicted 10-60% increase in plasma methadone AUC was less than the 2-fold increase in plasma alfentanil AUC, due to nonhepatic routes of methadone elimination and potential non-CYP3A-mediated hepatic metabolism, nonetheless, such changes should be detectable in clinical studies, particularly for S-methadone. Additionally, 2-fold changes in EDDP-related ratios were detectable in other methadone studies.27 A post-hoc power analysis, using a paired t-test, 19% coefficient of variation for IV methadone AUC (indinavir/control) ratios, α=0.05, and 80% power, suggested that 12 subjects would be sufficient to detect a 17% change in the AUC ratio, and that 31 subjects would be needed to detect a 10% difference. Therefore, indinavir effects are inconsistent with those predicted by CYP3A-dependent methadone metabolism and clearance, while indinavir effects on alfentanil disposition were exactly those predicted by CYP3A mediating alfentanil metabolism and clearance. These results do not support a role for CYP3A in clinical N-demethylation and clearance of single-dose methadone.
Several previous investigations also contravene the notion, based on extrapolation of in vitro methadone N-demethylation by CYP3A4,11,18,22,65,66 that clinical methadone metabolism and clearance are mediated by CYP3A4,8,13-16,22,67,68 interindividual variability in CYP3A4 is a major factor in variable methadone bioavailability,7 and methadone interactions with antiretroviral and other drugs are due to CYP3A4.8,17 Despite profound inhibition of hepatic (>70%) and first-pass (>95%) CYP3A, ritonavir actually induced methadone N-demethylation and clearance.27,28 Nelfinavir increased methadone systemic clearance, hepatic clearance, hepatic extraction ratios, and apparent oral clearance 1.6- to 2-fold, despite 50% and >75% inhibition of hepatic and first-pass CYP3A.29 Ritonavir/indinavir had no significant effects on methadone plasma concentrations, systemic or apparent oral clearance, hepatic clearance, or bioavailability, despite 90% and 97% inhibition of hepatic and first-pass CYP3A.30 None of these investigations showed a significant correlation between CYP3A activity and methadone clearance or N-demethylation. In the present investigation, there was no increase in methadone renal clearance, which was suggested to offset and explain previous absent effects of CYP3A inhibition.17 In aggregate, these investigations do not support a significant role for CYP3A in clinical single-dose methadone metabolism and clearance.
An alternative explanation is that CYP2B6 (vide infra) predominates at low methadone concentrations after a single low dose, while CYP3A4 predominates at higher steady-state methadone concentrations. Evidence does not appear to support this. CYP2B6 but not CYP3A4 catalyzes methadone N-demethylation stereoselectively,18-22 while methadone clearance is stereoselective regardless of dose.27,29,30,69 At both “lower” (0.5-1 μM) and “higher” (≥2.5 μM) methadone concentrations, CYP2B6 had equivalent or greater activity than CYP3A4.18-22 Finally, both methadone concentration ranges are so much lower than Km values for CYP2B6 (60 and 16 μM for R- and S-methadone, respectively) and CYP3A4 (137 and 149 μM), and the difference between ranges small relative to the large difference between concentrations and Km, that plasma concentration differences would not drive isoform-specificity.
Methadone disposition has been repeatedly investigated, and yet remains persistently enigmatic, if not misunderstood. For over a decade, methadone metabolism and clearance have been variably and variously attributed to CYPs 1A2, 2B6, 2C8, 2C9, 2C19, 2D6, 3A4, 3A5 and 19,11-16,18-22,66,70,71 although most frequently to CYP3A4.7,8,13-17,22 A now consistent finding is that CYP2B6 and CYP3A4 have the highest activity towards methadone metabolism in vitro, and that CYP2B6 is stereoselective whilst CYP3A4 is not.18-22 Additionally, there is growing evidence that CYP2B6 is a major determinant of methadone metabolism and clearance. Rifampin and efavirenz, which induce CYP2B6 as well as CYP3A4, and ritonavir, which induces CYP2B6 while inhibiting CYP3A4, all doubled methadone clearance.19,27,72 Voriconazole-increased R- and S-methadone AUCs 47% and 103%, respectively, attributed to CYP3A inhibition.73 Nonetheless, it was recently shown that voriconazole also inhibits CYP2B6.74 Modulating activity of CYP2B6, which metabolizes methadone stereoselectively,18-22 altered clinical R/S methadone plasma ratios,19,27 while inhibiting CYP3A4, which metabolizes methadone non-stereoselectively, had no such effect.29,30 Whereas CYP3A inhibition did not affect methadone disposition, CYP2B6 inhibition by ticlopidine75 decreased clinical methadone N-demethylation (Unpublished observation, Evan Kharasch, M.D., Ph.D., Department of Anesthesiology, Washington University, St. Louis, Missouri, 2010).
The last major purpose of this investigation was to evaluate indinavir effects on methadone pharmacodynamics. Previous investigations showed that drug interactions could significantly alter R-methadone concentration-effect relationships. Specifically, rifampin, ritonavir, nelfinavir, and efavirenz, but not ritonavir/indinavir, caused a leftward and upward shift of the concentration-response curves, consistent with an increase in apparent potency and efficacy.19,27,29,30 These effects were considered consistent with blood-brain barrier active methadone influx or efflux, and modulation by transporter-mediated interactions. One candidate transporter is P-gp, as altered blood-brain barrier P-gp activity changed brain methadone access.24 The present investigation shows that indinavir alone had no effect on methadone pharmacodynamics. If P-gp mediates methadone brain access in humans,76 then poor activity of indinavir as a P-gp inhibitor60,61,77 would be consistent with the lack of indinavir effects on methadone pharmacodynamics. A preliminary experiment using a human blood-brain barrier model (cocultured endothelial cells, pericytes, and astrocytes) showed no effect of indinavir on methadone transport, whereas known P-gp inhibitors (cyclosporine and ritonavir) reduced methadone efflux (Unpublished observation, Scott Campbell, Ph.D. and Evan Kharasch, M.D., Ph.D., Department of Anesthesiology, Washington University, St. Louis, Missouri, 2010).
There are potential limitations with this investigation. Methadone was studied after a single low dose rather than steady-state, because the risk of causing addiction of healthy volunteers makes the latter neither possible nor ethical. Doses were small, more like those used for treating pain, than opiate addiction. Nonetheless, methadone kinetics are independent of dose.13 Results with a single methadone dose might theoretically differ from those at steady-state, although indinavir also had no effect on steady-state methadone disposition.63 Indinavir effects were evaluated in healthy volunteers, not HIV-infected patients. This was deliberate, because antiretroviral therapy involves polypharmacy, thereby precluding a mechanistic evaluation and attribution of results to any one specific drug.
In summary, despite significant inhibition of hepatic and intestinal CYP3A activity, and modulation of intestinal transporters, indinavir had no effect on clinical methadone N-demethylation or clearance. This, together with previous observations, does not support a significant role for CYP3A or certain intestinal transporters in single-dose methadone disposition.
Acknowledgments
Supported by grants R01-DA14211, K24-DA00417, and R01-GM63674 (to Dr. Kharasch) and M01-RR00037 (University of Washington General Clinical Research Center) from the National Institutes of Health, Bethesda, Maryland
Footnotes
Governale L: Methadone utilization in the US 2002 - 2006, FDA Center for Drug Evaluation and Research, 2007, www.dpt.samhsa.gov/ppt/FINAL%20Methadone%20Governale%20SAMHSA%207-20-07.ppt, last accessed May 31, 2011.
Methadone mortality – A reassessment, Drug Enforcement Administration, Office of Enforcement Operations, Pharmaceutical Investigations Section, Targeting and Analysis Unit, 2007, http://www.dpt.samhsa.gov/ppt/methadone%20ARCOS%2007-20-07.ppt, last accessed May 21, 2011.
Methadone-associated overdose deaths: Factors contributing to increased deaths and efforts to prevent them. United States Government Accountability Office Report GAO-09-341. 2009, http://www.gao.gov/new.items/d09341.pdf, last accessed May 31, 2011.
Guidance for industry: Drug interaction studies, Food and drug administration (September 2006), (http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072101.pdf), last accessed June 1, 2011.
References
- 1.Nicholson AB. Methadone for cancer pain. Cochrane Database Syst Rev. 2007:CD003971. doi: 10.1002/14651858.CD003971.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, Donovan MI, Fishbain DA, Foley KM, Fudin J, Gilson AM, Kelter A, Mauskop A, O’Connor PG, Passik SD, Pasternak GW, Portenoy RK, Rich BA, Roberts RG, Todd KH, Miaskowski C. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113–30. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lobmaier P, Gossop M, Waal H, Bramness J. The pharmacological treatment of opioid addiction--A clinical perspective. Eur J Clin Pharmacol. 2010;66:537–45. doi: 10.1007/s00228-010-0793-6. [DOI] [PubMed] [Google Scholar]
- 4.Kharasch ED. Intraoperative methadone: Rediscovery, reappraisal, and reinvigoration? Anesth Analg. 2011;112:13–6. doi: 10.1213/ANE.0b013e3181fec9a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark JD. Understanding methadone metabolism: A foundation for safer use. Anesthesiology. 2008;108:351–2. doi: 10.1097/ALN.0b013e318164937c. [DOI] [PubMed] [Google Scholar]
- 6.Sims SA, Snow LA, Porucznik CA. Surveillance of methadone-related adverse drug events using multiple public health data sources. J Biomed Inform. 2007;40:382–9. doi: 10.1016/j.jbi.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Ferrari A, Coccia CP, Bertolini A, Sternieri E. Methadone--metabolism, pharmacokinetics and interactions. Pharmacol Res. 2004;50:551–9. doi: 10.1016/j.phrs.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 8.McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, Methadone and buprenorphine, and other frequently prescribed medications: A review. Am J Addict. 2010;19:4–16. doi: 10.1111/j.1521-0391.2009.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saber-Tehrani AS, Bruce RD, Altice FL. Pharmacokinetic drug interactions and adverse consequences between psychotropic medications and pharmacotherapy for the treatment of opioid dependence. Am J Drug Alcohol Abuse. 2011;37:1–11. doi: 10.3109/00952990.2010.540279. [DOI] [PubMed] [Google Scholar]
- 10.de Vos JW, Geerlings PJ, van den Brink W, Ufkes JGR, van Wilgenburg H. Pharmacokinetics of methadone and its primary metabolite in 20 opiate addicts. Eur J Clin Pharmacol. 1995;48:361–6. doi: 10.1007/BF00194951. [DOI] [PubMed] [Google Scholar]
- 11.Iribarne C, Berthou F, Baird S, Dréano Y, Picart D, Bail JP, Beaune P, Ménez JF. Involvement of cytochrome P450 3A4 enzyme in the N-demethylation of methadone in human liver microsomes. Chem Res Toxicol. 1996;9:365–73. doi: 10.1021/tx950116m. [DOI] [PubMed] [Google Scholar]
- 12.Moody DE, Alburges ME, Parker RJ, Collins JM, Strong JM. The involvement of cytochrome P450 3A4 in the N -demethylation of L-a-acetylmethadol (LAAM), norLAAM, and methadone. Drug Metab Dispos. 1997;25:1347–53. [PubMed] [Google Scholar]
- 13.Eap CB, Buclin T, Baumann P. Interindividual variability of the clinical pharmacokinetics of methadone: Implications for the treatment of opioid dependence. Clin Pharmacokinet. 2002;41:1153–93. doi: 10.2165/00003088-200241140-00003. [DOI] [PubMed] [Google Scholar]
- 14.Crettol S, Deglon JJ, Besson J, Croquette-Krokkar M, Gothuey I, Hammig R, Monnat M, Huttemann H, Baumann P, Eap CB. Methadone enantiomer plasma levels, CYP2B6, CYP2C19, and CYP2C9 genotypes, and response to treatment. Clin Pharmacol Ther. 2005;78:593–604. doi: 10.1016/j.clpt.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Crettol S, Deglon JJ, Besson J, Croquette-Krokar M, Hammig R, Gothuey I, Monnat M, Eap CB. ABCB1 and cytochrome P450 genotypes and phenotypes: Influence on methadone plasma levels and response to treatment. Clin Pharmacol Ther. 2006;80:668–81. doi: 10.1016/j.clpt.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Shiran MR, Lennard MS, Iqbal MZ, Lagundoye O, Seivewright N, Tucker GT, Rostami-Hodjegan A. Contribution of the activities of CYP3A, CYP2D6, CYP1A2 and other potential covariates to the disposition of methadone in patients undergoing methadone maintenance treatment. Br J Clin Pharmacol. 2009;67:29–37. doi: 10.1111/j.1365-2125.2008.03312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gruber VA, McCance-Katz EF. Methadone, buprenorphine, and street drug interactions with antiretroviral medications. Curr HIV/AIDS Rep. 2010;7:152–60. doi: 10.1007/s11904-010-0048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerber JG, Rhodes RJ, Gal J. Stereoselective metabolism of methadone N-demethylation by cytochrome P4502B6 and 2C19. Chirality. 2004;16:36–44. doi: 10.1002/chir.10303. [DOI] [PubMed] [Google Scholar]
- 19.Kharasch ED, Hoffer C, Whittington D, Sheffels P. Role of hepatic and intestinal cytochrome P450 3A and 2B6 in the metabolism, disposition and miotic effects of methadone. Clin Pharmacol Ther. 2004;76:250–69. doi: 10.1016/j.clpt.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Totah RA, Allen KE, Sheffels P, Whittington D, Kharasch ED. Enantiomeric metabolic interactions and stereoselective human methadone metabolism. J Pharmacol Exp Ther. 2007;321:389–99. doi: 10.1124/jpet.106.117580. [DOI] [PubMed] [Google Scholar]
- 21.Totah RA, Sheffels P, Roberts T, Whittington D, Thummel K, Kharasch ED. Role of CYP2B6 in stereoselective human methadone metabolism. Anesthesiology. 2008;108:363–74. doi: 10.1097/ALN.0b013e3181642938. [DOI] [PubMed] [Google Scholar]
- 22.Chang Y, Fang WB, Lin SN, Moody DE. Stereo-selective metabolism of methadone by human liver microsomes and cDNA-expressed cytochrome P450s: A reconciliation. Basic Clin Pharmacol Toxicol. 2010;108:55–62. doi: 10.1111/j.1742-7843.2010.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson SJ, Koszdin KK, Bernards CM. Opiate-induced analgesia as increased and prolonged in mice lacking P-glycoprotein. Anesthesiology. 2000;92:1392–99. doi: 10.1097/00000542-200005000-00030. [DOI] [PubMed] [Google Scholar]
- 24.Bauer B, Yang X, Hartz AM, Olson ER, Zhao R, Kalvass JC, Pollack GM, Miller DS. In vivo activation of human pregnane X receptor tightens the blood-brain barrier to methadone through P-glycoprotein up-regulation. Mol Pharmacol. 2006;70:1212–9. doi: 10.1124/mol.106.023796. [DOI] [PubMed] [Google Scholar]
- 25.Crettol S, Digon P, Golay KP, Brawand M, Eap CB. In vitro P-glycoprotein-mediated transport of (R)-, (S)-, (R,S)-methadone, LAAM and their main metabolites. Pharmacology. 2007;80:304–11. doi: 10.1159/000107104. [DOI] [PubMed] [Google Scholar]
- 26.Ortega I, Rodriguez M, Suarez E, Perez-Ruixo JJ, Calvo R. Modeling methadone pharmacokinetics in rats in presence of P-glycoprotein inhibitor valspodar. Pharm Res. 2007;24:1299–308. doi: 10.1007/s11095-007-9251-2. [DOI] [PubMed] [Google Scholar]
- 27.Kharasch ED, Bedynek PS, Park S, Whittington D, Walker A, Hoffer C. Mechanism of ritonavir changes in methadone pharmacokinetics and pharmacodynamics. I. Evidence against CYP3A mediation of methadone clearance. Clin Pharmacol Ther. 2008;84:497–505. doi: 10.1038/clpt.2008.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kharasch ED, Bedynek PS, Walker A, Whittington D, Hoffer C. Mechanism of ritonavir changes in methadone pharmacokinetics and pharmacodynamics. II. Ritonavir effects on CYP3A and P-glycoprotein activities. Clin Pharmacol Ther. 2008;84:506–12. doi: 10.1038/clpt.2008.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kharasch ED, Walker A, Whittington D, Hoffer C, Bedynek PS. Methadone metabolism and clearance are induced by nelfinavir despite inhibition of cytochrome P4503A (CYP3A) activity. Drug Alcohol Depend. 2009;101:158–68. doi: 10.1016/j.drugalcdep.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kharasch ED, Hoffer C, Whittington D, Walker A, Bedynek PS. Methadone pharmacokinetics are independent of cytochrome P4503A (CYP3A) activity and gastrointestinal drug transport: Insights from methadone interactions with ritonavir/indinavir. Anesthesiology. 2009;110:660–72. doi: 10.1097/ALN.0b013e3181986a9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kharasch ED, Walker A, Hoffer C, Sheffels P. Intravenous and oral alfentanil as in vivo probes for hepatic and first-pass cytochrome P450 3A activity: Noninvasive assessment using pupillary miosis. Clin Pharmacol Ther. 2004;76:452–66. doi: 10.1016/j.clpt.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Kharasch ED, Walker A, Isoherranen N, Hoffer C, Sheffels P, Thummel K, Whittington D, Ensign D. Influence of CYP3A5 genotype on the pharmacokinetics and pharmacodynamics of the cytochrome P4503A probes alfentanil and midazolam. Clin Pharmacol Ther. 2007;82:410–26. doi: 10.1038/sj.clpt.6100237. [DOI] [PubMed] [Google Scholar]
- 33.Gorski JC, Vannaprasaht S, Hamman MA, Ambrosius WT, Bruce MA, Haehner-Daniels B, Hall SD. The effect of age, sex, and rifampin administration on intestinal and hepatic cytochrome P450 3A activity. Clin Pharmacol Ther. 2003;74:275–87. doi: 10.1016/S0009-9236(03)00187-5. [DOI] [PubMed] [Google Scholar]
- 34.Fahmi OA, Maurer TS, Kish M, Cardenas E, Boldt S, Nettleton D. A combined model for predicting CYP3A4 clinical net drug-drug interaction based on CYP3A4 inhibition, inactivation, and induction determined in vitro. Drug Metab Dispos. 2008;36:1698–708. doi: 10.1124/dmd.107.018663. [DOI] [PubMed] [Google Scholar]
- 35.Fahmi OA, Ripp SL. Evaluation of models for predicting drug-drug interactions due to induction. Expert Opin Drug Metab Toxicol. 2010;6:1399–416. doi: 10.1517/17425255.2010.516251. [DOI] [PubMed] [Google Scholar]
- 36.Rostami-Hodjegan A, Tucker G. ‘In silico’ simulations to assess the ‘in vivo’ consequences of ‘in vitro’ metabolic drug–drug interactions. Drug Discov Today Technol. 2004;1:441–8. doi: 10.1016/j.ddtec.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Kirby BJ, Unadkat JD. Impact of ignoring extraction ratio when predicting drug-drug interactions, fraction metabolized, and intestinal first-pass contribution. Drug Metab Dispos. 2010;38:1926–33. doi: 10.1124/dmd.110.034736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirby BJ, Collier AC, Kharasch ED, Whittington D, Thummel KE, Unadkat JD. Complex drug interactions of HIV protease inhibitors 1: Inactivation, induction, and inhibition of cytochrome P450 3A by ritonavir or nelfinavir. Drug Metab Dispos. 2011;39:1070–8. doi: 10.1124/dmd.110.037523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galetin A, Gertz M, Houston JB. Contribution of intestinal cytochrome P450-mediated metabolism to drug-drug inhibition and induction interactions. Drug Metab Pharmacokinet. 2010;25:28–47. doi: 10.2133/dmpk.25.28. [DOI] [PubMed] [Google Scholar]
- 40.Chiba M, Hensleigh M, Nishime JA, Balani SK, Lin JH. Role of cytochrome P450 3A4 in human metabolism of MK-639, a potent human immunodeficiency virus protease inhibitor. Drug Metab Dispos. 1996;24:307–14. [PubMed] [Google Scholar]
- 41.Iribarne C, Berthou F, Carlhant D, Dreano Y, Picart D, Lohezic F, Riche C. Inhibition of methadone and buprenorphine N-dealkylations by three HIV-1 protease inhibitors. Drug Metab Dispos. 1998;26:257–60. [PubMed] [Google Scholar]
- 42.Unadkat JD, Wang Y. In: Protease inhibitors, Metabolic drug interactions. Levy RH, Thummel KE, Trager WF, Hansten PD, Eichelbaum M, editors. Williams & Wilkins; Philadelphia, Lippincott: 2000. pp. 647–52. [Google Scholar]
- 43.Ernest CS, II, Hall SD, Jones DR. Mechanism-based inactivation of cytochrome P450 3A (CYP3A) by HIV protease inhibitors. J Pharmacol Exp Ther. 2005;312:583–91. doi: 10.1124/jpet.104.075416. [DOI] [PubMed] [Google Scholar]
- 44.Granfors MT, Wang JS, Kajosaari LI, Laitila J, Neuvonen PJ, Backman JT. Differential inhibition of cytochrome P450 3A4, 3A5 and 3A7 by five human immunodeficiency virus (HIV) protease inhibitors in vitro. Basic Clin Pharmacol Toxicol. 2006;98:79–85. doi: 10.1111/j.1742-7843.2006.pto_249.x. [DOI] [PubMed] [Google Scholar]
- 45.Labroo RB, Thummel KE, Kunze KL, Podoll T, Trager WF, Kharasch ED. Catalytic role of cytochrome P4503A4 in multiple pathways of alfentanil metabolism. Drug Metab Dispos. 1995;23:490–6. [PubMed] [Google Scholar]
- 46.Klees TM, Sheffels P, Dale O, Kharasch ED. Metabolism of alfentanil by cytochrome P4503A (CYP3A) enzymes. Drug Metab Dispos. 2005;33:303–11. doi: 10.1124/dmd.104.002709. [DOI] [PubMed] [Google Scholar]
- 47.Burger D, Boyd M, Duncombe C, Felderhof M, Mahanontharit A, Ruxrungtham K, Ubolyam S, Stek M, Cooper D, Lange J, Phanupak P, Reiss P. Pharmacokinetics and pharmacodynamics of indinavir with or without low-dose ritonavir in HIV-infected Thai patients. J Antimicrob Chemother. 2003;51:1231–8. doi: 10.1093/jac/dkg198. [DOI] [PubMed] [Google Scholar]
- 48.Kraft WK, McCrea JB, Winchell GA, Carides A, Lowry R, Woolf EJ, Kusma SE, Deutsch PJ, Greenberg HE, Waldman SA. Indinavir and rifabutin drug interactions in healthy volunteers. J Clin Pharmacol. 2004;44:305–13. doi: 10.1177/0091270003262807. [DOI] [PubMed] [Google Scholar]
- 49.Anderson PL, Brundage RC, Bushman L, Kakuda TN, Remmel RP, Fletcher CV. Indinavir plasma protein binding in HIV-1-infected adults. AIDS. 2000;14:2293–7. doi: 10.1097/00002030-200010200-00010. [DOI] [PubMed] [Google Scholar]
- 50.Kharasch ED, Whittington D, Ensign D, Hoffer C, Bedynek PS, Campbell S, Stubbert K, Crafford A, London A, Kim T. Mechanism of efavirenz influence on methadone pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2011 doi: 10.1038/clpt.2011.276. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gertz M, Harrison A, Houston JB, Galetin A. Prediction of human intestinal first-pass metabolism of 25 CYP3A substrates from in vitro clearance and permeability data. Drug Metab Dispos. 2010;8:1147–58. doi: 10.1124/dmd.110.032649. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Unadkat JD. Enzymes in addition to CYP3A4 and 3A5 mediate N-demethylation of dextromethorphan in human liver microsomes. Biopharm Drug Dispos. 1999;20:341–6. doi: 10.1002/(sici)1099-081x(199910)20:7<341::aid-bdd195>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 53.de Maat MMR, Ekhart GC, Huitema ADR, Koks CHW, Mulder JW, Beijnen JH. Drug interactions between antiretroviral drugs and comedicated agents. Clin Pharmacokinet. 2003;42:223–82. doi: 10.2165/00003088-200342030-00002. [DOI] [PubMed] [Google Scholar]
- 54.Gass RJ, Gal J, Fogle PW, Detmar-Hanna D, Gerber JG. Neither dapsone hydroxylation nor cortisol 6ß-hydroxylation detects the inhibition of CYP3A4 by HIV-1 protease inhibitors. Eur J Clin Pharmacol. 1998;54:741–7. doi: 10.1007/s002280050545. [DOI] [PubMed] [Google Scholar]
- 55.Eagling VA, Back DJ, Barry MG. Differential inhibition of cytochrome P450 isoforms by the protease inhibitors, ritonavir, saquinavir and indinavir. Br J Clin Pharmacol. 1997;44:190–4. doi: 10.1046/j.1365-2125.1997.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Purkins L, Wood N, Kleinermans D, Love ER. No clinically significant pharmacokinetic interactions between voriconazole and indinavir in healthy volunteers. Br J Clin Pharmacol. 2003;56:62–8. doi: 10.1046/j.1365-2125.2003.02001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tucker GT, Houston JB, Huang S-M. Optimizing drug development: Strategies to assess drug metabolism/transporter interaction potential-toward a consensus. Clin Pharmacol Ther. 2001;70:103–14. doi: 10.1067/mcp.2001.116891. [DOI] [PubMed] [Google Scholar]
- 58.Cvetkovic M, Leake B, Fromm MF, Wilkinson GR, Kim RB. OATP and P-glycoprotein transporters mediate the cellular uptake and excretion of fexofenadine. Drug Metab Dispos. 1999;27:866–71. [PubMed] [Google Scholar]
- 59.Bailey DG, Dresser GK, Leake BF, Kim RB. Naringin is a major and selective clinical inhibitor of organic anion-transporting polypeptide 1A2 (OATP1A2) in grapefruit juice. Clin Pharmacol Ther. 2007;81:495–502. doi: 10.1038/sj.clpt.6100104. [DOI] [PubMed] [Google Scholar]
- 60.Polli JW, Wring SA, Humphreys JE, Huang L, Morgan JB, Webster LO, Serabjit-Singh CS. Rational use of in vitro P-glycoprotein assays in drug discovery. J Pharmacol Exp Ther. 2001;299:620–8. [PubMed] [Google Scholar]
- 61.Perloff MD, Störmer E, von Moltke LL, Greenblatt DJ. Rapid assessment of P-glycoprotein inhibition and induction in vitro. Pharm Res. 2003;20:1177–83. doi: 10.1023/a:1025092829696. [DOI] [PubMed] [Google Scholar]
- 62.Swift B, Tian X, Brouwer KL. Integration of preclinical and clinical data with pharmacokinetic modeling and simulation to evaluate fexofenadine as a probe for hepatobiliary transport function. Pharm Res. 2009;26:1942–51. doi: 10.1007/s11095-009-9909-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maas B, Kerr T, Fairbairn N, Montaner J, Wood E. Pharmacokinetic interactions between HIV antiretroviral therapy and drugs used to treat opioid dependence. Expert Opin Drug Metab Toxicol. 2006;2:533–43. doi: 10.1517/17425255.2.4.533. [DOI] [PubMed] [Google Scholar]
- 64.Fahmi OA, Hurst S, Plowchalk D, Cook J, Guo F, Youdim K, Dickins M, Phipps A, Darekar A, Hyland R, Obach RS. Comparison of different algorithms for predicting clinical drug-drug interactions, based on the use of CYP3A4 in vitro data: Predictions of compounds as precipitants of interaction. Drug Metab Dispos. 2009;37:1658–66. doi: 10.1124/dmd.108.026252. [DOI] [PubMed] [Google Scholar]
- 65.Foster DJ, Somogyi AA, Bochner F. Methadone N-demethylation in human liver microsomes: Lack of stereoselectivity and involvement of CYP3A4. Br J Clin Pharmacol. 1999;47:403–12. doi: 10.1046/j.1365-2125.1999.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang J-S, DeVane CL. Involvement of CYP3A4, CYP2C8, and CYP2D6 in the metabolism of (R)- and (S)-methadone in vitro. Drug Metab Dispos. 2003;31:742–7. doi: 10.1124/dmd.31.6.742. [DOI] [PubMed] [Google Scholar]
- 67.Foster DJ, Somogyi AA, Dyer KR, White JM, Bochner F. Steady-state pharmacokinetics of (R)- and (S)-methadone in methadone maintenance patients. Br J Clin Pharmacol. 2000;50:427–40. doi: 10.1046/j.1365-2125.2000.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shinderman M, Maxwell S, Brawand-Amey M, Golay KP, Baumann P, Eap CB. Cytochrome P4503A4 metabolic activity, methadone blood concentrations, and methadone doses. Drug Alcohol Depend. 2003;69:205–11. doi: 10.1016/s0376-8716(02)00320-4. [DOI] [PubMed] [Google Scholar]
- 69.Foster DJ, Somogyi AA, White JM, Bochner F. Population pharmacokinetics of (R)-, (S)- and rac-methadone in methadone maintenance patients. Br J Clin Pharmacol. 2004;57:742–55. doi: 10.1111/j.1365-2125.2004.02079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Fazio S, Gallelli L, De Siena A, De Sarro G, Scordo MG. Role of CYP3A5 in abnormal clearance of methadone. Ann Pharmacother. 2008;42:893–7. doi: 10.1345/aph.1K539. [DOI] [PubMed] [Google Scholar]
- 71.Lu WJ, Bies R, Kamden LK, Desta Z, Flockhart DA. Methadone: A substrate and mechanism-based inhibitor of CYP19 (aromatase) Drug Metab Dispos. 2010;38:1308–13. doi: 10.1124/dmd.110.032474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kharasch ED, Mitchell D, Coles R, Blanco R. Rapid clinical induction of hepatic cytochrome P4502B6 activity by ritonavir. Antimicrob Agents Chemother. 2008;52:1663–9. doi: 10.1128/AAC.01600-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu P, Foster G, LaBadie RR, Gutierrez MJ, Sharma A. Pharmacokinetic interaction between voriconazole and efavirenz at steady state in healthy male subjects. J Clin Pharmacol. 2008;48:73–84. doi: 10.1177/0091270007309703. [DOI] [PubMed] [Google Scholar]
- 74.Jeong S, Nguyen PD, Desta Z. Comprehensive in vitro analysis of voriconazole inhibition of eight cytochrome P450 (CYP) enzymes: Major effect on CYPs 2B6, 2C9, 2C19, and 3A. Antimicrob Agents Chemother. 2009;53:541–51. doi: 10.1128/AAC.01123-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Turpeinen M, Tolonen A, Uusitalo J, Jalonen J, Pelkonen O, Laine K. Effect of clopidogrel and ticlopidine on cytochrome P450 2B6 activity as measured by bupropion hydroxylation. Clin Pharmacol Ther. 2005;77:553–9. doi: 10.1016/j.clpt.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 76.Coller JK, Barratt DT, Dahlen K, Loennechen MH, Somogyi AA. ABCB1 genetic variability and methadone dosage requirements in opioid-dependent individuals. Clin Pharmacol Ther. 2006;80:682–90. doi: 10.1016/j.clpt.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 77.Bachmeier CJ, Spitzenberger TJ, Elmquist WF, Miller DW. Quantitative assessment of HIV-1 protease inhibitor interactions with drug efflux transporters in the blood-brain barrier. Pharm Res. 2005;22:1259–68. doi: 10.1007/s11095-005-5271-y. [DOI] [PubMed] [Google Scholar]