Abstract
Racial/ethnic origin plays an important role in fracture risk. Racial/ethnic differences in fracture rates cannot be fully explained by bone mineral density (BMD). Studies examining the influence of bone geometry and strength on fracture risk have focused primarily on older adults and have not included people from diverse racial/ethnic backgrounds. Our goal was to explore racial/ethnic differences in hip geometry and strength in a large sample of midlife women. We performed Hip Structure Analysis (HSA) on hip DXA scans from 1942 pre- and early peri-menopausal women. The sample included Caucasian (50%), African American (27%), Chinese (11%) and Japanese (12%) women, age 42–52 years. HSA was performed using software developed at John’s Hopkins University. African American women had higher conventional (8.4–9.7%) and HSA BMD (5.4–19.8%) than other groups with the exception being Japanese women who had the highest HSA BMD (9.7–31.4%). HSA indices associated with more favorable geometry and greater strength and resistance to fracture were more prevalent in African American and Japanese women. Femurs of African American women had a smaller outer diameter, a larger cross-sectional area and section modulus, and a lower buckling ratio. Japanese women presented a different pattern with a higher section modulus and lower buckling ratio, similar to African American women, but a wider outer diameter; this was offset by a greater cross-sectional area and a more centrally located centroid. Chinese women had similar conventional BMD as Caucasian women but a smaller neck region area and HSA BMD at both regions. They also had a smaller cross-sectional area and section modulus, a more medially located centroid, and a higher buckling ratio than Caucasian women. The observed biomechanical differences may help explain racial/ethnic variability in fracture rates. Future research should explore the contribution of hip geometry to fracture risk across all race/ethnicities.
Keywords: Hip Structure Analysis, Bone Geometry, Bone Mineral Density, Ethnicity, Women
Introduction
Racial/ethnic origin plays an important role in the risk of osteoporotic fractures, especially of the hip. Most studies to date have examined differences between US women of Caucasian and African heritage to seek explanations for lower hip fracture rates in the latter group (1, 2). Although differences in rates of falls may help explain differences in fracture rates, it is more likely that those who suffer fewer fractures have a mechanical advantage that either strengthens the hip or reduces the effects of trauma when falls do occur. Both factors seem to play a role in lower fracture rates among African Americans. While their femur geometry does not appear to better withstand common axial and bending forces, they possess thicker cortices which should reduce susceptibility to local cortical buckling (3). Moreover, greater obesity among African Americans may lessen severity of hip trauma during a fall due to soft tissue padding effects (4).
While low bone mineral density (BMD) is a major contributor to fracture risk, it is not itself a mechanical property and it does not account for all of the statistical variability observed in fracture rates. Furthermore, racial/ethnic differences in BMD do not consistently parallel racial/ethnic patterns in fracture rates, perhaps in part because of body size confounding effects. Consequently, more attention is being paid to bone structural dimensions (i.e., geometry) and how they influence fracture risk. Methods have been developed to assess geometry at the proximal femur using dual energy x-ray absorptiometry (DXA) and Quantitative Computed Tomography (QCT). Recent studies employing these techniques in white and non-white samples suggest that important geometric differences exist that might help explain racial/ethnic disparities in fracture rates (2, 3, 5–9).
As part of an ancillary study to the Study of Women’s Health Across the Nation (SWAN), we conducted Hip Structure Analysis (HSA) on archived baseline DXA scans to examine racial/ethnic differences in femoral hip structure to gain insights into mechanical factors that might account for lower risk of hip fracture in some racial/ethnic groups compared to others. As in the WHI study (3), we compared African Americans to Caucasians using the latter as a reference group. However, the SWAN study also recruited women of Chinese and Japanese ancestry, enabling cross-ethnic comparisons across four major subgroups of pre-/early peri-menopausal women, in contrast to the exclusively postmenopausal Women’s Health Initiative (WHI) cohort.
Materials and Methods
Study Sample
The Study of Women’s Health Across the Nation (SWAN) is a multi-center, longitudinal study designed to characterize changes that occur during the menopausal transition in a community-based sample of 3302 women of varying racial/ethnic background. Enrolled women were 42–52 years of age, were pre-menopausal or early peri-menopausal, had an intact uterus and at least one ovary, had experienced at least one menstrual cycle in the 3 months prior to screening, were not using hormone contraceptives or hormone therapy at baseline, and self-identified as a member of one of five eligible racial/ethnic groups (Caucasian, African American, Hispanic, Chinese and Japanese). Details of eligibility and recruitment are published elsewhere (10). Women completed their baseline clinic visit during 1996–1997.
A bone density study was conducted at five of the seven SWAN centers including Boston, MA; Detroit, MI; Los Angeles, CA; Oakland, CA; and Pittsburgh, PA. Areal BMD (aBMD) of the proximal femur and spine was measured by Dual Energy X-ray Absorptiometry (DXA) using a QDR 4500 or QDR 2000 (Hologic Inc., Waltham, MA). All sites enrolled Caucasian women. Each site additionally enrolled women who self-identified as belonging to one pre-specified minority ethnic group: African American (Boston, MA; Detroit, MI; Pittsburgh, PA), Japanese (Los Angeles, CA), and Chinese (Oakland, CA). A total of 2413 women were enrolled into the bone density study.
The Hip Strength Across the Menopausal Transition study is ancillary to SWAN. The primary objective of the ancillary study was to examine the association between various indices of bone strength, structure, and BMD and fracture in women transitioning through the menopause. To examine cross-sectional racial/ethnic differences in hip structure and strength, we performed HSA using archived baseline hip DXA scans on 1942 women who had two or more hip DXA scans during follow-up study visits. We were unable to perform HSA on an additional 468 (19.4%) participants due to missing or corrupted scans or software incompatibilities. The DXA scans of women reporting current Tamoxifen use (n=3) were also excluded. Age and hip (total, femoral neck, and intertrochanter) BMD were not different between women whether or not their data was analyzed by HSA. However, women who did not have HSA weighed more (77.7 kg versus 72.6 kg) and represented a greater proportion of African Americans (22% of African Americans versus 12-18% of other race/ethnicity) than women who had HSA. The SWAN and ancillary study protocols were approved by each center’s Institutional Review Board and written informed consent was obtained from all participants.
Measures
Demographic Information
Standard, self-administered questionnaires and interviews were used to obtain demographic, lifestyle and medical history data. Baseline menopausal status was assigned based on menstrual bleeding: 1) pre-menopause: monthly bleeding with no perceived change in cycle interval and 2) early peri-menopause: monthly bleeding with a perceived change in cycle interval, but at least one menstrual period within the past 3 months. Height and weight were measured using a fixed stadiometer and a balance beam scale, respectively, with participants in light clothing and without shoes. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared (kg/m2). Smoking was evaluated by questionnaire and was coded as never, past, or current. Physical activity was assessed using a version of the Kaiser Permanente Activity Survey (KPAS) (11), a modification of the Baecke questionnaire (12). A total physical activity score with a possible range of 3 (lowest) to 15 (highest) was calculated based on questions about physical activity in various domains including sports and exercise, active living, and household/caregiving. Dietary calcium (mg/day), supplemental calcium (indicator for supplemental use; yes/no) and vitamin D (alone or in combination with something else; yes/no), and alcohol (none, ≤1, and >1 serving/day) intakes were assessed by a food frequency questionnaire (13). Women were classified as diabetic at baseline if they had a fasting serum glucose level ≥126 mg/dl or reported anti-diabetic medication use.
DXA Scanning
Conventional areal BMD (g/cm2) of the proximal femur and lumbar spine was measured by DXA using a QDR 4500 or a QDR 2000 (Hologic Inc., Waltham, MA) with Osteodyne’s Hip Positioner System (Osteodyne, Inc.). Areal BMD does not account for potential racial/ethnic differences in bone size; therefore we also calculated bone mineral apparent density (BMAD) using the formula BMAD (g/cm3)=BMC/area2 as an estimate of volumetric BMD to partially account for these differences. All sites employed a standard quality control (QC) program that included daily measurement of a Hologic anthropomorphic spine phantom; cross-site calibration with a single anthropomorphic spine phantom; local review of scan images; and monthly and quarterly review of QC scans plus all flagged scans by Synarc, Inc. (Waltham, MA). Duplicate scans of the proximal femur and spine, with complete repositioning, conducted on five women (ages 42–52 years) at each site yielded short-term in vivo standard deviation values of 0.014 g/cm2 (1.4%) and 0.016 g/cm2 (2.2%), respectively. Details of the QC program are published elsewhere (14). Scan images were then exported and shipped to John’s Hopkins University (JHU) for Hip Structure Analysis.
Hip Structure Analysis (HSA)
Scan files were analyzed by Hip Structure Analysis software developed at the Johns Hopkins School of Medicine (15). A calibration step phantom was circulated to all study sites prior to analysis to generate a scanner-specific calibration that was employed during the HSA process. All personal subject identifiers were removed prior to shipment to JHU or were automatically removed prior to analysis by the HSA software and were only restored after all analysis was complete. The HSA method uses the principle that a line of pixels across the bone axis is a projection of the mineral in a cross-section from which certain geometric properties can be measured (16). Geometry is calculated from 5 profiles spaced 1 pixel apart and then averaged at each region. The Narrow-Neck (NN) region traverses the femoral neck at its narrowest point and the intertrochanter (IT) is centered across the bisector of the angle between neck and shaft axes. The shaft region was not used in the present study as it is a rare site of hip fracture. Average pixel value in region profiles is reported as BMD, and outer diameter (OD) is measured from outer profile margins corrected for image blur. To determine the bone surface in the cross-section, pixel values are divided by the average mineral density of normal adult cortical bone (1.053 g/cm3), yielding a linear thickness; the profile integral is thus area (CSA) in cm2. The center of mass (COM) of the profile is determined, then the cross-sectional moment of inertia (CSMI) is measured as the integral weighted by the square of distance of each pixel from the COM. The COM was itself used as an outcome by dividing the distance from the COM to the medial margin by the outer diameter. This value called Centroid Position, which reveals asymmetry of the mass in the cross-section, was found to be smaller (more medially located) among hip fracture cases in the Study of Osteoporotic Fractures (17). Maximum bending stress in a cross-section is a function of section modulus (SM), computed as CSMI divided by the maximum distance to the medial or lateral profile margin (dmax). Research has suggested that homeostatic mechanisms tend to preserve SM in aging bone cross-sections (15, 18) but in a progressively thinner cortical shell. If cortices become thin enough to buckle (fold) under compressive loads the SM will overestimate the actual strength. This complex phenomenon cannot be fully characterized by the limited information in a DXA scan but an estimate of the buckling ratio (BR) can suggest that a cross-section may be susceptible. The BR is used in engineering designs incorporating hollow tubes; ideally the ratio of outer radius to wall thickness should be kept below ~10 to avoid strength loss due to local buckling (19). BR is estimated in HSA by modeling the cross-section as a hollow circular (NN) or elliptical (IT) annulus with a fixed proportion (60% and 70%, respectively) of the CSA in the cortical shell. Though undeniably crude, this estimate appears to provide the mechanical explanation for why conventional BMD predicts hip fractures (20).
The HSA program also measures the femoral neck length (NL) as the distance from the center of the femoral head to intersection of the neck and shaft axes, as well as the angle (NSA) between them. These parameters influence the moment arm of forces causing bending of the proximal femur. Useable data were obtained from 1942 participants as outlined earlier.
Statistical Analysis
Demographic and clinical characteristics were described using measures of central tendency (means, standard deviation, frequencies, etc.). HSA indices were normally distributed; therefore parametric testing was employed. Values of characteristics in each of the ethnic groups were compared using chi-square statistics (categorical variables), analysis of variance and the Wilcoxon test (continuous variables). To test for ethnic differences in mean HSA measurements, multiple linear regression modeling was used. Preliminary data from a pilot study we conducted indicated that there were no differences in geometry as assessed by HSA between pre- and early peri-menopausal women. Therefore, we did not stratify our analyses by menopausal status. All models (except for neck shaft angle, neck length and centroid position) were adjusted for variables that differed significantly across race/ethnicity and are known to influence bone mass including age, height, weight, menopausal status, physical activity, smoking, dietary calcium intake, calcium supplement, vitamin D supplement, daily alcohol consumption, corticosteroid use, diabetic status, arthritis, and study site. Neck length is body (bone) size dependent but should not be influenced by other (modifiable) factors, thus was adjusted for height and weight alone. Neck shaft angle and centroid position are not body size dependent and were not adjusted for other factors. First, all groups were compared to Caucasians. Dunnett adjustment was used to adjust p-values for multiple comparisons. Second, further subgroup (i.e., African American vs. Japanese, African American vs. Chinese, and Japanese vs. Chinese) testing was conducted using Tukey-Kramer adjustment methods. All analyses were performed using SAS 9.2 (SAS Institute, Inc., Cary, NC).
Results
Demographic and Clinical Characteristics
The final cohort included 1942 pre- and early peri-menopausal women, average age 46.4 (±2.7) years, from diverse racial/ethnic backgrounds including Caucasian (51.5%), African American (26.6%), Chinese (11.3%) and Japanese (12.3%). There were a number of significant racial/ethnic differences in demographic and clinical characteristics in our sample (Table 1). Japanese women were slightly older than women in the other racial/ethnic groups, and both Chinese and Japanese women were more likely to be in early peri-menopause at baseline as compared to Caucasian and African American women. Chinese and Japanese women were also significantly shorter and weighed on average more than 15 kilograms less than Caucasian and African American women. African American women were more likely to be current smokers, taking corticosteroids, be classified as diabetic, and self-report a diagnosis of arthritis than women in the other racial/ethnic groups. A higher proportion of Caucasian and Japanese women reported consuming one or more drinks of alcohol daily as compared to African American and Chinese women. Physical activity scores were similar between Caucasian and Japanese women, and higher than African American and Chinese women. Japanese women had the lowest dietary calcium intake but were more likely to report calcium supplement use than other racial/ethnic groups. Vitamin D supplement use was lowest among the Chinese women.
Table 1.
Demographic and clinical characteristics a by ethnicity of women in the SWAN Hip Strength study
| Total (n=1942) | Caucasian (n=966) | African-American (n=517) | Chinese (n=220) | Japanese (n=239) | P value | |
|---|---|---|---|---|---|---|
|
| ||||||
| Age in years | 46.4 (2.7) | 46.4 (2.7) | 46.5 (2.6) | 46.1 (2.6) | 46.7 (2.6) | 0.03 |
|
| ||||||
| Site, n (%) | <0.0001 | |||||
| Michigan | 428 (22.0) | 176 (18.2) | 252 (48.7) | 0 (0) | 0 (0) | |
| MGH | 335 (17.3) | 197 (20.4) | 138 (26.7) | 0 (0) | 0 (0) | |
| UC-Davis | 391 (20.1) | 171 (17.7) | 0 (0) | 220 (100) | 0 (0) | |
| UCLA | 421 (21.7) | 182 (18.8) | 0 (0) | 0 (0) | 239 (100) | |
| Pittsburgh | 367 (18.9) | 240 (24.8) | 127 (24.6) | 0 (0) | 0 (0) | |
|
| ||||||
| Menopausal Status, n (%) | 0.0045 | |||||
| Pre-menopausal | 850 (43.8) | 434 (44.9) | 247 (47.9) | 81 (36.8) | 88 (36.8) | |
| Early Peri-menopausal | 1091 (56.2) | 532 (55.1) | 269 (52.1) | 139 (63.2) | 151 (63.2) | |
|
| ||||||
| Height (cm) | 162.3 (6.5) | 164.1 (6.2) | 163.3 (6.1) | 157.8 (5.5) | 157.1 (4.7) | <0.0001 |
|
| ||||||
| Weight (kg) | 72.6 (19.3) | 74.3 (18.2) | 83.1 (19.3) | 57.9 (10.7) | 56.7 (8.9) | < 0.0001 |
|
| ||||||
| BMI (kg/m2) | 27.5 (6.8) | 27.6 (6.5) | 31.2 (7.2) | 23.3 (3.9) | 23.0 (3.6) | <0.0001 |
|
| ||||||
| Dietary Calcium Intake (mg/d) | 742.2 (428.1) | 818.1 (437.8) | 699.6 (437.6) | 687.2 (372.0) | 643.5 (357.2) | < 0.0001 |
|
| ||||||
| Calcium Supplement, n (%) | 875 (45.1) | 463 (48.0) | 197 (38.1) | 81 (36.8) | 134 (56.3) | <0.0001 |
|
| ||||||
| Vitamin D Supplement, n (%) | 749 (38.6) | 394 (40.9) | 185 (35.8) | 61 (27.7) | 109 (45.8) | 0.0002 |
|
| ||||||
| Physical Activity Score (range 3–14) | 7.8 (1.8) | 8.1 (1.8) | 7.3 (1.8) | 7.3 (1.7) | 7.9 (1.6) | < 0.0001 |
|
| ||||||
| Daily Alcohol Servings, n (%) | 998 (51.5) | 393 (40.8) | 292 (56.5) | 174 (79.1) | 139 (58.4) | <0.0001 |
| None | 826 (42.6) | 498 (51.2) | 203 (39.3) | 42 (19.1) | 83 (34.9) | |
| <1 drink/day | 115 (5.9) | 73 (7.6) | 22 (4.3) | 4 (1.8) | 16 (6.7) | |
| ≥1 drink/day | ||||||
|
| ||||||
| Smoking, n (%) | <0.0001 | |||||
| Never | 1145 (59.4) | 520 (54.1) | 268 (52.8) | 206 (93.6) | 151 (63.7) | |
| Past | 492 (25.5) | 317 (33.0) | 106 (20.9) | 12 (5.5) | 57 (24.1) | |
| Current | 290(15.1) | 125 (13.0) | 134 (26.4) | 2 (0.9) | 29 (12.2) | |
|
| ||||||
| Diabetes, n (%) | 82 (4.2) | 37 (3.8) | 42 (8.1) | 3 (1.4) | 0 | <0.0001 |
|
| ||||||
| Arthritis, n (%) | 354 (18.2) | 185 (19.1) | 126 (24.4) | 26 (11.8) | 17 (7.1) | <0.0001 |
|
| ||||||
| Corticosteroid Use, n (%) | 21 (1.1) | 10 (1.0) | 11 (2.1) | 0 | 0 | 0.016 |
Mean (SD) unless otherwise noted.
Hip BMD and HSA Indices
Table 2 displays bone mineral density (DXA and HSA-derived) and the structural geometry (HSA) by racial/ethnic group (mean, SE) adjusted for age, height, weight, menopausal status, physical activity, smoking, dietary calcium intake, calcium supplement, vitamin D supplement, daily alcohol consumption, corticosteroid use, diabetic status, arthritis, and study site.
Table 2.
Adjusteda mean (standard error) of bone mineral density and hip structure analysis (HSA) indices by race/ethnicity
| Caucasian (n=966) | African-American (n=517) | Chinese (n=220) | Japanese (n=239) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| DXA – Femoral Neck | ||||||
| BMD (g/cm2) | 0.82 (0.003) | 0.90 (0.005)e | 0.83 (0.08) | 0.83 (0.007) | ||
| BMAD (g/cm3) | 0.17 (0.001) | 0.19 (0.001)e | 0.18 (0.002)d | 0.17 (0.002) | ||
| BMC (g) | 3.91 (0.02) | 4.19 (0.03)e | 3.86 (0.04) | 4.10 (0.04)e | ||
| Neck region area (cm2) | 4.80 (0.01) | 4.69 (0.02)e | 4.63 (0.03)e | 4.94 (0.02)e | ||
|
| ||||||
| Hip Structure Analysis | ||||||
| Neck-shaft angleb | 131.5 (0.16) | 130.5 (0.23)d | 132.1 (0.36) | 129.4 (0.34)e | ||
| Neck length (cm)b | 4.83 (0.02) | 4.87 (0.02) | 4.65 (0.03)e | 4.97 (0.03)d | ||
|
| ||||||
| Narrow Neck | ||||||
| BMD (g/cm2) | 0.95 (0.005) | 1.03 (0.007)e | 0.86 (0.01)e | 1.13 (0.01)e | ||
| Cross-sectional area (cm2) | 2.68 (0.012) | 2.87 (0.02)e | 2.40 (0.03)e | 3.28 (0.03)e | ||
| Outer diameter (cm) | 2.96 (0.006) | 2.92 (0.001)e | 2.93 (0.01)c | 3.07 (0.01)e | ||
| Section modulus (cm3) | 1.22 (0.008) | 1.27 (0.01)c | 1.06 (0.02)e | 1.62 (0.02)e | ||
| Buckling ratio | 8.91 (0.06) | 8.06 (0.09)e | 9.94 (0.13)e | 7.42 (0.12)e | ||
| Centroid position | 0.466 (0.000) | 0.468 (0.001) | 0.462 (0.001)e | 0.469 (0.001)d | ||
|
| ||||||
| Intertrochanter | ||||||
| BMD (g/cm2) | 0.93 (0.005) | 0.98 (0.008)e | 0.84 (0.02)e | 1.11 (0.01)e | ||
| Cross-sectional area (cm2) | 4.54 (0.03) | 4.69 (0.04)d | 3.99 (0.06)e | 5.63 (0.06)e | ||
| Outer diameter (cm) | 5.10 (0.01) | 5.03 (0.02)d | 4.94 (0.02)e | 5.35 (0.02)e | ||
| Section modulus (cm3) | 3.72 (0.03) | 3.69 (0.04) | 3.13 (0.06)e | 4.94 (0.05)e | ||
| Buckling ratio | 7.67 (0.05) | 7.33 (0.07)d | 8.29 (0.11)e | 6.18 (0.10)e | ||
| Centroid position | 0.436 (0.000) | 0.436 (0.001) | 0.434 (0.001) | 0.448 (0.001)e | ||
Adjusted for age (yrs.), height (cm), weight (kg), menopausal status (pre-/early peri-menopausal), physical activity score (range 3–15), smoking (never, past, current), total dietary calcium intake (mg/d), calcium supplement (yes/no), vitamin D supplement (yes/no), daily alcohol consumption (drinks/d, none, ≤1, >1), corticosteroid use (yes/no), diabetic status (yes/no), arthritis (yes/no) and study site;
Neck shaft angle and centroid position are unadjusted and neck length is adjusted for height only.
p<0.05;
p<0.001;
p<0.0001 vs. Caucasian.
Caucasian vs. African-American Women
African American women had significantly higher (9.8%, p<0.0001) conventional femoral neck BMD than Caucasian women, attributable both to a greater BMC and a smaller region area. They also had higher BMAD than Caucasian women. Consistently, African American women had narrower (outer diameter, OD) NN and IT regions but a greater amount of bone (cross-sectional area, CSA) in those regions; hence a greater HSA BMD at those regions (8% and 5%, respectively, p<0.0001). Section modulus (SM) was significantly greater (4%, p<0.05) in African American women at the NN region only. Buckling ratio (BR) at both regions was significantly lower (10%, p<0.0001 at NN; 4%, p<0.001 at IT) among African American women. Caucasian and African American women had a similar neck length (NL) but Caucasian women had a significantly greater neck shaft angle (NSA). Centroid position was shifted toward the thicker medial cortex at both regions to a similar extent in both groups.
Caucasian vs. Chinese Women
Chinese women had similar conventional femoral neck BMD as Caucasian women, attributable to significantly smaller region area containing a non-significantly smaller BMC. BMAD was higher in Chinese than Caucasian women. HSA BMD was significantly lower among Chinese women at the NN (9%, p<0.0001) and IT (10%, p<0.0001) regions due to a significantly smaller (narrower) neck region as well as a significantly smaller CSA (10% NN; 12% IT). Section modulus was lower (13% at NN; 16% at IT) and buckling ratios were higher (12% NN; 8% IT) at both regions. Chinese women also had a larger NSA but smaller NL than Caucasian women. Centroid position was more medially located at the narrow neck indicating greater asymmetry in the distribution of neck mass than seen is Caucasians.
Caucasian vs. Japanese Women
Japanese women had similar conventional femoral neck BMD but significantly greater BMC and region area as Caucasian women. BMAD was also similar between Japanese and Caucasian women. However, Japanese women had significantly greater HSA BMD (19%, p<0.0001) at both regions, primarily due to a higher CSA (22–24%, p<0.0001) opposed by a wider outer diameter (4–5%, p<0.0001). Their wider femurs led to significantly greater SM (33%, p<0.0001) but despite wider outer diameters the apparently thicker cortices led to lower BR at the NN (17%, p<0.0001) and IT (19%, p<0.0001) regions than Caucasian women. NSA was smaller and NL longer in Japanese women as compared to their Caucasian counterparts. Centroids were more centrally located in Japanese women than Caucasian women at both regions.
Other Ethnic Comparisons
African American women had greater conventional BMD than Chinese and Japanese women and this was due primarily to a greater BMC than the other groups. Japanese women had the largest FN region area which together with a similar BMC as African American women resulted in a conventional BMD similar to Chinese women. The difference in BMAD was significant between all groups; African American women had the highest BMAD followed by the Chinese and the Japanese women. HSA BMD at the NN and IT regions was significantly higher in Japanese women than African American and Chinese women primarily due to their larger cross-sectional area at both regions. Like African American women, Chinese women had similarly narrower outer diameter and FN region areas but slightly narrower IT diameters vs. Japanese women. Compared to other groups, Chinese women had a smaller cross-sectional area, a lower section modulus and a higher buckling ratio at both regions. Japanese women had a smaller neck shaft angle but a longer femoral neck than women in the other racial/ethnic groups.
Discussion
Racial/ethnic differences in fracture rates cannot be fully explained by BMD differences, partially because ethnic groups vary considerably in body size and shape in ways that independently influence BMD and the underlying mechanical strength that it attempts to represent. Some evidence that racial/ethnic differences in hip geometry may influence fracture propensity exists (3), but data are limited primarily to older adults and black-white comparisons (1, 2). The present study used Hip Structure Analysis (HSA) on archived DXA scans of pre- and early peri-menopausal ethnically diverse women in the Study of Women’s Health Across the Nation (SWAN) to assess racial/ethnic differences in femoral structure and strength properties.
Our results suggest that African American and Japanese women have structural advantages at the hip over Caucasian and Chinese women that may confer reduced susceptibility to hip fracture. Figure 1 summarizes the significant differences in geometry with Caucasian women as the referent, shown with factors related to cross-section dimensions separated from those related to the bending moment. The parameters were separated because the ethnic differences tend to trend in opposite directions with respect to mechanical implications.
Figure 1.
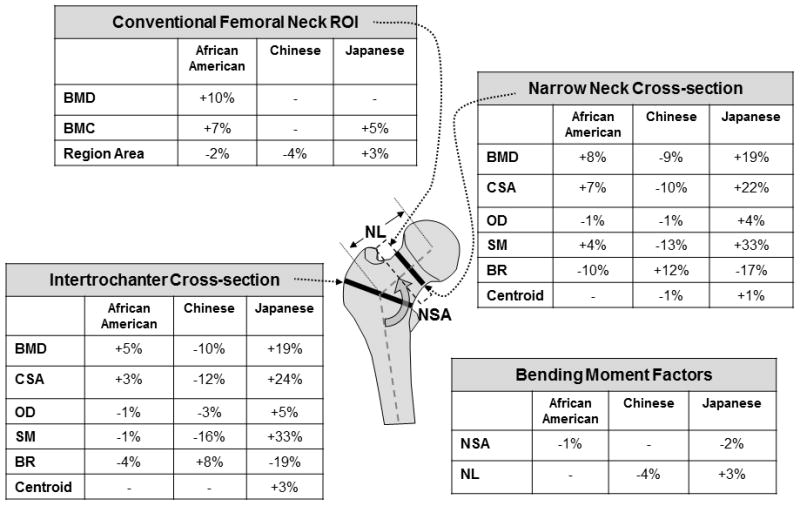
Measurement regions for conventional DXA and Hip Structure Analysis: Mean Differences vs. Caucasians. BMD=bone mineral density; BMC=bone mineral content; CSA=cross-sectional area; OD=outer diameter; SM=section modulus; BR=buckling ratio; NSA=neck shaft angle; NL=neck length.
Cross-section Dimensions
Cross-sectional area (CSA) and section modulus (SM) of a cross-section are critical because they govern its resistance to axial compression and bending forces, respectively. At the femoral neck CSA and SM were higher than Caucasians among African Americans and Japanese but lower in Chinese. The same pattern is evident at the intertrochanter except that African Americans have a slightly weaker SM than Caucasians. These differences at both regions among African Americans were previously observed in the Women’s Health Initiative (WHI) study (3). Narrower femoral necks (smaller OD) among African Americans have also been noted in previous studies of both men and women using DXA methods (1–3, 5). Because size-adjusted proximal femurs of African Americans are significantly narrower, their CSA needs to be disproportionately larger to achieve a higher SM than Caucasians. From a mechanical perspective, the higher CSA among African American women at both regions should better resist axially directed forces. Chinese women also had significantly narrower femurs than Caucasians but it was not offset by a larger CSA; thus their SM was lower than that of Caucasian women at both regions. Smaller values of CSA and SM should lead to lower strength in axial compression and bending, respectively. Other studies reported significantly lower CSA and SM in Chinese men and women vs. Caucasian counterparts (6, 9). Japanese women presented a different picture; their femurs were significantly wider (larger OD) than other groups but also contained a greater mass (CSA) resulting in a SM that was one-third greater at both regions than Caucasians and also larger than in other groups.
The distribution of bone surface within cross-sections also influences bending resistance. For the same diameter, SM is greatest when the centroid is at the center equidistant from outer surfaces. If the cortex is thicker on one side the centroid shifts toward that side and farther from the opposite surface. This reduces the section modulus and increases bending stress on the farther surface. The relative centroid position is 0.5 if located in the center of the cross-section. Compared to other groups, centroids were more centrally located in Japanese but more medially located in Chinese, contributing to their greater and lesser SM, respectively.
Neck Bending Moment
African American women had a smaller NSA vs. Caucasians which should increase bending in a stance mode. Adaptation to greater bending may explain why their SM was 4% greater than Caucasians at the narrow neck, although SM was 1% smaller at the intertrochanter. These findings are consistent with those of the Women’s Health Initiative (3). Longer neck lengths (NL) should also increase neck bending. As in WHI, we observed slightly (1%) longer NL in African Americans vs. Caucasians but unlike WHI, differences did not reach significance (p=0.20).
Consistent with other reports, Chinese women had shorter NL which should reduce bending moments and adaptation to them may explain their significantly smaller SM than Caucasians at both regions. Previous studies reported significantly shorter HAL and NL in Chinese men and women recruited in Changsha Xi’an and Shanghai compared to Caucasians, consistent with the present study (6, 9). In a study of premenopausal women of Chinese, Indian, Polynesian and European ancestry, HAL and NL were greatest among Polynesians, followed by Europeans, Chinese (New Zealand born or immigrants from Taiwan, Hong Kong, Malaysia or mainland China) and Indians (21). Some caution is needed when comparing our work to previous reports using HAL. HAL measures from the inner pelvic margin to the lateral greater trochanter margin. HAL, while also measured along the neck axis length, is not directly comparable to neck length (NL).
Japanese women in our study had significantly longer NL and smaller NSA both of which should increase bending in normal ambulation. Consistent with expectations of adaptation, their SM may be larger than that of other groups to better resist this greater bending. Contrary to our findings, height-adjusted NL were significantly shorter in native Japanese women compared to US women of European ancestry (22).
Relevance of Ethnic Differences to Hip Fracture
An important consideration for fracture risk is that the bending resulting from a fall impacting on the greater trochanter opposes that in normal physiologic stance. In normal stance the loading forces concentrate compressive stress along the thicker inferior-medial cortex of the proximal femur, while those along the thinner superior-lateral cortex are greatly reduced due to stress-shielding effects of bipedalism (23). Bending in a fall concentrates high compressive stresses on the thinner cortex (24). Fracture initiation appeared to initiate on this surface under high speed video imaging of experimental fractures simulating a fall mode by De Bakker et al. (25). QCT data demonstrated thinner superior-lateral cortices among hip fracture cases vs. controls in both men and women in the AGES-REYKJAVIK Study (26). While the specifics remain unclear, local failure of thinned cortices by crushing or local buckling appears likely. Thus factors that increase the concentration of compressive stresses on the superior-lateral neck cortex or reduce the resistance to them in a fall should increase hip fracture risk. A longer or more vertically oriented (larger NSA) femur neck would thus increase fall-mode bending. A larger NSA was evident among fracture cases vs. controls in the Study of Osteoporotic fractures (SOF), although NL was not significantly longer (17). A case-control study of hip fractures in Korean men and women reported that longer HAL or greater NSA were independently associated with increased odds of intertrochanter but not femoral neck fracture (27).
In the present study Japanese had a significantly smaller NSA but a significantly longer NL than Caucasians; these effects on the bending moment in a fall are opposing and evaluation of their net effect would require more sophisticated modeling than used here. An earlier study also showed smaller NSA in Japanese compared to Caucasian women (22). Contrary to our findings Japanese women in that study had shorter NL than their Caucasian counterparts which persisted after scaling by height. Reasons for the observed difference in NL are unclear, but women in the other study were recruited in Japan and were on average older, shorter and weighed less than in our study. African American women in our study also had a significantly smaller NSA vs. Caucasians, with similar NL, which may have a net positive effect on fracture risk. NSA of Chinese women were similar to that of Caucasian women, but their shorter NL should confer an advantage by reducing bending in a fall.
Factors that should increase fall mode stresses on the superior-lateral cortex would include a smaller section modulus or more medially shifted centroid both of which were evident in Chinese women. Here Japanese women have advantageously larger section moduli and more centrally located centroids. Finally greater thinning of the superior lateral cortex would increase susceptibility to local failure. Cortical thinning cannot be directly evaluated by current DXA methods although indirect evidence is available in the centroid position and the estimated mean cortical thickness incorporated into the buckling ratio (BR). As compared to Caucasians, buckling ratios at both regions were significantly lower in Japanese and African Americans. In contrast, a higher BR in Chinese women suggests greater susceptibility to local buckling. Our findings in Chinese women are contrary to that of Zhang et al. who reported significantly lower BR in Chinese vs. Caucasian men and women (6). While a more sophisticated analysis method would provide a clearer perspective on fracture susceptibility overall both African American and Japanese have structural advantages that should reduce hip fracture susceptibility vs. Caucasians while the opposite may be the case among Chinese women.
The differences in hip geometric parameters observed between Chinese and Japanese women in the present study are particularly interesting because superficially they appear similar with nearly identical values of conventional BMD, height, weight and BMI. But the forces that cause fracture are transferred through dimensions (geometry) and the dimensions are quite different in the two groups. We suspect that these important dimensional differences will help explain the variability in hip fracture rates observed between Asian subgroups (28, 29).
Relevance of Racial/Ethnic Differences in Body Size and Shape
In our sample, as compared to Caucasians, African American women were shorter and weighed more while Chinese and Japanese women were both shorter and weighed less. Linear regression models used to adjust for differences in body size and shape may not adequately compensate for ethnic differences in body habitus or in underlying hip mechanics. Adjustment generally attenuated differences between African American and Chinese vs. Caucasians but increased differences between Japanese and Caucasian women. This unique finding will need to be studied in more detail in this sample (longitudinal data is available) and verified in other Japanese populations. We should note that a recent publication by Ishii et al. using the same data set also evaluated ethnic differences in hip strength indices using a different method (30). After adjustment for covariates strength indices in Chinese, Japanese and African Americans were all significantly higher than Caucasians. Consistent with our findings, strength indices were greatest in Japanese followed by African Americans, but unlike our findings, indices in Chinese were higher than Caucasians rather than lower as we observed. We suspect the differences in findings are related to different size scaling effects in the two studies.
Strengths and limitations
Our study has a number of strengths. SWAN is a large, well-characterized sample of women from diverse racial/ethnic backgrounds transitioning through the menopause. We were able to include a large number of relevant variables in our analyses and extend our findings to include women of Chinese and Japanese origin. The HSA methodology has been used successfully in other cohorts and the structural indices have distinguished fracture cases from controls (31–35) and are predictive of fracture in longitudinal studies (17, 20, 36). The technical limitations of HSA have been well-described (37). Most importantly, it is more prone to precision error than BMD on the same data 3-D bones are evaluated from 2-D images. Positioning error was minimized in SWAN by use of the Osteodyne Hip Positioning System during scanning. Although we used height and weight to adjust for racial/ethnic differences in body shape and size, ethnic differences in total body lean mass, which were not evaluated, may permit better adjustment as in the WHI study (4). Our study was cross-sectional and did not directly evaluate hip fracture risk. However, ongoing analyses are examining the association between geometry and fracture in this sample. We were unable to obtain HSA measurements on nearly 20% of our sample which may limit generalizability; however, the primary reasons for the missing measurements in the current study have not been routinely encountered in other studies that have utilized HSA. Furthermore, there were no differences in age or areal BMD between women who did and did not undergo HSA measurement. Our study lacked sufficient samples of women of Hispanic or American Indian ethnicity. Finally, our sample was limited to pre-/early peri-menopausal women at select study sites, so results may not generalize to women in the larger SWAN cohort or to women of other ages.
Our results are in general agreement with those of other studies that observed a more favorable structural geometry at the hip in African American and Japanese, as compared to Caucasian and Chinese American women. The observed biomechanical differences should translate to differences in hip fracture risk and may help to explain racial/ethnic differences in fracture rates. Future research should focus on more realistic models of hip mechanics to better emulate physiologic and traumatic loading conditions yielding a more easily understood strength estimate. We are currently exploring whether such a model can be derived with the limited data provided by SWAN and similar studies.
Acknowledgments
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495, AG026463). The bone strength and geometry data (HSA) are from John’s Hopkins University. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Winifred Rossi, 2012 – Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Kim Sutton-Tyrrell, Co-PI 2001 – present; Maria Mori Brooks Co-PI 2012; New England Research Institutes, Watertown, MA – Sonja McKinlay, Principal Investigator 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair; Chris Gallagher, Former Chair.
We thank the study staff at each site and all the women who participated in SWAN.
Authors’ roles: Study design: MED, TJB, and JAC. Study conduct: MED, TJB, and JAC. Data collection: JAC, MED, TJB, and JAC. Data analysis: TJB, YL and KR. Data interpretation: MED, TJB, YL, ASK, GAG, KR, JL, SG, MV, and JAC. Drafting of manuscript: MED, TJB, and YL. Revising manuscript content: MED, TJB, YL, ASK, GAG, KR, JL, SG, and JAC. Approving final version of manuscript: MED, TJB, YL, ASK, GAG, KR, JL, SG, MV, and JAC. MED takes responsibility for the integrity of the data analysis.
Footnotes
Disclosures:
Drs. Danielson, Cauley, Greendale, Greenspan, Karlamangla, Lo, Ruppert and Vuga and Ms. Lian have no conflicts to report. Dr. Beck is co-founder of Beck Radiological Innovations, Inc., a company that develops software and hardware methods for measuring bone structure as well as other products. His former employer the John’s Hopkins University licensed the HSA software used in this paper to Hologic, Inc. and he receives a share of the royalties.
References
- 1.Nelson DA, Barondess DA, Hendrix SL, Beck TJ. Cross-sectional geometry, bone strength, and bone mass in the proximal femur in black and white postmenopausal women. J Bone Miner Res. 2000;15(10):1992–7. doi: 10.1359/jbmr.2000.15.10.1992. Epub 2000/10/12. [DOI] [PubMed] [Google Scholar]
- 2.Nelson DA, Pettifor JM, Barondess DA, Cody DD, Uusi-Rasi K, Beck TJ. Comparison of cross-sectional geometry of the proximal femur in white and black women from Detroit and Johannesburg. J Bone Miner Res. 2004;19(4):560–5. doi: 10.1359/JBMR.040104. Epub 2004/03/10. [DOI] [PubMed] [Google Scholar]
- 3.Nelson D, Beck T, Wu G, Lewis C, Bassford T, Cauley J, et al. Ethnic differences in femur geometry in the women’s health initiative observational study. Osteoporosis International. 2010;22(5):1377–88. doi: 10.1007/s00198-010-1349-4. Epub 2010 Aug 5. [DOI] [PubMed] [Google Scholar]
- 4.Beck TJ, Petit MA, Wu G, LeBoff MS, Cauley JA, Chen Z. Does obesity really make the femur stronger? BMD, geometry, and fracture incidence in the women’s health initiative-observational study. J Bone Miner Res. 2009;24(8):1369–79. doi: 10.1359/jbmr.090307. Epub 2009/03/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Travison T, Beck T, Esche G, Araujo A, McKinlay J. Age trends in proximal femur geometry in men: variation by race and ethnicity. Osteoporosis International. 2008;19(3):277–87. doi: 10.1007/s00198-007-0497-7. [DOI] [PubMed] [Google Scholar]
- 6.Zhang F, Tan LJ, Lei SF, Deng HW. The differences of femoral neck geometric parameters: effects of age, gender and race. Osteoporosis International. 2010;21(7):1205–14. doi: 10.1007/s00198-009-1057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X-F, Duan Y, Beck TJ, Seeman E. Varying contributions of growth and ageing to racial and sex differences in femoral neck structure and strength in old age. Bone. 2005;36(6):978–86. doi: 10.1016/j.bone.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Marshall LM. Race and ethnic variation in proximal femur structure and BMD among older men. J Bone Miner Res. 2008;23:121–30. doi: 10.1359/JBMR.070908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan L, Crabtree NJ, Reeve J, Zhou B, Dequeker J, Nijs J, et al. Does hip strength analysis explain the lower incidence of hip fracture in the People’s Republic of China? Bone. 2004;34(3):584–8. doi: 10.1016/j.bone.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Sowers MR, Greendale GA, Bondarenko I, Finkelstein JS, Cauley JA, Neer RM, et al. Endogenous hormones and bone turnover markers in pre- and perimenopausal women: SWAN. Osteoporos Int. 2003;14(3):191–7. doi: 10.1007/s00198-002-1329-4. Epub 2003/05/06. [DOI] [PubMed] [Google Scholar]
- 11.Sternfeld B, Ainsworth BE, Quesenberry CP. Physical Activity Patterns in a Diverse Population of Women. Preventive Medicine. 1999;28(3):313–23. doi: 10.1006/pmed.1998.0470. [DOI] [PubMed] [Google Scholar]
- 12.Baecke J, Burema J, Frijters J. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. The American Journal of Clinical Nutrition. 1982;36(5):936–42. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 13.Huang MH, Schocken M, Block G, Sowers M, Gold E, Sternfeld B, et al. Variation in nutrient intakes by ethnicity: results from the Study of Women’s Health Across the Nation (SWAN) Menopause. 2002;9(5):309–19. doi: 10.1097/00042192-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Finkelstein JS, Lee M-LT, Sowers M, Ettinger B, Neer RM, Kelsey JL, et al. Ethnic variation in bone density in premenopausal and early perimenopausal women: effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab. 2002;87(7):3057–67. doi: 10.1210/jc.87.7.3057. [DOI] [PubMed] [Google Scholar]
- 15.Beck TJ, Looker AC, Ruff CB, Sievanen H, Wahner HW. Structural trends in the aging femoral neck and proximal shaft: analysis of the Third National Health and Nutrition Examination Survey dual-energy X-ray absorptiometry data. J Bone Miner Res. 2000;15(12):2297–304. doi: 10.1359/jbmr.2000.15.12.2297. Epub 2000/12/29. [DOI] [PubMed] [Google Scholar]
- 16.Martin RB, Burr DB. Non-invasive measurement of long bone cross-sectional moment of inertia by photon absorptiometry. Journal of Biomechanics. 1984;17(3):195–201. doi: 10.1016/0021-9290(84)90010-1. [DOI] [PubMed] [Google Scholar]
- 17.Kaptoge S, Beck TJ, Reeve J, Stone KL, Hillier TA, Cauley JA, et al. Prediction of incident hip fracture risk by femur geometry variables measured by hip structural analysis in the study of osteoporotic fractures. J Bone Miner Res. 2008;23(12):1892–904. doi: 10.1359/jbmr.080802. Epub 2008/08/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayhew PM, Thomas CD, Clement JG, Loveridge N, Beck TJ, Bonfield W, et al. Relation between age, femoral neck cortical stability, and hip fracture risk. The Lancet. 2005;366(9480):129–35. doi: 10.1016/s0140-6736(05)66870-5. [DOI] [PubMed] [Google Scholar]
- 19.Young WC. Elastic stability formulas for stress and strain. In: HC, ST, editors. Roark’s Formulas for Stress and Strain. 6. New York, NY: McGraw-Hill; 1989. p. 688. [Google Scholar]
- 20.Rivadeneira F, Zillikens MC, De Laet CE, Hofman A, Uitterlinden AG, Beck TJ, et al. Femoral neck BMD is a strong predictor of hip fracture susceptibility in elderly men and women because it detects cortical bone instability: the Rotterdam Study. J Bone Miner Res. 2007;22(11):1781–90. doi: 10.1359/jbmr.070712. Epub 2007/07/20. [DOI] [PubMed] [Google Scholar]
- 21.Chin K, Evans MC, Cornish J, Cundy T, Reid IR. Differences in hip axis and femoral neck length in premenopausal women of Polynesian, Asian and European origin. Osteoporosis International. 1997;7(4):344–7. doi: 10.1007/bf01623775. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura T, Turner CH, Yoshikawa T, Slemenda CW, Peacock M, Burr DB, et al. Do variations in hip geometry explain differences in hip fracture risk between japanese and white americans? Journal of Bone and Mineral Research. 1994;9(7):1071–6. doi: 10.1002/jbmr.5650090715. [DOI] [PubMed] [Google Scholar]
- 23.Lovejoy CO. Evolution of human walking. Sci Am. 1988;259:118–25. doi: 10.1038/scientificamerican1188-118. [DOI] [PubMed] [Google Scholar]
- 24.Verhulp E, van Rietbergen B, Huiskes R. Load distribution in the healthy and osteoporotic human proximal femur during a fall to the side. Bone. 2008;42(1):30–5. doi: 10.1016/j.bone.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 25.de Bakker PM, Manske SL, Ebacher V, Oxland TR, Cripton PA, Guy P. During sideways falls proximal femur fractures initiate in the superolateral cortex: Evidence from high-speed video of simulated fractures. J Biomech. 2009;42(12):1917–25. doi: 10.1016/j.jbiomech.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Johannesdottir F, Poole KES, Reeve J, Siggeirsdottir K, Aspelund T, Mogensen B, et al. Distribution of cortical bone in the femoral neck and hip fracture: A prospective case-control analysis of 143 incident hip fractures; the AGES-REYKJAVIK Study. Bone. 2011;48(6):1268–76. doi: 10.1016/j.bone.2011.03.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Im G, Lim M. Proximal hip geometry and hip fracture risk assessment in a Korean population. Osteoporosis International. 2011;22(3):803–7. doi: 10.1007/s00198-010-1301-7. [DOI] [PubMed] [Google Scholar]
- 28.Cauley JA, El-Hajj Fuleihan G, Arabi A, Fujiwara S, Ragi-Eis S, Calderon A, et al. Official Positions for FRAXR Clinical Regarding International Differences: From Joint Official Positions Development Conference of the International Society for Clinical Densitometry and International Osteoporosis Foundation on FRAXR. Journal of Clinical Densitometry. 2011;14(3):240–62. doi: 10.1016/j.jocd.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Cheng S, Levy A, Lefaivre K, Guy P, Kuramoto L, Sobolev B. Geographic trends in incidence of hip fractures: a comprehensive literature review. Osteoporosis International. 2011;22(10):2575–86. doi: 10.1007/s00198-011-1596-z. [DOI] [PubMed] [Google Scholar]
- 30.Ishii S, Cauley J, Greendale G, Danielson M, Safaei Nili N, Karlamangla A. Ethnic differences in composite indices of femoral neck strength. Osteoporosis International. 2012;23(4):1381–90. doi: 10.1007/s00198-011-1723-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crabtree NJ, Kroger H, Martin A, Pols HAP, Lorenc R, Nijs J, et al. Improving Risk Assessment: Hip Geometry, Bone Mineral Distribution and Bone Strength in Hip Fracture Cases and Controls. The EPOS Study. Osteoporosis International. 2002;13(1):48–54. doi: 10.1007/s198-002-8337-y. [DOI] [PubMed] [Google Scholar]
- 32.Pulkkinen P, Partanen J, Jalovaara P, Jämsä T. Combination of bone mineral density and upper femur geometry improves the prediction of hip fracture. Osteoporosis International. 2004;15(4):274–80. doi: 10.1007/s00198-003-1556-3. [DOI] [PubMed] [Google Scholar]
- 33.Gnudi S, Sitta E, Fiumi N. Bone density and geometry in assessing hip fracture risk in post-menopausal women. Br J Radiol. 2007;80(959):893–7. doi: 10.1259/bjr/37401526. [DOI] [PubMed] [Google Scholar]
- 34.Szulc P, Duboeuf F, Schott A, Dargent-Molina P, Meunier P, Delmas P. Structural determinants of hip fracture in elderly women: re-analysis of the data from the EPIDOS study. Osteoporosis International. 2006;17(2):231–6. doi: 10.1007/s00198-005-1980-7. [DOI] [PubMed] [Google Scholar]
- 35.Melton LJ, Beck TJ, Amin S, Khosla S, Achenbach SJ, Oberg AL, et al. Contributions of bone density and structure to fracture risk assessment in men and women. Osteoporosis International. 2005;16(5):460–7. doi: 10.1007/s00198-004-1820-1. [DOI] [PubMed] [Google Scholar]
- 36.Lacroix AZ, Beck TJ, Cauley JA, Lewis CE, Bassford T, Jackson R, et al. Hip structural geometry and incidence of hip fracture in postmenopausal women: what does it add to conventional bone mineral density? Osteoporos Int. 2009 doi: 10.1007/s00198-009-1056-1. Epub 2009/09/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beck T. Measuring the structural strength of bones with dual-energy X-ray absorptiometry: principles, technical limitations, and future possibilities. Osteoporos Int. 2003;14(Suppl 5):81–8. doi: 10.1007/s00198-003-1478-0. [DOI] [PubMed] [Google Scholar]


