Abstract
Diastolic dysfunction is characterized by slow or incomplete relaxation of the ventricles during diastole, and is an important contributor to heart failure pathophysiology. Clinical symptoms include fatigue, shortness of breath, and pulmonary and peripheral edema, all contributing to decreased quality of life and poor prognosis. There are currently no therapies available that directly target the heart pump defects in diastolic function. Calcium mishandling is a hallmark of heart disease and has been the subject of a large body of research. Efforts are ongoing in a number of gene therapy approaches to normalize the function of calcium handling proteins such as sarcoplasmic reticulum calcium ATPase. An alternative approach to address calcium mishandling in diastolic dysfunction is to introduce calcium buffers to facilitate relaxation of the heart. Parvalbumin is a calcium binding protein found in fast-twitch skeletal muscle and not normally expressed in the heart. Gene transfer of parvalbumin into normal and diseased cardiac myocytes increases relaxation rate but also markedly decreases contraction amplitude. Although parvalbumin binds calcium in a delayed manner, it is not delayed enough to preserve full contractility. Factors contributing to the temporal nature of calcium buffering by parvalbumin are discussed in relation to remediation of diastolic dysfunction.
Keywords: diastolic dysfunction, heart failure, parvalbumin, gene therapy, calcium
1. Heart Failure and Calcium Handling
Heart failure is a clinical syndrome of heart pump insufficiency and is a leading cause of death worldwide [1]. Heart failure is characterized by poor prognosis and decreased quality of life, with primary symptoms of breathlessness, fatigue, and edema of the lungs and lower extremities [2]. Clinically, heart failure is divided into two categories [3–5]: 1) heart failure with preserved ejection fraction (HFpEF), and 2) heart failure with reduced ejection fraction (HFrEF). Diastolic dysfunction, the decreased ability of the heart to relax in diastole, is a key pathogenic feature of HFpEF, but can also be present in HFrEF. Despite the high percentage of patients thought to have diastolic dysfunction, the pumping action of the heart occurring during systole has for decades been the main focus of therapeutic development. Although mortality remains high, aggressive utilization of ACE inhibitors, beta-blockers and diuretics has improved survival in patients with reduced ejection fraction [6]. In comparison, these therapies have largely proven ineffective at extending lifespan in heart failure patients exhibiting primarily diastolic dysfunction (HFpEF) [3].
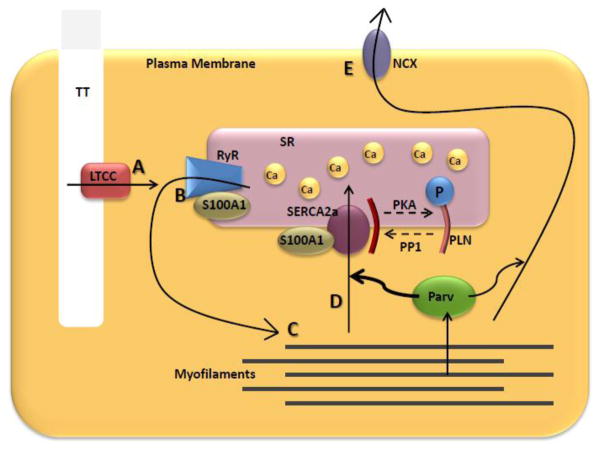
Normal heart pump function is driven by the intricately regulated cyclic release and reuptake of calcium (Ca2+) to and from the cytoplasm of cardiac myocytes in the process of excitation-contraction (E-C) coupling. During normal E-C coupling, depolarization of the myocyte sarcolemma by an action potential induces a small influx of Ca2+ through the L-type Ca2+ channels (LTCC). This, in turn, triggers a much larger release of Ca2+ from the sarcoplasmic reticulum (SR) by stimulating the ryanodine receptor (RyR) Ca2+ release channel. Ca2+ then interacts with sarcomeric troponin C and allows for actin-myosin engagement and force production. Finally, Ca2+ is removed from the cytoplasm, primarily via the SR Ca2+ ATPase (SERCA2a) and the sarcolemmal sodium-calcium exchanger (NCX), leading to relaxation (Figure 1). Defects in any of these mechanisms driving Ca2+ flux can lead to profound cardiac dysfunction.
Figure 1.
Calcium Cycling in the Cardiac Myocyte. Arrows represent Ca2+ flux during one contractile cycle. A) Action potential allows Ca2+ entry through the LTCC, which B) triggers a much larger Ca2+ release from the SR through the RyR. C) Ca2+ binds to myofilament proteins, leading to force production. D) Ca2+ is then taken back up into the SR via SERCA2a, or E) pumped out of the cell via NCX. S100A1 and PLN act as modulators of Ca2+ transport protein function. Parv buffers Ca2+ away from myofilaments after force production.
In diastolic dysfunction, there may be an increase in sarcomere Ca2+ sensitivity and a decrease in the rate of Ca2+ reuptake via SERCA2a and NCX, all leading to a state of Ca2+ overload. This leads to a slow or incomplete relaxation of the ventricles, causing pulmonary edema and hypertension. Chronically, increased diastolic Ca2+ also activates cell death pathways and alters metabolic profile. A number of reviews contain detailed discussions of these topics [7–10].
Given the challenges of extending lifespan in heart failure patients with preserved ejection fraction, there is considerable need for effective therapeutics directly targeting heart relaxation performance. Most existing heart failure therapies act by reducing functional demand on the myocardium rather than directly restoring myocyte function. Although some diastolic dysfunction can be attributed to systemic dysfunction, a principal defect of this disease is a heart-intrinsic inability to effectively relax, of which defects in Ca2+ handling play an important role. This review focuses on experimental therapies specifically targeted at improving Ca2+ homeostasis in an effort to improve relaxation performance in diastolic dysfunction. The first section presents an overview of how improving the expression, function, and activity of several endogenous Ca2+ handling proteins in the heart affects diastolic function in experimental models of heart failure. The second section presents an additional strategy for normalizing Ca2+ handling through the introduction of a cytoplasmic Ca2+ buffering system to enhance relaxation. Both sections emphasize gene therapeutic strategies.
2. Endogenous Calcium Handling Proteins
2.1. Phospholamban and SERCA2a
Reduced Ca2+ reuptake into the SR is a hallmark of the failing heart and an important contributor to Ca2+ mishandling and slow relaxation in diastolic dysfunction. Therefore, SERCA2a and its regulator phospholamban (PLN) are attractive therapeutic targets, particularly as SERCA2a activity can be decreased in failing hearts [11]. Early work using adenoviral gene transfer of SERCA2a and PLN in isolated cardiac myocytes demonstrated a role for SERCA2a to increase the rate of Ca2+ transient decay and decrease diastolic Ca2+ levels, with PLN counteracting this function [12, 13]. Phosphorylation of PLN by Protein Kinase A (PKA) on serine-16 relieves its inhibition on SERCA2a, allowing increased Ca2+ uptake into the SR (Figure 1).
Multiple approaches have been utilized to alter the function of both PLN and SERCA2a with the goal of improving Ca2+ handling and thus diastolic function. The phospho-mimetic PLN(S16E) has been utilized in several models to relieve PLN inhibition of SERCA2a including the BIO14.6 dilated cardiomyopathic hamster model [14], a coronary artery ligation model [15], and a rapid pacing model in sheep [16]. Improvements in diastolic function including left ventricular end diastolic pressure (LVEDP) and the load independent time constant tau were found with PLN(S16E) treatment in these studies. An alternative means to relieve SERCA2a inhibition is to inactivate PLN or reduce PLN expression. The former was accomplished by expressing an antibody against PLN, which increased relaxation rate, measured by the negative pressure derivative (−dP/dt), in isolated hearts from diabetic mice [17]. Reducing the amount of PLN protein was achieved by injecting adeno-associated virus (AAV9) expressing short hairpin RNA (shRNA) against PLN in aortic-banded rats, which resulted in significantly improved LVEDP and −dP/dt at one and three months post injection [18]. However, another study showed that a five-fold knockdown of PLN using AAV6 shRNA-PLN injected into the left ventricle of dogs caused an increase in serum cTnI 3–6 weeks after treatment, and a decrease in fractional shortening from 4–10 weeks after treatment. It was suggested that shRNA-PLN may interfere with endogenous microRNA pathways [19].
Recently, inhibition of Protein Phosphatase 1 (PP1), which dephosphorylates PLN and thus decreases SERCA2a activity (Figure 1), was studied in Muscle Lim Protein (MLP) knockout mice, a model of genetic dilated cardiomyopathy. Mice were injected with AAV9 carrying PP1 shRNA, which resulted in a 25% decrease in PP1 protein expression. Compared to controls, AAV9-shPP1 injected animals showed improved diastolic function measured by tau and LVEDP, as well as improved fractional shortening, LV dimensions, and LV posterior wall thickness 12 weeks after gene transfer. Isolated cell studies provide evidence that normalized Ca2+ handling was a major contributor to the results seen [20].
The concept of increasing the SERCA to PLN ratio to ameliorate heart failure has been tested by expressing SERCA in various models of heart failure. In aortic-banded rats, increasing Ca2+ reuptake into the SR with adenoviral gene delivery of SERCA improved several measures of systolic function and energy status, but did not normalize banding-induced increases in heart weight. Relaxation performance was not evaluated [21]. In another model, rat hearts with permanent ligations of the coronary artery were injected with lentiviral vectors harboring SERCA2a. Two months later, rat hearts receiving SERCA2a had increased fractional shortening and improved left ventricular volumes. In addition, in vivo hemodynamics showed increased ejection fraction and decreased tau six months after gene transfer of SERCA2a. Six month survival after ligation was also increased in animals with lentiviral SERCA2a [22].
SERCA2a activity is ATP-dependent and therefore its applicability for treatment of the energy compromised failing heart has been explored. NMR spectroscopy was used to measure 31P in two studies. SERCA2a AAV increased the phosphocreatine to ATP ratio (PCr:ATP) in hearts of aortic banded mice [21]. A separate study reported little change in energetics measurements between hearts of aortic constricted mice with and without overexpression of SERCA2a [23]. Another study in rats undergoing aortic-banding showed SERCA2a improved energy-efficiency of hearts measured by oxygen consumption [24]. All of these studies used ex vivo hearts acutely perfused with a glucose-containing buffer.
The strategy of SERCA2a delivery in heart failure has advanced to clinical trials. AAV1-SERCA2a was injected via intracoronary infusion into nine patients with heart failure in a Phase 1 clinical trial to assess safety. To date, there have been no safety concerns reported [25]. Thus, AAV1-SERCA2a treatment was also investigated in a larger Phase 2 trial where the highest dose (1×1013 DNAse resistant particles) reduced the number of recurrent clinical events compared to placebo after 12 months [26]. It will be important to continue monitoring this cohort beyond the 12 month endpoint of this study to fully address SERCA2a as a therapeutic treatment for the failing human heart.
Na+/Ca2+ exchanger
The Na+/Ca2+ exchanger (NCX) is a sarcolemmal protein which can function to transport Ca2+ into or out of the cell (Figure 1). In heart failure patients, mRNA and protein expression of NCX can be increased [27], possibly a compensatory mechanism to decreased SERCA2a activity. Increased NCX expression has been correlated to increased diastolic function in failing human heart explants [28] and therefore has been studied experimentally as a way to improve heart function in healthy and diseased models. In a rabbit model of heart failure, modest improvements in fractional shortening and maximum pressure derivative (+dP/dt) were shown two weeks after administration of adenovirus containing NCX (Ad-NCX), but diastolic parameters including −dP/dt and LVEDP were not improved. Interestingly, isolated cells from these Ad-NCX treated healthy and failing hearts had decreased contractility compared to controls [29]. Myocyte relaxation measurements were not reported in this rabbit model, however overexpressing NCX in a mouse hypertrophy model rescued the hypertrophy-induced prolongation of the Ca2+ transient in isolated myocytes [30]. In contrast to NCX overexpression, NCX inhibition with NCX-shRNA protected isolated rat cardiac myocytes from calcium overload [31]. In the future, it will be important to understand the cross-talk between SERCA2a and NCX in the regulation of Ca2+ handling and the roles these proteins play in different species and models of heart failure.
2.4. S100A1
The Ca2+ binding protein S100A1 has been shown to interact with various Ca2+ handling proteins (such as RyR and SERCA) (Figure 1) and affect their activities [32]. S100A1 expression is decreased in heart failure. Isolated human failing cardiac myocytes treated with Ad-S100A1 exhibited an increased rate of Ca2+ decay and decreased RyR Ca2+ leak, presumably by directly interacting with RyR. Ad-S100A1 treatment also improved PCr:ATP in myocytes [33]. Cryoinfarcted hearts injected with Ad-S100A1 showed a significant improvement in contractility and relaxation seven days after gene transfer. Cells isolated from these rat hearts had an increased rate of cell relengthening, and a decrease in both diastolic Ca2+ and Ca2+ leak [34]. In another study, AAV6-S100A1 was injected 10 weeks after cryoinfarct. Cardiac function assessed eight weeks later showed improved −dP/dt and LVEDP under basal conditions and with isoproterenol. Additionally, mRNA expression of SERCA2a and PLN were normalized [35]. In a large animal model of heart failure, pig hearts were occluded for two hours via balloon catheter, and two weeks later AAV9-S100A1 was retrogradely injected into the coronary artery. Hearts injected with AAV9-S100A1 showed significantly improved whole heart function, including diastolic parameters such as −dP/dt, LVEDP, and LV end diastolic diameter (LVEDD). Diastolic Ca2+ and SR Ca2+ leak were increased in isolated myocytes from occluded hearts, both of which were corrected by S100A1. AAV9-S100A1 also improved the PCr:ATP and NADH/NAD ratios, suggesting improved energetics [36].
3. Ca2+ Buffering
The introduction of a cytosolic Ca2+ buffering system offers an alternative approach to normalize aberrant Ca2+ handling in diastolic dysfunction. Conceptually, a Ca2+ buffer with maximal buffering capacity occurring during diastole could facilitate a faster removal of Ca2+ from myofilaments, thus improving the rate of relaxation in the face of slow Ca2+ reuptake. A buffer could act as a temporary storage depot for Ca2+ until it is taken up into the SR by SERCA2a or transported out of the cell by NCX. In the case of a buffering system, increased relaxation rate could occur without the need for increased maximal energy production in an energy compromised failing heart. Additionally, a novel buffer may act to correct multiple mechanisms of Ca2+ mishandling as opposed to targeting the activity of one protein. These potential advantages provide incentive to explore Ca2+ buffers as an additional or alternative approach to improving the expression or activity of endogenous Ca2+-handling proteins as discussed in the previous section.
Proteins containing EF-hands are integral to the maintenance of normal intracellular Ca2+ handling. EF-hands function as Ca2+ sensors and buffers, affecting multiple regulatory pathways and controlling free cytosolic Ca2+ concentrations. They contain a 12-amino acid cation binding loop flanked by two α-helices in an approximately perpendicular arrangement. EF-hands were so named because their shape approximates that of a hand with the thumb and forefinger held approximately 90° apart as first illustrated by Kretsigner and Nockolds in 1973 [37].
Parvalbumin (Parv) is a ~12 kDa protein containing two functional EF-hands. There are three distinct domains within Parv. The AB domain (amino acids 1–40) at the N-terminus does not bind Ca2+. The CD (amino acids 41–70) and EF (amino acids 80–108) domains each contain an EF-hand that is capable of binding both Ca2+ and magnesium (Mg2+). Amino acids 1, 3, 5, 7, 9, and 12 of Parv’s EF hand binding loops form electrostatic interactions with Ca2+ and Mg2+. Calcium is bound by the EF-hand binding loops via seven coordinating oxygen atoms in a pentagonal bipyrimidal geometry. Magnesium binding differs in that it features only six coordination sites. The coordinating oxygen atoms come from the side chains of amino acids 1, 3, 5, and 12, the backbone carbonyl group of amino acid 7, and either a side chain oxygen atom from amino acid 9 (CD loop) or a water molecule (EF loop). The 12th amino acid side chain is unique in that it provides two coordinating oxygen atoms for Ca2+ and one for Mg2+, allowing each to attain their preferred coordination geometry [38].
Parvalbumin has α and β isoforms, which are differentially expressed in vivo. The α-Parv isoform is found in fast-twitch skeletal muscle, GABAergic neurons, endocrine glands, brain, bone and kidney. The β-Parv isoform (also referred to as oncomodulin in mammals) is expressed in the thymus in chickens [39], and hair cells of the organ of corti in mammals [40]. It has also been identified in human placental tissue [41]. Parvalbumin is not expressed in detectable amounts in the cardiac tissue of most species. In humans, the α and β isoforms contain approximately 50% sequence homology [41].
The primary function of Parv is to buffer Ca2+. In fast twitch muscle, Parv facilitates rapid relaxation by buffering Ca2+ away from myofilaments after a contraction. An example of this buffering action is found in the toadfish Opsanus tau, which has the highest known concentration of Parv in its swimbladder muscle, 1.5–3.0 mM [42], enabling it to contract and relax up to 200 times per second to produce its distinctive “boatwhistle” mating call [43]. Parv Ca2+ and Mg2+ binding affinities vary between species and isoforms, but are generally in the range of KCa2+ = 107–109 M−1 and KMg2+ = 103–105 M−1, respectively [44, 45]. In a resting (non-contracted) cell, Mg2+ concentration (~1 mM) is much higher than Ca2+ concentration (10–100 nM). As a result, the EF-hands in Parv are primarily occupied by Mg2+ at baseline. When the muscle cell contracts and Ca2+ concentration in the cell is high, Ca2+ displaces Mg2+ in the EF-hand binding loops. The capability of Parv to bind Mg2+ is imperative for the temporal nature of its Ca2+ buffering function. The time required for Mg2+ dissociation prevents Ca2+ from binding to Parv immediately after Ca2+ release from the SR, making it a delayed Ca2+ buffer [45].
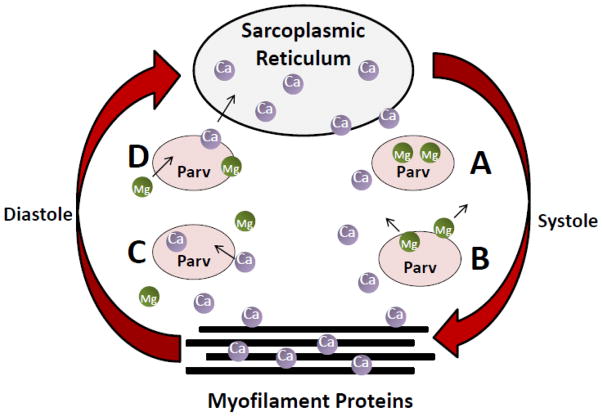
Because of the important role for Ca2+ mishandling in heart failure pathophysiology [46], the therapeutic potential of Parv has been tested for increasing the relaxation rate of the heart in states of diastolic dysfunction. A working model for this concept is pictured in Figure 2. When cardiac myocytes are in the relaxed state in diastole, Mg2+ is bound to the EF-hand loops of Parv. Upon depolarization at the start of systole, excitation contraction coupling causes a rapid release of Ca2+ from the SR, raising cytosolic Ca2+ to ~1 μM and leading to contraction of the myofilaments. The rise in Ca2+ concentration facilitates the removal of Mg2+ from and the subsequent binding of Ca2+ to the EF-hands of Parv. Because of the relatively slow off-rate of Mg2+ from Parv, Ca2+ is not instantly buffered. This delay is vital for myofilament cross-bridge cycling to be able to occur. The “delayed” Ca2+ buffering effect of Parv ideally takes place when Ca2+ is beginning to dissociate from myofilament proteins at the end of systole. The buffering capability of Parv provides temporary storage for Ca2+, enabling more rapid and complete relaxation of the myofilaments, even in the case of slow Ca2+ reuptake.
Figure 2.
Working Model for Parv Function in the Heart. A) In the relaxed state, cytosolic Ca2+ is low and Mg2+ is bound to Parv. B) At the beginning of systole, Ca2+ is released from the SR and binds to the myofilament protein cTnC to facilitate contraction. Increased cytosolic Ca2+ concentration causes dissociation of Mg2+ from Parv. C) In diastole, Parv facilitates rapid dissociation of Ca2+ from cTnC by buffering Ca2+ away from the myofilaments, thus facilitating relaxation. D) As Ca2+ is returned to the SR, Ca2+ dissociates from Parv and is replaced by Mg2+.
This working model has been tested extensively through a combination of primary cell culture and in vivo physiological studies. Early work focused on understanding Parv-induced changes in cellular function by using adenoviral gene transfer of Parv in isolated adult rat cardiac myocytes. Parvalbumin expression at levels approximating those found in rat superior vastus lateralis muscle [47] significantly enhanced the rate of relaxation and Ca2+ transient decay in cells from healthy rats and those with hypothyroidism [48] and hypertension induced cardiac hypertrophy [49]. In a dual gene transfer strategy, Parv rescued the delayed relaxation phenotype displayed by both the A63V and E180G mutations of α-tropomyosin [50]. Parv-transduced cardiac myocytes from canines with descending thoracic aortic coarctation, a large animal model of diastolic dysfunction, demonstrated a marked improvement in relaxation time [51].
In vivo studies have confirmed findings from cellular studies. Direct intramyocardial adenoviral gene delivery of Parv into the left ventricle of rats with and without hypothyroidism decreased left ventricular isovolumic relaxation time (LV IVRT) assessed by echocardiography. LV IVRT was inversely correlated to Parv protein expression. Additionally, times to 50% and 90% relaxation and −dP/dt were improved during measurement by in vivo hemodynamics micromanometry [52]. Aged Fisher 344 x BN F1 rats have evidence of diastolic dysfunction without significant fibrosis. Adenoviral delivery of Parv in these rats resulted in a significant improvement in the load independent time constant tau [53]. A similar study in aged Fisher 344 rats, which show significant evidence of fibrosis, also revealed improvement in diastolic dysfunction [54] providing evidence for the utility of Parv to improve diastolic dysfunction with different etiologies.
Along with the hastened rate of relaxation and Ca2+ transient decay, a decrease in contraction amplitude in both cellular and in vivo studies has been observed with Parv. The Parv-induced increase in relaxation rate is inversely related to contraction amplitude. This is thought to occur when the maximal buffering capacity of Parv occurs prior to the end of systole, resulting in sequestration of Ca2+ away from cTnC too early in the contractile cycle. Several factors may be involved in this process and are discussed in the following paragraphs.
First, changing the amount of Parv expression changes the maximal buffering capacity. Increasing Parv concentrations in isolated cardiac myocytes is achieved by increasing the number of days cells are cultured after adenoviral gene transfer [47]. As demonstrated in isolated cell experiments, and corroborated by mathematical modeling, Parv concentrations below 0.01 mM have minimal effects on cellular function, and concentrations greater than 0.1 mM greatly increase relaxation rate, but at the expense of contraction amplitude. Between 0.01 and 0.1 mM, Parv has modest beneficial effects on relaxation without adversely diminishing contraction amplitude in isolated cardiac myocyte experiments [47, 55], in which function is generally measured by pacing cells at 0.2–1Hz.
Heart rate is another factor affecting the buffering capacity of parvalbumin. In a study of Parv transgenic mice [56], the dependence of Parv function on pacing was demonstrated. Ex vivo analysis of whole hearts from transgenic mice revealed modest increases in relaxation rate and no effect on contraction amplitude at a pacing rate of 7 Hz. However, as pacing was decreased to 2.5 Hz, transgenic hearts had a more robust increase in relaxation rate, but the contraction amplitude was severely diminished. Mathematical modeling gives insight into the mechanism behind this effect. The rapid contraction-relaxation cycle that occurs in hearts paced at 7 Hz does not allow enough time during diastole for Ca2+ to completely dissociate from Parv and be transported back into the SR before the beginning of the next contractile cycle. The relatively slow off-rate of Ca2+ from Parv results in a percentage of EF-hand binding sites being continuously occupied by Ca2+ under conditions of fast pacing. This is similar to the results in skeletal muscle found by Robertson et al. [57] where upon fast pacing the Parv binding sites became saturated and lost buffering capacity.
Finally, intrinsic Ca2+ and Mg2+ affinities of Parv affect physiological function. This was demonstrated by comparing β-Parv from carp with human α-Parv [49]. Carp β-Parv differs from human α-Parv by having 16% increased Mg2+ affinity and 218% increased Ca2+ affinity [49]. Comparing these two isoforms side by side, β-Parv was found to decrease relaxation rate and contraction amplitude to a greater extent than α-Parv in isolated cells from Dahl salt-sensitive rats, a model of hypertension-induced diastolic dysfunction. Extensive work has been done with Parv isoforms to understand the role of various binding loop amino acids to influence Ca2+ and Mg2+ affinities [58–61]. Knowledge gleaned from this work may be of importance in the identification of a calcium buffer with optimized kinetics for human heart physiology.
4. Conclusion
Diastolic dysfunction is a prominent feature in HFpEF, and can be present in HFrEF as well. Although there are many precipitating causes of diastolic dysfunction, Ca2+ mishandling is an important factor in its development and progression. Because of the importance of Ca2+, targeting Ca2+ handling proteins through gene therapeutic strategies is a major area of study. This review has presented several different and potentially complementary approaches to normalizing Ca2+ handling in diastole. One approach is to target the expression, function or activity of endogenous Ca2+ handling proteins in the heart. This offers the opportunity to compensate for specific defects in Ca2+ reuptake in diastole. The introduction of a cytosolic Ca2+ buffering system is another approach to improve relaxation performance in diastole. Ca2+ buffering does not target the expression or function of a specific Ca2+ handling protein, but rather provides a temporary storage depot for excess Ca2+ regardless of the primary defect in Ca2+ handling. Buffering allows for efficient removal of Ca2+ from myofilaments in diastole, facilitating rapid relaxation, without increasing maximal energy expenditure. The primary drawback of wild type Parv as a Ca2+ buffer is its potential to reach maximal buffering capacity too early in the contraction-relaxation cycle, thus inhibiting contraction amplitude. Future work will explore the factors affecting the temporal nature of Ca2+ binding by Parv to understand how to harness the therapeutic potential of Ca2+ buffers for the improvement of relaxation, while not compromising contractile function.
Highlights.
Parvalbumin, a calcium buffer, facilitates rapid relaxation in fast-twitch muscle.
Viral gene transfer of parvalbumin in cardiac myocytes hastens relaxation.
Parvalbumin buffering of calcium is “delayed”, occurring primarily in diastole.
Parvalbumin represents a potential gene therapeutic for diastolic heart failure.
Abbreviations
- AAV
adeno-associated virus
- Ad
adenovirus
- Ca2+
calcium
- cTnC
cardiac troponin C
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- LVEDD
left ventricular end diastolic dimension
- LVEDP
left ventricular end diastolic pressure
- LV IVRT
left ventricular isovolumic relaxation time
- LTCC
L-type calcium channel
- Mg2+
magnesium
- Parv
parvalbumin
- PCr
phosphocreatine
- PLN
phospholambam
- +dP/dt, −dP/dt
positive and negative pressure derivatives
- PKA
protein kinase A
- PP1
protein phosphatase 1
- RyR
ryanodine receptor
- SERCA2a
sarcoplasmic reticulum calcium ATPase 2a
- NCX
sodium calcium exchanger
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 2.Barker WH, Mullooly JP, Getchell W. Changing incidence and survival for heart failure in a well-defined older population, 1970–1974 and 1990–1994. Circulation. 2006;113:799–805. doi: 10.1161/CIRCULATIONAHA.104.492033. [DOI] [PubMed] [Google Scholar]
- 3.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 4.Tribouilloy C, Rusinaru D, Mahjoub H, Souliere V, Levy F, Peltier M, Slama M, Massy Z. Prognosis of heart failure with preserved ejection fraction: a 5 year prospective population-based study. Eur Heart J. 2008;29:339–347. doi: 10.1093/eurheartj/ehm554. [DOI] [PubMed] [Google Scholar]
- 5.McCullough PA, Khandelwal AK, McKinnon JE, Shenkman HJ, Pampati V, Nori D, Sullivan RA, Sandberg KR, Kaatz S. Outcomes and prognostic factors of systolic as compared with diastolic heart failure in urban America. Congest Heart Fail. 2005;11:6–11. doi: 10.1111/j.1527-5299.2005.03731.x. [DOI] [PubMed] [Google Scholar]
- 6.Jhund PS, Macintyre K, Simpson CR, Lewsey JD, Stewart S, Redpath A, Chalmers JW, Capewell S, McMurray JJ. Long-term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: a population study of 5.1 million people. Circulation. 2009;119:515–523. doi: 10.1161/CIRCULATIONAHA.108.812172. [DOI] [PubMed] [Google Scholar]
- 7.Schaub MC, Heizmann CW. Calcium, troponin, calmodulin, S100 proteins: from myocardial basics to new therapeutic strategies. Biochem Biophys Res Commun. 2008;369:247–264. doi: 10.1016/j.bbrc.2007.10.082. [DOI] [PubMed] [Google Scholar]
- 8.Kranias EG, Hajjar RJ. Modulation of cardiac contractility by the phospholamban/SERCA2a regulatome. Circ Res. 2012;110:1646–1660. doi: 10.1161/CIRCRESAHA.111.259754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gustafsson AB, Gottlieb RA. Heart mitochondria: gates of life and death. Cardiovasc Res. 2008;77:334–343. doi: 10.1093/cvr/cvm005. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz-Meana M, Fernandez-Sanz C, Garcia-Dorado D. The SR-mitochondria interaction: a new player in cardiac pathophysiology. Cardiovasc Res. 2010;88:30–39. doi: 10.1093/cvr/cvq225. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt U, Hajjar RJ, Helm PA, Kim CS, Doye AA, Gwathmey JK. Contribution of abnormal sarcoplasmic reticulum ATPase activity to systolic and diastolic dysfunction in human heart failure. J Mol Cell Cardiol. 1998;30:1929–1937. doi: 10.1006/jmcc.1998.0748. [DOI] [PubMed] [Google Scholar]
- 12.Hajjar RJ, Kang JX, Gwathmey JK, Rosenzweig A. Physiological effects of adenoviral gene transfer of sarcoplasmic reticulum calcium ATPase in isolated rat myocytes. Circulation. 1997;95:423–429. doi: 10.1161/01.cir.95.2.423. [DOI] [PubMed] [Google Scholar]
- 13.Hajjar RJ, Schmidt U, Kang JX, Matsui T, Rosenzweig A. Adenoviral gene transfer of phospholamban in isolated rat cardiomyocytes. Rescue effects by concomitant gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase. Circ Res. 1997;81:145–153. doi: 10.1161/01.res.81.2.145. [DOI] [PubMed] [Google Scholar]
- 14.Hoshijima M, Ikeda Y, Iwanaga Y, Minamisawa S, Date MO, Gu Y, Iwatate M, Li M, Wang L, Wilson JM, Wang Y, Ross J, Jr, Chien KR. Chronic suppression of heart-failure progression by a pseudophosphorylated mutant of phospholamban via in vivo cardiac rAAV gene delivery. Nat Med. 2002;8:864–871. doi: 10.1038/nm739. [DOI] [PubMed] [Google Scholar]
- 15.Iwanaga Y, Hoshijima M, Gu Y, Iwatate M, Dieterle T, Ikeda Y, Date MO, Chrast J, Matsuzaki M, Peterson KL, Chien KR, Ross J., Jr Chronic phospholamban inhibition prevents progressive cardiac dysfunction and pathological remodeling after infarction in rats. J Clin Invest. 2004;113:727–736. doi: 10.1172/JCI18716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaye DM, Preovolos A, Marshall T, Byrne M, Hoshijima M, Hajjar R, Mariani JA, Pepe S, Chien KR, Power JM. Percutaneous cardiac recirculation-mediated gene transfer of an inhibitory phospholamban peptide reverses advanced heart failure in large animals. J Am Coll Cardiol. 2007;50:253–260. doi: 10.1016/j.jacc.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 17.Meyer M, Belke DD, Trost SU, Swanson E, Dieterle T, Scott B, Cary SP, Ho P, Bluhm WF, McDonough PM, Silverman GJ, Dillmann WH. A recombinant antibody increases cardiac contractility by mimicking phospholamban phosphorylation. FASEB J. 2004;18:1312–1314. doi: 10.1096/fj.03-1231fje. [DOI] [PubMed] [Google Scholar]
- 18.Suckau L, Fechner H, Chemaly E, Krohn S, Hadri L, Kockskamper J, Westermann D, Bisping E, Ly H, Wang X, Kawase Y, Chen J, Liang L, Sipo I, Vetter R, Weger S, Kurreck J, Erdmann V, Tschope C, Pieske B, Lebeche D, Schultheiss HP, Hajjar RJ, Poller WC. Long-term cardiac-targeted RNA interference for the treatment of heart failure restores cardiac function and reduces pathological hypertrophy. Circulation. 2009;119:1241–1252. doi: 10.1161/CIRCULATIONAHA.108.783852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bish LT, Sleeper MM, Reynolds C, Gazzara J, Withnall E, Singletary GE, Buchlis G, Hui D, High KA, Gao G, Wilson JM, Sweeney HL. Cardiac gene transfer of short hairpin RNA directed against phospholamban effectively knocks down gene expression but causes cellular toxicity in canines. Hum Gene Ther. 2011;22:969–977. doi: 10.1089/hum.2011.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyazaki Y, Ikeda Y, Shiraishi K, Fujimoto SN, Aoyama H, Yoshimura K, Inui M, Hoshijima M, Kasahara H, Aoki H, Matsuzaki M. Heart failure-inducible gene therapy targeting protein phosphatase 1 prevents progressive left ventricular remodeling. PLoS One. 2012;7:e35875. doi: 10.1371/journal.pone.0035875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.del Monte F, Williams E, Lebeche D, Schmidt U, Rosenzweig A, Gwathmey JK, Lewandowski ED, Hajjar RJ. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase in a rat model of heart failure. Circulation. 2001;104:1424–1429. doi: 10.1161/hc3601.095574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niwano K, Arai M, Koitabashi N, Watanabe A, Ikeda Y, Miyoshi H, Kurabayashi M. Lentiviral vector-mediated SERCA2 gene transfer protects against heart failure and left ventricular remodeling after myocardial infarction in rats. Mol Ther. 2008;16:1026–1032. doi: 10.1038/mt.2008.61. [DOI] [PubMed] [Google Scholar]
- 23.Pinz I, Tian R, Belke D, Swanson E, Dillmann W, Ingwall JS. Compromised myocardial energetics in hypertrophied mouse hearts diminish the beneficial effect of overexpressing SERCA2a. J Biol Chem. 2011;286:10163–10168. doi: 10.1074/jbc.M110.210757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakata S, Lebeche D, Sakata N, Sakata Y, Chemaly ER, Liang LF, Tsuji T, Takewa Y, del Monte F, Peluso R, Zsebo K, Jeong D, Park WJ, Kawase Y, Hajjar RJ. Restoration of mechanical and energetic function in failing aortic-banded rat hearts by gene transfer of calcium cycling proteins. J Mol Cell Cardiol. 2007;42:852–861. doi: 10.1016/j.yjmcc.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, Borow K, Dittrich H, Zsebo KM, Hajjar RJ. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail. 2009;15:171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, Yaroshinsky A, Zsebo KM, Dittrich H, Hajjar RJ. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation. 2011;124:304–313. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Studer R, Reinecke H, Bilger J, Eschenhagen T, Bohm M, Hasenfuss G, Just H, Holtz J, Drexler H. Gene expression of the cardiac Na(+)-Ca2+ exchanger in end-stage human heart failure. Circ Res. 1994;75:443–453. doi: 10.1161/01.res.75.3.443. [DOI] [PubMed] [Google Scholar]
- 28.Hasenfuss G, Schillinger W, Lehnart SE, Preuss M, Pieske B, Maier LS, Prestle J, Minami K, Just H. Relationship between Na+-Ca2+-exchanger protein levels and diastolic function of failing human myocardium. Circulation. 1999;99:641–648. doi: 10.1161/01.cir.99.5.641. [DOI] [PubMed] [Google Scholar]
- 29.Munch G, Rosport K, Baumgartner C, Li Z, Wagner S, Bultmann A, Ungerer M. Functional alterations after cardiac sodium-calcium exchanger overexpression in heart failure. Am J Physiol Heart Circ Physiol. 2006;291:H488–495. doi: 10.1152/ajpheart.01324.2005. [DOI] [PubMed] [Google Scholar]
- 30.Stagg MA, Malik AH, MacLeod KT, Terracciano CM. The effects of overexpression of the Na+/Ca2+ exchanger on calcium regulation in hypertrophied mouse cardiac myocytes. Cell Calcium. 2004;36:111–118. doi: 10.1016/j.ceca.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Maddaford TG, Dibrov E, Hurtado C, Pierce GN. Reduced expression of the Na+/Ca2+ exchanger in adult cardiomyocytes via adenovirally delivered shRNA results in resistance to simulated ischemic injury. Am J Physiol Heart Circ Physiol. 2010;298:H360–366. doi: 10.1152/ajpheart.00932.2009. [DOI] [PubMed] [Google Scholar]
- 32.Ritterhoff J, Most P. Targeting S100A1 in heart failure. Gene Ther. 2012;19:613–621. doi: 10.1038/gt.2012.8. [DOI] [PubMed] [Google Scholar]
- 33.Brinks H, Rohde D, Voelkers M, Qiu G, Pleger ST, Herzog N, Rabinowitz J, Ruhparwar A, Silvestry S, Lerchenmuller C, Mather PJ, Eckhart AD, Katus HA, Carrel T, Koch WJ, Most P. S100A1 genetically targeted therapy reverses dysfunction of human failing cardiomyocytes. J Am Coll Cardiol. 2011;58:966–973. doi: 10.1016/j.jacc.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Most P, Pleger ST, Volkers M, Heidt B, Boerries M, Weichenhan D, Loffler E, Janssen PM, Eckhart AD, Martini J, Williams ML, Katus HA, Remppis A, Koch WJ. Cardiac adenoviral S100A1 gene delivery rescues failing myocardium. J Clin Invest. 2004;114:1550–1563. doi: 10.1172/JCI21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pleger ST, Most P, Boucher M, Soltys S, Chuprun JK, Pleger W, Gao E, Dasgupta A, Rengo G, Remppis A, Katus HA, Eckhart AD, Rabinowitz JE, Koch WJ. Stable myocardial-specific AAV6-S100A1 gene therapy results in chronic functional heart failure rescue. Circulation. 2007;115:2506–2515. doi: 10.1161/CIRCULATIONAHA.106.671701. [DOI] [PubMed] [Google Scholar]
- 36.Pleger ST, Shan C, Ksienzyk J, Bekeredjian R, Boekstegers P, Hinkel R, Schinkel S, Leuchs B, Ludwig J, Qiu G, Weber C, Raake P, Koch WJ, Katus HA, Muller OJ, Most P. Cardiac AAV9-S100A1 gene therapy rescues post-ischemic heart failure in a preclinical large animal model. Sci Transl Med. 2011;3:92ra64. doi: 10.1126/scitranslmed.3002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kretsinger RH, Nockolds CE. Carp muscle calcium-binding protein. II. Structure determination and general description. J Biol Chem. 1973;248:3313–3326. [PubMed] [Google Scholar]
- 38.Permyakov EA. Parvalbumin. In: Uversky VN, editor. Molecular Anatomy and Physiology of Proteins. Nova Science Publishers, Inc; New York: 2007. [Google Scholar]
- 39.Hapak RC, Zhao H, Boschi JM, Henzl MT. Novel avian thymic parvalbumin displays high degree of sequence homology to oncomodulin. J Biol Chem. 1994;269:5288–5296. [PubMed] [Google Scholar]
- 40.Sakaguchi N, Henzl MT, Thalmann I, Thalmann R, Schulte BA. Oncomodulin is expressed exclusively by outer hair cells in the organ of Corti. J Histochem Cytochem. 1998;46:29–40. doi: 10.1177/002215549804600105. [DOI] [PubMed] [Google Scholar]
- 41.Fohr UG, Weber BR, Muntener M, Staudenmann W, Hughes GJ, Frutiger S, Banville D, Schafer BW, Heizmann CW. Human alpha and beta parvalbumins. Structure and tissue-specific expression. Eur J Biochem. 1993;215:719–727. doi: 10.1111/j.1432-1033.1993.tb18084.x. [DOI] [PubMed] [Google Scholar]
- 42.Hamoir G, Gerardin-Otthiers N, Focant B. Protein differentiation of the superfast swimbladder muscle of the toadfish Opsanus tau. J Mol Biol. 1980;143:155–160. doi: 10.1016/0022-2836(80)90129-1. [DOI] [PubMed] [Google Scholar]
- 43.Fine ML. Seasonal and geographical variation of the mating call of the oyster toadfish Opsanus tau L. Oecologia. 1978;36:45–57. doi: 10.1007/BF00344570. [DOI] [PubMed] [Google Scholar]
- 44.Pauls TL, Cox JA, Berchtold MW. The Ca2+(-)binding proteins parvalbumin and oncomodulin and their genes: new structural and functional findings. Biochim Biophys Acta. 1996;1306:39–54. doi: 10.1016/0167-4781(95)00221-9. [DOI] [PubMed] [Google Scholar]
- 45.Falke JJ, Drake SK, Hazard AL, Peersen OB. Molecular tuning of ion binding to calcium signaling proteins. Q Rev Biophys. 1994;27:219–290. doi: 10.1017/s0033583500003012. [DOI] [PubMed] [Google Scholar]
- 46.Periasamy M, Janssen PM. Molecular basis of diastolic dysfunction. Heart Fail Clin. 2008;4:13–21. doi: 10.1016/j.hfc.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coutu P, Metzger JM. Optimal range for parvalbumin as relaxing agent in adult cardiac myocytes: gene transfer and mathematical modeling. Biophys J. 2002;82:2565–2579. doi: 10.1016/S0006-3495(02)75599-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wahr PA, Michele DE, Metzger JM. Parvalbumin gene transfer corrects diastolic dysfunction in diseased cardiac myocytes. Proc Natl Acad Sci U S A. 1999;96:11982–11985. doi: 10.1073/pnas.96.21.11982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodenbaugh DW, Wang W, Davis J, Edwards T, Potter JD, Metzger JM. Parvalbumin isoforms differentially accelerate cardiac myocyte relaxation kinetics in an animal model of diastolic dysfunction. Am J Physiol Heart Circ Physiol. 2007;293:H1705–1713. doi: 10.1152/ajpheart.00232.2007. [DOI] [PubMed] [Google Scholar]
- 50.Coutu P, Bennett CN, Favre EG, Day SM, Metzger JM. Parvalbumin corrects slowed relaxation in adult cardiac myocytes expressing hypertrophic cardiomyopathy-linked alpha-tropomyosin mutations. Circ Res. 2004;94:1235–1241. doi: 10.1161/01.RES.0000126923.46786.FD. [DOI] [PubMed] [Google Scholar]
- 51.Hirsch JC, Borton AR, Albayya FP, Russell MW, Ohye RG, Metzger JM. Comparative analysis of parvalbumin and SERCA2a cardiac myocyte gene transfer in a large animal model of diastolic dysfunction. Am J Physiol Heart Circ Physiol. 2004;286:H2314–2321. doi: 10.1152/ajpheart.01137.2003. [DOI] [PubMed] [Google Scholar]
- 52.Szatkowski ML, Westfall MV, Gomez CA, Wahr PA, Michele DE, DelloRusso Turner C, II, Hong KE, Albayya FP, Metzger JM. In vivo acceleration of heart relaxation performance by parvalbumin gene delivery. J Clin Invest. 2001;107:191–198. doi: 10.1172/JCI9862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michele DE, Szatkowski ML, Albayya FP, Metzger JM. Parvalbumin gene delivery improves diastolic function in the aged myocardium in vivo. Mol Ther. 2004;10:399–403. doi: 10.1016/j.ymthe.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt U, Zhu X, Lebeche D, Huq F, Guerrero JL, Hajjar RJ. In vivo gene transfer of parvalbumin improves diastolic function in aged rat hearts. Cardiovasc Res. 2005;66:318–323. doi: 10.1016/j.cardiores.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 55.Coutu P, Metzger JM. Genetic manipulation of calcium-handling proteins in cardiac myocytes. I. Experimental studies. Am J Physiol Heart Circ Physiol. 2005;288:H601–612. doi: 10.1152/ajpheart.00424.2004. [DOI] [PubMed] [Google Scholar]
- 56.Day SM, Coutu P, Wang W, Herron T, Turner I, Shillingford M, Lacross NC, Converso KL, Piao L, Li J, Lopatin AN, Metzger JM. Cardiac-directed parvalbumin transgene expression in mice shows marked heart rate dependence of delayed Ca2+ buffering action. Physiol Genomics. 2008;33:312–322. doi: 10.1152/physiolgenomics.00302.2007. [DOI] [PubMed] [Google Scholar]
- 57.Robertson SP, Johnson JD, Potter JD. The time-course of Ca2+ exchange with calmodulin, troponin, parvalbumin, and myosin in response to transient increases in Ca2+ Biophys J. 1981;34:559–569. doi: 10.1016/S0006-3495(81)84868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cates MS, Berry MB, Ho EL, Li Q, Potter JD, Phillips GN., Jr Metal-ion affinity and specificity in EF-hand proteins: coordination geometry and domain plasticity in parvalbumin. Structure. 1999;7:1269–1278. doi: 10.1016/s0969-2126(00)80060-x. [DOI] [PubMed] [Google Scholar]
- 59.Henzl MT, Davis ME, Tan A. Leucine 85 is an important determinant of divalent ion affinity in rat beta-parvalbumin (Oncomodulin) Biochemistry. 2008;47:13635–13646. doi: 10.1021/bi8014899. [DOI] [PubMed] [Google Scholar]
- 60.Lee YH, Tanner JJ, Larson JD, Henzl MT. Crystal structure of a high-affinity variant of rat alpha-parvalbumin. Biochemistry. 2004;43:10008–10017. doi: 10.1021/bi0492915. [DOI] [PubMed] [Google Scholar]
- 61.Henzl MT, Ndubuka K. Low-affinity signature of the rat beta-parvalbumin CD site. Evidence for remote determinants. Biochemistry. 2007;46:23–35. doi: 10.1021/bi061421h. [DOI] [PubMed] [Google Scholar]