Abstract
The potential efficacy of total body center of mass (COM) acceleration for feedback control of standing balance by functional neuromuscular stimulation (FNS) following spinal cord injury (SCI) was investigated. COM acceleration may be a viable alternative to conventional joint kinematics due to its rapid responsiveness, focal representation of COM dynamics, and ease of measurement. A computational procedure was developed using an anatomically-realistic, three-dimensional, bipedal biomechanical model to determine optimal patterns of muscle excitations to produce targeted effects upon COM acceleration from erect stance. The procedure was verified with electromyographic data collected from standing able-bodied subjects undergoing systematic perturbations. Using 16 muscle groups targeted by existing implantable neuroprostheses, data were generated to train an artificial neural network (ANN)-based controller in simulation. During forward simulations, proportional feedback of COM acceleration drove the ANN to produce muscle excitation patterns countering the effects of applied perturbations. Feedback gains were optimized to minimize upper extremity (UE) loading required to stabilize against disturbances. Compared to the clinical case of maximum constant excitation, the controller reduced UE loading by 43% in resisting external perturbations and by 51% during simulated one-arm reaching. Future work includes performance assessment against expected measurement errors and developing user-specific control systems.
Keywords: Acceleration, Balance, Center of Mass, Feedback, Control System, Functional Neuromuscular Stimulation, Neuroprosthesis, Posture, Rehabilitation, Spinal Cord Injury, Standing
I. INTRODUCTION
This study investigated the use of the acceleration of total body center of mass (COM) as alternative feedback to conventional joint kinematics for continuously adjusting stimulation to muscles following spinal cord injury (SCI) for maintaining stable standing against perturbations to postural balance. Neuroprostheses employing functional neuromuscular stimulation (FNS) have effectively restored basic standing function following SCI using pre-programmed stimulation to facilitate sit-to-stand maneuvers and continuous, constant stimulation to maintain upright posture [1,2]. Because stimulation is applied at constant levels for standing maintenance, the user is required to exert significant upper extremity (UE) effort upon an assistive device (e.g., walker) to stabilize against postural disturbances. Sustained UE effort compromises the utility of standing with FNS by limiting reach and manual function, and reduces standing time by expediting the onset of upper body fatigue.
Standard joint angle feedback has been extensively investigated for closed-loop control of standing with FNS. It has been implemented in isolation for individual joints including the knees [3, 4], hips [5, 6], and ankles [7, 8]. These studies showed measures of improvement in disturbance response but effectively constrained the standing system to single planes of movement. Comprehensive (ankles, knees, hips, and trunk) three-dimensional control of standing with FNS based on joint feedback has been investigated in simulation [9]. Although it demonstrated a significant reduction in upper extremity effort during postural perturbations when compared to constant, maximal stimulation, this system required tuning 18 separate gain parameters for the proportional and derivative feedback from nine individual joints. This required instrumentation at each joint under active control, which may be cumbersome and impractical for routine clinical deployment. Furthermore, in order to effectively compensate for the delay between stimulus onset and peak muscle force generation, standard proportional-derivative joint feedback gains may be undesirably high, leaving the control system prone to instability.
Acceleration has been previously suggested as an effective means for assessing balance [10, 11, 12] and offers several potential advantages over joint-based control for standing with FNS. First, it is sensitive to the inertial effects of rapidly acting perturbations and can respond before significant changes in standing posture can occur, thereby providing a more potent initial feedback signal than position-based control. Acceleration of the system COM provides a representation of global system dynamics that are critical for standing control [13]. Finally, adequate measurement of COM acceleration may be plausible with only a few well placed accelerometers. This is because perturbed standing can be represented with a minimal number of synergies [14, 15] and nearly 75% of body mass is concentrated centrally across the pelvis, abdomen, and trunk [16].
The primary objective of this study was to develop and evaluate, in simulation, a feedback control system for FNS standing that uses gain-modulated COM acceleration inputs to produce optimal muscle excitation patterns which counter the effects of postural disturbances. A model-based approach was employed to determine the feasibility and basic operating characteristics of the controller prior to online testing with SCI subjects. The controller consisted of using proportional COM acceleration feedback to drive an artificial neural network (ANN). This ANN was trained on muscle excitation patterns optimized to produce target changes in COM acceleration from the neutral, erect standing posture. To validate the optimal acceleration-excitation synergy represented by the data used to train the ANN, electromyographic (EMG) data were collected during systematic perturbation of able-bodied standing subjects. The COM acceleration directions in which certain muscle groups were most active following a perturbation from neutral standing were compared across both data sets. Controller performance was evaluated according to the reduction in UE effort necessary to stabilize the model against disturbances with active controller modulation of muscle excitation levels compared to the case of constant excitation levels analogous to clinical stimulation paradigms.
II. Methods
The overall system (Figure 1) included two parallel controllers (FNS muscle control, UE loading) acting on a three-dimensional model of SCI bipedal standing (Section II.A) to maintain an erect, neutral setpoint position. The setpoint was defined as a single reference position that the control system was designed to maintain. The most erect posture corresponding to the highest vertical COM position above the center of the base of support (BOS) was selected as the desired setpoint for the model. The FNS control system employed negative feedback of measured COM acceleration changes thereby driving an ANN to modulate muscle excitation levels to counter the effects produced by postural disturbances. Volitional UE loading was represented by PID control of shoulder position (Section II.B) corresponding to the setpoint. The objective of both the FNS and UE control systems was to resist disturbances imposed upon the standing model while in the setpoint posture. The FNS controller was evaluated according to the reduction in shoulder position controller output (i.e., reduction in UE loading) under various postural disturbances using feedback controller modulation of the muscle excitation levels compared to the constant muscle excitation levels described in Section II.C.
Figure 1.
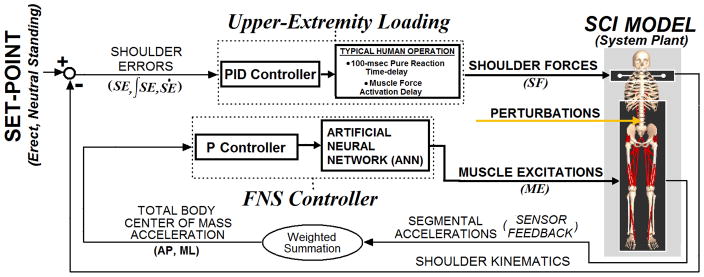
Overall model system. Two parallel controllers act to maintain the 3-D model of bipedal SCI stance at setpoint position against postural perturbations: (1) A FNS controller modulates trunk, lower-extremity muscle excitations according to COM acceleration feedback in the AP and ML directions driving an ANN. The ANN is trained to output muscle excitation changes that counter measured effects induced by disturbances and net recovery responses upon the COM. (2) An upper-extremity (UE) controller, representing user volitional loading, produces three-dimensional point forces at the shoulders according to position errors relative to the shoulder set-point posture. COM acceleration and shoulder positional errors are expressed in globally-fixed 3-D Cartesian coordinates. The gains for UE control are determined according to Ziegler-Nichols tuning rules. FNS controller gains are optimized using global-search algorithm to minimize UE controller output (“loading”) against perturbations.
In creating the data space that governs FNS controller action, the model was used to determine instantaneous changes in COM acceleration induced across the anterior-posterior (AP) and medial-lateral (ML) dimensions by changes in activation level from the setpoint stance for each muscle group available for FNS control. Optimal patterns of muscle activation were then formulated (Section II.D) to produce target changes in COM acceleration about the erect setpoint position that were feasible subject to force-generating capabilities of the included muscle groups. This is similar to the concept of “induced accelerations” introduced in [17] to determine the net effect of changes in muscle activation upon joint angular accelerations given a particular system state. This model-based optimization procedure for coordinating muscle activity according to changes in COM acceleration was validated using the EMG data collected from able-bodied individuals undergoing disturbances while standing (Section II.E). The net (across AP and ML dimensions) COM acceleration directions along which muscle groups were most active were compared between the EMG and model-based data (Section II.F).
The optimization procedure for producing optimal changes in muscle activation in accordance to targeted changes in COM acceleration from erect stance was applied to create data representing a synergy used to train the ANN (Section II.G). Each two dimensional (AP, ML) COM acceleration target represented a single training point of inputs, and the corresponding optimal excitation levels represented a single training point of outputs. For the purposes of ANN training, muscle activation, the muscle state variable determining force output level, was assumed to be directly proportional to excitation, the actual control input and analog for FNS stimulation level, for ANN training. Excitation-activation coupling was subsequently addressed during forward simulations with specified perturbations (Section II.H) with optimal tuning of the feedback controller gains (Section II.I) to minimize UE loading in the presence of activation dynamics [18]. Control system performance was observed during resistance of disturbances under two-arm and one-arm support conditions and during simulated one-arm reaching and manipulation of a weighted object (Section II.J). The models for SCI bipedal standing and volitional UE loading, determination of baseline excitation levels, and test perturbations were originally described in [9].
A. Three-Dimensional Model of SCI Stance
A three-dimensional computer model of human bipedal stance was developed in SIMM (Software for Interactive Musculoskeletal Modeling, Musculographics, Inc., Santa Rosa, CA) and adapted from a previously described representation of the lower extremities [19] and trunk [20]. This model consisted of nine segments (two feet, two thighs, two shanks, pelvis-lumbar component, and head-arm-trunk complex) with 15 anatomical degrees of freedom (DOFs) representing bilateral motions of ankle plantar/dorsiflexion (PF/DF), ankle inversion/eversion (Inv/Ev), knee flexion/extension (F/E), hip F/E internal/external rotation (Int/Ext), hip ab/adduction (Ab/Ad), and trunk roll-pitch-yaw. Passive moment properties [21] due to SCI were included at these DOFs. Both feet were in constant contact with the ground, defining a closed-chain which effectively reduced the number of independent DOFs to six [22]. The LEs were in series with a single three-DOF trunk joint at the lumbrosacral (L5-S1) region. A total of 58 muscle elements were defined across the trunk and lower extremities. When representing SCI standing by FNS, the only muscle groups actively controlled in the model were consistent with those targeted by existing 16-channel implanted FNS systems [23] and are listed in Table I. It is expected that these implanted systems will be used for individuals with complete thoracic-level SCI for restoring standing balance. Elements within each muscle group were constrained to act synchronously at the same level of excitation as if co-activated by a single stimulus output at a common motor point (e.g., femoral nerve innervating vasti). Excitation is a normalized quantity (0 to 1). Muscles were represented as Hill-type actuators with nonlinear force dynamics that included excitation-activation coupling and conventional length-tension and force-velocity properties [18]. The peak force parameter for each SCI muscle group was scaled from able-bodied values to produce the maximum isometric joint moments generated by individuals with complete thoracic-level SCI in response to electrical stimulation [24].
TABLE I.
STIMULATED MUSCLES AND CORRESPONDING SCI JOINT MOMENTS AND BASELINE EXCITATION LEVELS
| Muscle Group (stimulated by single channel) | Primary Anatomical Actions | SCI Joint Moment (N-m) from [24] | Optimal, Maximal Excitation Level from [9] |
|---|---|---|---|
| Soleus, Gastrocnemius | Ankle Plantarflexion | 55 | 0.049, 0.262 |
| Tibialis Anterior | Ankle Dorsiflexion | 15 | 0.000, 1.000 |
| Vasti (Medialis, Intermedius, Lateralis) | Knee Extension | 80 | 0.960, 1.000 |
| Adductor Magnus | Hip Extension, Hip Adduction | 63, 30 | 0.767, 1.000 |
| Gluteus Maximus | Hip Extension | 63 | 1.000, 1.000 |
| Gluteus Medius | Hip Abduction | 44 | 0.281, 1.000 |
| Semimembranosus | Hip Extension, Hip Adduction | 63, 30 | 0.467, 1.000 |
| Erector Spinae | Trunk Extension | 70 | 0.645, 1.000 |
B. Upper Extremity Controller
“To approximate UE loading that a standing neuroprosthesis user may need to exert on an assistive support to resist postural perturbations, three-dimensional stabilization forces were applied to each shoulder position. PID controller output defined the shoulder force (SF) in each dimension ‘j’ (anterior-posterior, medial-lateral, or inferior-superior defined in globally fixed reference frame) according to input shoulder position errors (SE) relative to the reference positions at the setpoint posture as follows:
| (1) |
UE controller output acted on shoulder position since the current model does not explicitly include dynamic representations of the arms, which would still otherwise produce reaction loads at the shoulders. The three PID gains (Kp, Ki and Kd) were determined according to Ziegler-Nichols 2nd method tuning rules [25] against a 100 N, 200 msec forward test pulse at the thorax COM. The same PID gains were used for all three dimensions since only a single Ziegler-Nichols ultimate gain was observed for the single test perturbation. This test pulse induced a model trunk acceleration of ~2.5 m/sec2 which is less than that induced by “middle level” perturbations [26]. To approximate typical human operator response, 100 msec pure time delays [27] and muscle force activation delays [18] were applied to the shoulder force outputs. To simulate one-arm support conditions, as required to functionally reach on the contralateral side, only support side shoulder position controller forces were active.”1 The PID gains were reported and discussed in [9] and produce support loads typically observed in FNS standing systems [28].
C. Determining Optimal and Maximal Sets of Constant Excitation Levels for Baseline Performance
“To provide a comparative standard for controller performance across a range of sufficient but constant excitation levels for stable standing, the “optimal” and “maximal” muscle excitations (Table I) were determined for the desired setpoint posture using the optimizer from [29]. The “optimal” excitation levels represent the minimum constant excitation levels sufficient to support stable standing, while the “maximal” excitation levels represent the largest constant excitation levels supporting the same posture. The “optimal” hip (36.2N-m) and knee (11.5N-m) extension moment constraints were selected as those minimally necessary to support stable erect standing in energy efficient postures without joint contractures as reported in [30]. Joint moment constraints at the trunk (20.2N-m, E) and ankles (2.9N-m, PF) were subsequently selected such that the static UE loading was zero when the model shoulder positions were at the setpoint position. For comparison to clinically relevant systems applying supramaximal stimulation, the “maximal” set of constant excitations were specified as all muscles fully excited (excitation = 1.0) except the ankle plantarflexors, which were adjusted to 0.262 as part of the requirement to minimize static UE loading at the setpoint. The “maximal” set drove the knees, hips, and trunk slightly (< 5deg) into hyper-extension, i.e., past setpoint position defining full extension. Clinically, this is desired and commonly observed.”2
D. Procedure for Creating Optimal Muscle Activation Data According to COM Acceleration Targets
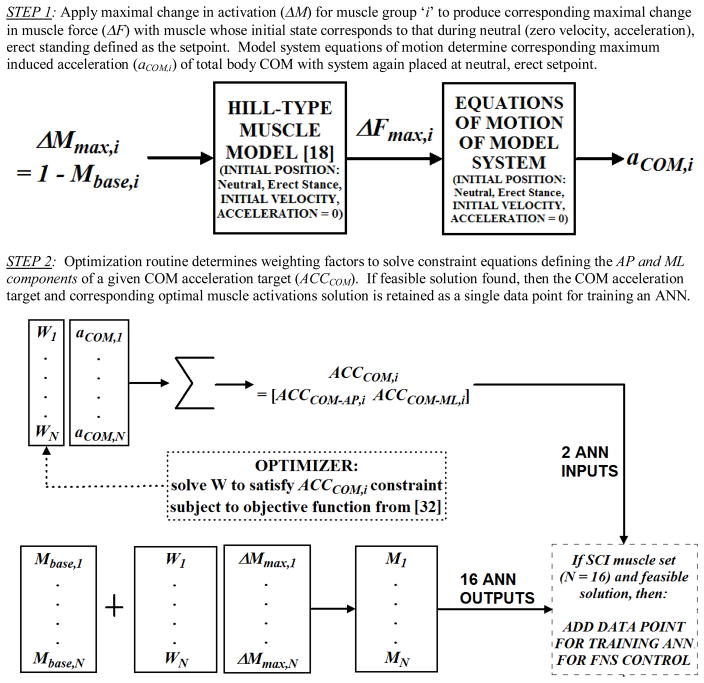
A model-based procedure was employed to generate the data used to train the ANN in the FNS control system. The procedure determined optimal muscle activation patterns in accordance with specified COM acceleration targets, expressed in Cartesian coordinates with respect to a globally fixed reference frame. The procedure was performed twice, each with a different muscle set. The first was restricted to the 16 muscle groups with SCI-adjusted force properties targeted for activation by an implanted neuroprosthesis. Data from this set were used to train the ANN and develop the FNS controller acting to resist postural disturbances. The second muscle set represented able-bodied (ABL) function and included all 58 muscle groups available across the trunk and lower extremities without SCI force adjustment. Results for this set were used to validate the procedure against EMG data collected for able-bodied subjects undergoing standing disturbances (section II.E). The procedure is depicted in Figure 2 and is outlined as follows:
Figure 2.
Two-step procedure for creating optimal muscle activation data in accordance with COM acceleration targets for training the ANN of the FNS controller.
-
STEP 1: Using the model system equations of motion (SD/FAST®, Symbolic Dynamics, Mountain View, CA), the maximal COM acceleration (aCOM) induced due to the maximal change in muscle activation was determined for each muscle group (i) with the initial position state at the setpoint position and zero initial velocity and acceleration for all muscle and skeletal states. The maximal change in muscle activation (ΔMmax) is the full activation level (normalized to equal 1) minus the baseline muscle activation level (Mbase) used for steady-state standing maintenance. In the SCI construction, the baseline activation levels were set equal to the “optimal” constant excitation set described in section II.C. For the able-bodied case, it was assumed that baseline activation of all 58 muscles was zero since during quiet standing, EMG activity was observed to be negligibly low [14]. It was also assumed that tibialis anterior does not produce significant accelerations upon the system since its isolated FNS activity produces net ankle DF, resulting in simple lifting of the anterior foot (i.e, “toe-off”) at neutral stance. Consequently, tibialis anterior was removed from the analysis for the SCI case. Ankle PF activity was restricted to soleus force output with other plantar-flexors (medial, lateral gastrocnemius) omitted despite being potentially accessible to FNS with a single stimulation channel at the triceps surae.
All three muscle heads of the triceps surae could be included, however, in this case, it was noted from pilot experimentation that optimal excitation levels to the ankle PF group were notably smaller compared to other muscle groups. This directly resulted from a lack of targeted musculature that could produce strong anterior shifts in COM acceleration. This includes certain hip and trunk flexors that pitch the body forward not being stimulated and ankle DF expectedly yielding toe-off from erect stance. In turn, the relatively strong posterior COM accelerations that can be induced from erect stance by the entire triceps surae muscle group were not as effectively balanced. Including only soleus essentially de-sensitized the optimal ankle PF actions to stimulus input and it was observed that this resulted in better overall standing performance (i.e., reduced UE loading).
STEP 2: Using the aCOM values for ΔMmax of individual muscle groups, an optimization was formulated to determine the optimal muscle activation solutions to produce a given COM acceleration target from the setpoint stance. Given proportionality between changes in muscle activation forces and the corresponding accelerations induced upon the system, the linear constraint equations to be satisfied by the optimizer yielded the desired net system COM acceleration (ACCCOM) targets as follows:
| (2) |
| (3) |
The net COM acceleration is defined here by only two components in the anterior-posterior (AP) and medial-lateral (ML) directions. The third dimension of COM acceleration (in the inferior-superior direction) was assumed to be small enough to be omitted provided the system does not collapse given sufficient baseline stimulation to produce basic constraints (e.g., knees do not buckle) typical for standing [31]. Each component target represents an optimization constraint that is equal to the weighted sum of the respective aCOM that can be induced by an individual muscle group from the baseline level. The weighting factor (Wi) is the normalized (0 to 1) change in activation from baseline for each muscle group. Only positive (i.e., increase) changes in activation from baseline levels were explored since it was assumed the baseline levels are fundamentally necessary to maintain basic standing with FNS. This assumption was necessary since this FNS control system is designed to operate about erect stance. With only COM acceleration feedback to modulate stimulation levels, some measure of FNS activation is necessary to maintain the erect setpoint when no significant accelerations (e.g., quiet standing) are present. Furthermore, without position feedback to produce alternate combinations of activation that can still preserve the basic, erect standing configuration, only increases from baseline activations are considered since decreases may overtly compromise the erect configuration about which these COM acceleration targets are being defined.
Using the Optimization Toolbox in MATLAB® (Mathworks, Natick, MA), the solution vectors (W) were determined within the maximum feasible space of COM acceleration targets. The maximum feasible target for each direction was simply the sum of the absolute values of the respective direction of aCOM (listed in Table II), multiplied by two (given the symmetry of the left and right side muscle groups). These maximum feasible values were 2.10, 0.48, and 1.74m/sec2 in the posterior, anterior and lateral directions, respectively. In creating a solution space encompassing these limits, COM acceleration targets were specified between +/−1.8 m/sec2 in the ML direction and 2.2 and 0.5m/sec2 in the AP direction at increments of 0.1m/sec2, yielding a total of 1036 targets. The solution vectors (W) were optimized according to minimization of an objective criterion developed for locomotion [32]:
| (4) |
TABLE II.
MAXIMUM ACCELERATION OF TOTAL BODY CENTER OF MASS (COM) INDUCED FROM QUIET ERECT STANCE FOR EACH MUSCLE GROUP TARGETED FOR STIMULATION
| Muscle Group (Right Side) | SCI aCOM-AP (m/sec2) | SCI aCOM-ML (m/sec2) | ABL aCOM-AP (m/sec2) | ABL aCOM-ML (m/sec2) |
|---|---|---|---|---|
| Soleus | −0.559 | 0.011 | −1.587 | 0.030 |
| Tibialis Anterior | N/A | N/A | 1.699 | −0.398 |
| Vasti | −0.064 | 0.004 | −2.661 | 0.016 |
| Adductor Magnus | −0.034 | 0.307 | −0.265 | 3.494 |
| Gluteus Maximus | 0.000 | 0.000 | −2.077 | −0.640 |
| Gluteus Medius | −0.394 | −1.333 | −0.703 | −2.378 |
| Semimembranosus | 0.228 | 0.078 | 0.726 | 0.247 |
| Erector Spinae | 0.009 | −0.006 | 0.048 | −0.032 |
Note: SCI = Spinal Cord Injury muscle set, ABL = Able-bodied muscle set. Positive values are either in the Anterior (Front) or Right Directions.
Optimization parameters included a maximum of 10,000 iterations, constraint equation tolerance of 0.01m/sec2 and function tolerance of 0.001 N2/m4. If the optimizer produced a solution that met the tolerance for both constraint equations for a given ACCCOM, then that COM acceleration target solution was classified as “feasible”. Only feasible solution points were retained for subsequent EMG analysis or ANN training. The two components of ACCCOM served as the INPUTS and the corresponding 16 absolute muscle activation solutions (Mi’s) served as the OUTPUTS for ANN training.
E. Collection of EMG Data of Able-bodied Individuals during Perturbed Bipedal Standing
To validate the general procedure used to create the muscle activation synergy from II.D The direction of the resultant COM acceleration for which activity was highest for different muscle groups was calculated for the model-based synergy and compared to a similar metric based on the EMG data collected from three able-bodied volunteers undergoing systematic external perturbations while standing. All able-bodied subjects signed informed consent forms approved by the Institutional Review Board of the Louis Stokes Cleveland Department of Veterans Affairs Medical Center. None showed nor reported a history of orthopedic or vestibular problems. Perturbations were applied using software developed in LabVIEW® (National Instruments, Austin, TX) to control electromagnetic linear actuators (STA2506, Copley Control, Canton, MA) mounted on customized framing (80/20 Inc., Columbia City, IN) rigidly fixed to either floor or wall surfaces. Subjects stood with arms crossed and wore a weight belt, approximately at COM level, and were positioned perpendicularly to four actuator complexes placed in front, back, right, and left of the subject. Four ropes were tied off on one end onto the belt with each rope connected and directly aligned with the piston of an actuator on the other end. A customized aluminum plate with attached rope cleat was used to quickly fasten, adjust for length, and release the rope from the actuator. All programmed disturbances were discrete force pulses, 250 msec in duration. The force pulse amplitude threshold that elicited stepping for each subject in each of the four directions was determined by trial and error. Perturbations were limited to 80% the stepping threshold. COM acceleration under these conditions was not a controlled variable, but was assumed to be close to the maximal values possible during stable bipedal standing and could be interpreted as proportionally equal in each direction. Thirty perturbations were applied upon each subject in each direction.
EMG signals were recorded bilaterally from muscles approximately coincident with those targeted for stimulation in neuroprosthesis recipients: tibialis anterior, soleus, vasti, semimembranosus, gluteus maximus, gluteus medius, adductor magnus, and lower erector spinae. EMG data were collected using disposable, self-adhesive surface electrodes placed according to SENIAM standards (www.seniam.org). Data were acquired with a Telemyo® 900 (Noraxon, Scottsdale, AZ) at a sampling frequency of 1500Hz. EMG signals were rectified and band-limited by a 50 Hz 4th-order low-pass Butterworth filter offline as specified in [15]. The mean amplitude (amp) of the processed EMG during the perturbation period across all trials was determined for each muscle (j) in each direction (k). For each muscle, the EMG activation vector ( ), representing which net direction it is most active, was calculated as follows:
| (5) |
Dk is a unit-direction vector in the opposite direction of the perturbation and is represented in XY-Cartesian coordinates where +/− X correspond to front/back and +/− Y corresponds to right/left. The opposite direction of the pull was used since it was assumed that muscle activity initially increases to resist COM acceleration effects produced by the disturbances. Final activation vectors are unity-normalized for graphical display since only net directional information is used for comparison between model and EMG results.
F. Comparing Able-Bodied EMG Data against Optimal Model-Based Data
Using standard conversion of Cartesian to polar coordinates, the angular coordinate (θEMG) of for each muscle was calculated to specify the primary direction of activation for each muscle group opposing the systematic perturbations during able-bodied bipedal standing. The polar angular coordinate (θSYN) serves as the primary direction of activation for each muscle group according to the model-based synergy and was determined from the following activation vector quantity:
| (6) |
ACCCOM are the COM acceleration targets and Xj is the corresponding muscle excitation solution from section II.D. Correspondence between the angular coordinates for the EMG and model-based vectors is observed for each muscle group.
G. Creating Artificial Neural Network for FNS Control
AP and ML components of each feasible COM acceleration target resulting from the simulations were the INPUTS and the corresponding 16 optimal muscle excitation levels were the OUTPUTS for a single ANN data point. Feasible data points were randomly assigned for training (70%), testing (20%), and validation (10%) of the ANN. The ANN was constructed with the Neural Network Toolbox in MATLAB (Mathworks®, Natick, MA). A three layer (input, hidden, output layers), feedforward ANN structure was employed for its universal mapping capability of nonlinear functions [33]. The number of hidden layer neurons was determined to be 18 by heuristically finding the number of neurons providing the lowest MSE after 1000 training epochs. All input and output data were normalized over [−1, +1] prior to training. The training function was the Levenberg-Marquardt algorithm [34]. A maximum of 10000 epochs were specified for training in lieu of an early stopping criterion specified as 250 consecutive epochs of increasing fitting error to the validation set. The ANN output sensitivity was calculated as the slope for ANN output excitation in each acceleration direction for each muscle group at neutral stance with zero acceleration input.
H. Perturbation Simulations
“In all, 978 perturbation simulations were conducted to optimally tune and evaluate the controller with respect to total UE loading. Total UE loading was the sum of the “net” force applied at the left and right shoulders. For each simulation, the computer model started at the desired erect setpoint, and UE loading was tracked during the perturbation and following recovery period (750msec). This recovery period was sufficient to sustain effective stabilization, defined as UE loading within 1% body-weight (BW) of its final steady-state value, across all simulations. Each perturbation simulation included a single pulse force disturbance applied at a single location. The location, direction, magnitude, and duration of the perturbation were varied with each simulation. Perturbations were applied at the COM locations of the thorax, pelvis, femur, or shank segment in the forward, backward, left, or right directions relative to a globally fixed Cartesian reference frame. These force disturbances ranged from 5% to 15% BW in magnitude and 50 to 500 msec in duration. Perturbations were also repeated at the system COM, also expressed in global three-dimensional coordinates.”3
I. Tuning COM Acceleration Feedback Controller
For dynamic controller action, each of the two acceleration inputs (‘i’) to the ANN was multiplied by its respective proportional gain (KP,i) as follows:
| (7) |
Gains were optimized to minimize the objective function criterion of the total two-arm UE loading necessary for stabilization during perturbation and recovery over all 978 simulations. The gains for both acceleration inputs were optimally tuned using an asynchronous parallel pattern set global search algorithm implemented in the APPSPACK [35] software package running on a FUSION A8 multi-processor computer (Western Scientific, Inc., San Diego CA). Algorithm parameters were determined such that solutions were found within 100 hours of computational time. These parameters include initial step size equal to 1, step tolerance equal to 0.01, and step contraction factor equal to 0.985. The gains were bounded between 0 and −10. The negative value indicates negative feedback whereby the control system acted to produce effects that counter the COM acceleration observed during perturbation and recovery. The initial gain values were based on manual tuning. This process involved stepwise increments of each feedback gain to minimize UE loading while holding the other feedback gain to zero. The test perturbation for manual tuning was a 100N, 200msec force pulse at the thorax.
J. Testing Controller Performance
External Force Pulse Perturbations
All 978 perturbation simulations were repeated with the feedback controller active and with constant baseline (optimal or maximal) excitation levels under two-arm and one-arm support conditions. Control systems were optimally tuned according to two-arm support but tested under both support conditions to observe general controller performance capabilities including disturbance rejection while potentially keeping one arm free for object manipulation. The fundamental synergy defined by the optimal acceleration-excitation data remains unchanged regardless of support condition. Even the net synergy after determination of optimally-tuned feedback gains remains symmetric since only one feedback gain is present for each test dimension. Furthermore, it is expected that with tuning of a similar FNS control system deployed under live conditions, the SCI subject would initially resist external perturbations under two-arm support prior to testing with one-arm support while performing functional tasks. Therefore, it was reasonable to tune under two-arm support conditions and test for both two-arm and one-arm support in simulation. The level of significance of any reduction in UE loading with the controller active compared to baseline was determined across perturbation direction, location, and magnitude by multiple analysis of variance (MANOVA).
“Functional Task Performance (FTP)
Functional implications of the controller were assessed in simulation with application of sinusoidal force loads at one shoulder to mimic postural disturbances due to weighted, voluntary single arm movements. Three-dimensional, sinusoidal force loading was applied at the left shoulder while UE control was applied only at the right shoulder (i.e., one-arm support). The applied sinusoid forces were as follows: Anterior/Posterior: 1 Hz, 10 N amplitude, 0 N offset; Right/Left: 1 Hz, 20 N amplitude, 0 N offset; Superior/Inferior: 0.5 Hz, 20 N amplitude, −50 N offset. These amplitude and frequency specifications were consistent with those observed in loaded (2.27kg) single arm voluntary movements described in [36].”4
III. RESULTS
A. Induced COM Acceleration Results
The maximum COM acceleration induced from neutral stance by each SCI muscle group targeted for stimulation is listed in Table II. The soleus and gluteus medius produced the largest induced COM accelerations in the posterior direction. This is explained by basic anatomical constraints of the ankles and hips being located below the COM whereby ankle plantar-flexion and hip extension in the sagittal plane would drive the system backwards. Gluteus medius and adductor magnus induced the largest COM accelerations in the medial-lateral dimension. This is also anatomically consistent given their primary articulations of hip abduction and adduction, whose effects were largest in the coronal plane. Gluteus maximus and tibialis anterior produced no changes in COM acceleration since gluteus maximus was already maximally activated at its initial level and tibialis anterior was assumed to produce toe-off at erect stance. Only semimembranosus and erector spinae induced COM accelerations in the anterior direction. While these muscles extend the hip and trunk, respectively, they both generate forward motion of the pelvis and lower torso. Anatomical constrains explain this with semimembranosus producing knee flexion in conjunction with hip extension while the erector spinae spans the entire mid-to-lower torso. Prolonged activity of these extensor muscles may drive the system posteriorly, but their instantaneous effects from quiet, neutral standing is to shift the COM anteriorly. Vasti and erector spinae effects were small relative to other muscles. The vasti were nearly maximally activated at baseline and erector spinae did not produce a very significant instantaneous acceleration at neutral standing.
B. EMG Validation of COM Acceleration Mapping
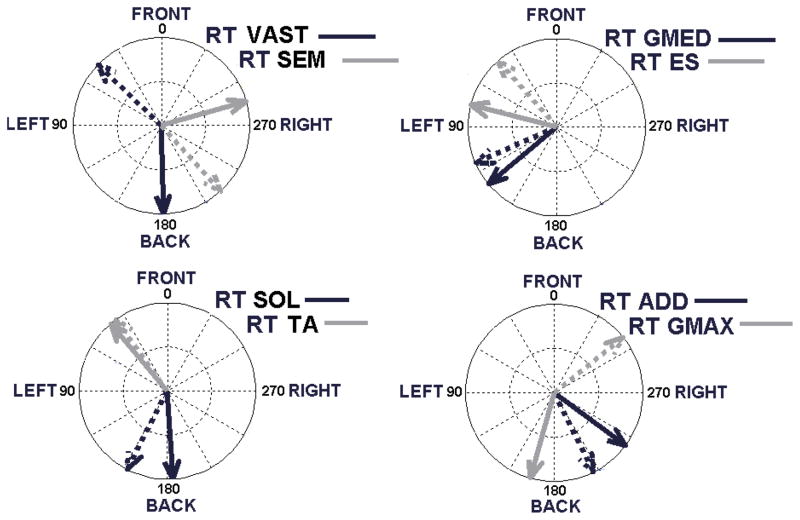
The primary activation directions for each right side muscle group from both the able-bodied EMG data set and the able-bodied model synergy are shown in Figure 3. The methodology for EMG collection and analysis was robust as the standard deviation (SD) about the mean θEMG for all muscle groups was < 20°, indicating that ±2 SD are encompassed within a single quadrant of the 2-dimensional direction space. The model polar angular coordinate θSYN was within the quadrant centered about θEMG for all muscle groups except vasti, semimembranosus, and gluteus maximus. These exceptions in θEMG can be attributed to the positional stabilization required to prevent collapse during perturbations applied at the lower torso in the live subject experiments. Specifically, the increases in EMG activity for semimembranosus and vasti were likely necessary to prevent hip and knee flexion induced by forward and backward disturbances, respectively. Higher gluteus maximus EMG against backward disturbances may be explained by a co-contraction response in conjunction with antagonist muscles to generally stiffen the hips. For the other 5 muscle groups, good correspondence in activation directions indicate that their first-response contributions to stabilize standing can be described in accordance to the initial COM acceleration direction induced by a perturbation from quiet bipedal standing.
Figure 3.
Compass diagrams displaying theoretical “net activation directions” for various muscle groups. Net activation direction suggests muscle group is most active when system COM is being accelerated in that direction as a result of all muscles activating to directly counter (i.e., oppose) disturbance from neutral standing. Activation direction (θ) results displayed for respective able-bodied model (solid arrow) and EMG (broken arrow) data for the following right-side (RT) muscle groups: SOL = Soleus (θ = 183°, 153°±13°), TA = Tibialis Anterior (θ = 40°, 35°±11°), VAST = Vastus Intermedius (θ = 181°, 46°±13°), SEM = Semimembranosus (θ = 286°, 222°±12°), ADD = Adductor Magnus (θ = 234°, 207°±13°), GMAX = Gluteus Maximus (θ = 164°, 319°±17°), GMED = Gluteus Medius (θ = 130°,114°±19°), ES = Erector Spinae (θ = 75°, 41°±12°).
C. Artificial Neural Network Results
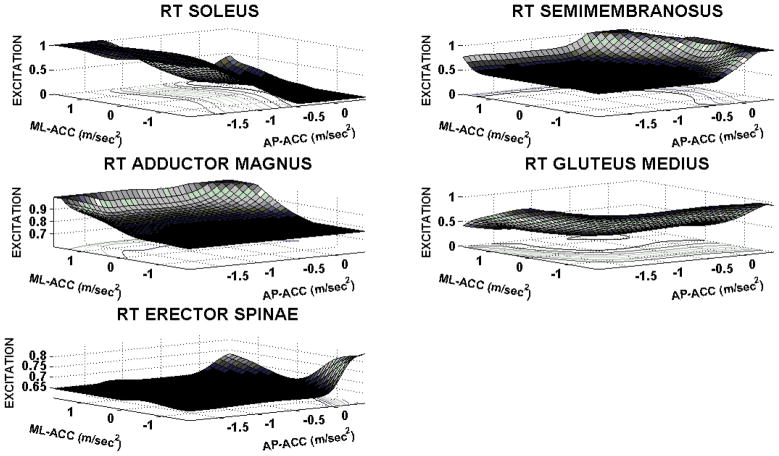
The artificial neural network was capable of accurately outputting the synergistic muscle excitation patterns optimized according to COM acceleration input targets. The mean errors (Table III) in outputs by the ANN for all feasible COM acceleration target inputs were less than 0.001. This demonstrates that the ANN was an effective structure to represent the synergy that is to be driven by feedback control in forward simulations. The ANN excitation surface outputs for 5 right side muscle groups are shown in Figure 4. Note that tibialis anterior was omitted due to potential toe-off, and gluteus maximus and vasti were nearly maximally activated simply to meet the specified optimal baseline requirements for standing. The output surfaces indicate that the soleus is prominent in accelerating the system COM backward across the entire feasible target space. The right semimembranosus and has observable increased activity in accelerating the COM forwards. The muscles groups most active in driving the system COM in the right and left directions were the right adductor magnus and right gluteus medius, respectively. This follows anatomical intuition with the ANN providing smooth output of the optimization space used for ANN training. Sensitivity results in Table III suggest all the targeted muscle groups in Figure 4, except erector spinae, would be recruited immediately in disturbance rejection as they undergo notable recruitment to even small (near zero-acceleration point) changes in acceleration. Erector spinae would be additionally recruited only with sufficiently increased AP and ML acceleration.
TABLE III.
ARTIFICIAL NEURAL NETWORK (ANN) EXCITATION OUTPUT RESULTS: SENSITIVITY AT ZERO ACCELERATION AND MEAN ANN PREDICTION ERROR
| Muscle Group (Right) |
|
|
Mean Error in ANN output (excitation) | ||
|---|---|---|---|---|---|
| Soleus | −0.415 | 0.000 | 1.57e-4 | ||
| Tibialis Anterior | N/A | N/A | N/A | ||
| Vasti | 0.000 | 0.000 | < 1e-5 | ||
| Adductor Magnus | −0.006 | 0.151 | 2.47e-4 | ||
| Gluteus Maximus | N/A | N/A | N/A | ||
| Gluteus Medius | −0.047 | −0.330 | 1.84e-4 | ||
| Semimembranosus | 1.095 | 0.172 | 3.94e-4 | ||
| Erector Spinae | 0.000 | 0.000 | 5.27e-5 |
N/A for muscle groups either not targeted for feedback control or always fully activated.
Figure 4.
ANN excitation output of select right (RT)-side muscles as a function of COM-acceleration inputs. Note: AP = Anterior-Posterior, ML = Medial-Lateral. Positive axes = Anterior, Right
D. Controller Gain Tuning
The final feedback gains that minimized total UE loading during external perturbations were −5.17e-2 and −0.99 for the AP and ML COM acceleration component inputs, respectively. The higher allowable feedback gain for the ML component can be explained by greater inherent stability of bipedal standing in that direction. The base of support is wider in the ML direction and the standing system approximates a 4-bar linkage [37] as compared to a more unstable inverted pendulum in the AP direction [38].
E. Controller Performance
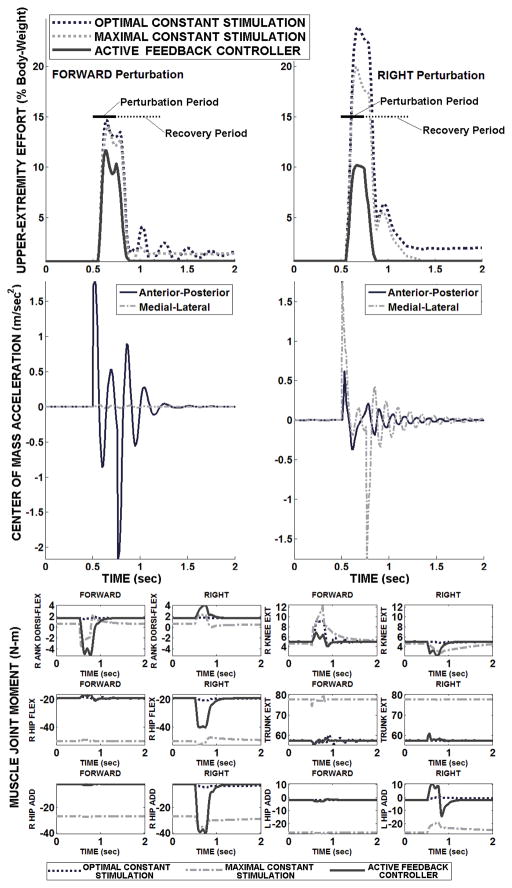
Typical two-arm UE loading and muscle induced joint moments for baseline and controller-active conditions are shown in Figure 5. In response to 15% BW, 250 msec force pulses applied at the model COM in the AP and lateral directions, the controller reduced total UE loading during the perturbation and recovery period by 43% and 66%, respectively. The controller provided robust return to the setpoint posture with near zero final UE loading. The joint moments produced by the controller during steady-state before and after the perturbation were lower than maximal baseline as expected and identical to optimal baseline as designed. The robust return to optimal baseline performance indicates that acceleration feedback control was transient as the controller did not produce instabilities requiring excessive UE loading. The peak UE loading produced with controller action was below that with maximum baseline stimulation in both perturbation directions.
Figure 5.
Two-arm UE loading, COM acceleration feedback, and muscle-induced joint moments to stabilize against perturbation pulse (15% body-weight, 250 msec) applied at model COM in either forward or side (i.e., right) direction.
Consistent with anatomical function, ankle plantar-flexion and hip extension were prominent in resisting a forward disturbance. Correspondingly, right hip abduction and left hip adduction were strongly activated to reject the rightward disturbance. Trunk extension was small even against a forward disturbance since it was applied at the system COM, which is too low to produce significant trunk flexion. Knee extension moments were small in all cases despite high vasti excitation because the knees were generally held in hyper-extension where length-tension properties limited force output. The largest (>5N-m) controller-mediated changes in joint moments occurred at the ankles and hips, reflecting the well described ankle and hip strategies [14] for stable standing.
Smooth UE loading and joint moment profiles were observed with controller feedback despite some oscillations (Figure 5, middle panels) in the COM acceleration feedback signal, indicating that the mass-inertia of the system and delays in muscle force actuation were able to sufficiently dampen those effects. For a sideward perturbation, ML acceleration was more prominent as expected, but the AP acceleration component was still notable. This further underscores how sensitive the SCI standing system is to destabilizing effects in the AP direction compared to the ML direction.
Composite simulation results for one-arm and two-arm resistance to perturbations are shown in Table IV. Maximal constant excitation always resulted in lower UE loading than optimal, but the acceleration feedback controller improved performance over either baseline case in all listed condition cases except backwards perturbations. This results from only semimembranosus being available to induce forward COM accelerations from neutral stance. UE loading also increased as perturbations were applied to more superiorly located segments. Perturbations applied to lower segments were more attenuated by muscle and inertial effects before greater UE stabilization was required. UE loading is significantly greater (i.e., standing is more unstable) during one-arm support under optimal or maximal baseline stimulation. Optimal baseline stimulation was further ineffective in one-arm support as the model COM occasionally failed to return to within 0.1m of its original position. With the ANN controller active, similar UE loading was expended in resisting perturbations with either one-arm (21N) or two-arm (20N) support, demonstrating the consistency and value of feedback control. The mean reduction in UE loading with the controller active compared to maximal baseline across all force pulse perturbations over both one-arm and two-arm conditions was 43%. The controller produced a statistically significant reduction in UE loading with rejection of the null hypothesis of equal means at p = 0.05 across all perturbation variables (direction, location, amplitude) as compared to baseline. During one-arm FTP, the controller kept the model erect and reduced UE loading by 51% compared to maximal baseline excitation.
TABLE IV.
UPPER EXTREMITY (UE) LOADING FOR STABILIZATION AGAINST POSTURAL DISTURBANCES
| Disturbance Condition | Mean Baseline | Mean Controller | % Reduction | |
|---|---|---|---|---|
| UE Loading (N) | UE Loading (N) | w/Controller | ||
| Direction | Optimal | Maximal | ||
| Forward | 66 | 47 | 27 | 44 |
| Backward | 32 | 22 | 25 | −14 |
| Side (Left or Right) | 51 | 36 | 14 | 61 |
| Segment Location | ||||
| Thorax | 61 | 48 | 34 | 27 |
| Pelvis | 58 | 41 | 20 | 49 |
| Thigh (Left or Right) | 52 | 35 | 19 | 44 |
| Shank (Left or Right) | 39 | 22 | 8 | 63 |
| Support Conditions | ||||
| Two-Arm Support | 32 | 28 | 20 | 29 |
| One-Arm Support | 73* | 44 | 21 | 52 |
| One-Arm FTP | 147* | 35 | 17 | 51 |
Results include simulations where model COM is unable to return within 0.1m of starting position following application of disturbance loading
IV. Discussion
To address inherent drawbacks to joint feedback, we proposed and evaluated the feasibility of COM acceleration feedback for control of FNS standing. Using a three-dimensional model of bipedal SCI standing, we developed a control system using COM acceleration feedback to modulate muscle excitation levels and reduce the upper extremity loading required to stabilize against postural disturbances. Use of COM acceleration as a feedback signal follows directly from previous studies that have implicated acceleration [10, 11, 12] and COM dynamics [13] in standing balance control. In this study, we demonstrated that COM acceleration is a potentially valuable feedback parameter for characterizing standing control specifically against perturbations.
We outlined a methodology to produce an optimal synergy that relates changes in muscle activation from neutral standing to changes in COM acceleration using our anatomically realistic model. The resultant synergy was validated by comparing which net direction certain muscle groups were most active to accelerate the system COM in opposing a disturbance for an able-bodied model synergy against EMG measurements recorded from the same muscle groups during systematic perturbation of able-bodied standing subjects. Five muscle groups (tibialis anterior, soleus, gluteus medius, adductor magnus, and lower erector spinae.) demonstrated high correspondence between the model-constructed synergy and live EMG data. This indicates that these muscle groups should be consistently targeted for FNS control under COM acceleration feedback to stabilize against disturbances.
However, the remaining three muscle groups (gluteus maximus, vasti, and semimembranosus) did not correspond well. It was postulated that activity of these muscle groups were modulated according to positional requirements for maintaining erect stance that are not considered in the construction of the activation-acceleration map. Thus, it may be best to reserve these muscle groups for position-based feedback or simply constant activation for basic standing support. In fact, these same muscle groups had relatively high baseline activation levels determined as optimal for sufficiently stable SCI standing (Table I) and have also been commonly targeted for stimulation to provide basic standing support during clinical application [1]. For FNS control of standing that utilizes feedback of only COM acceleration, position-based corrections would need to be made volitionally by the user, but assisted dynamically by modulation of stimulation levels for the muscles not reserved for basic standing support such that user effort was minimized.
For forward simulations of FNS feedback control, the same activation-acceleration mapping procedure was employed to construct another model-based synergy using only muscle groups targeted by a 16-channel implant [23] and reflecting typical FNS force generating capabilities following SCI [24]. In simulation, this SCI-specific synergy was represented by an artificial neural network driven by proportional COM acceleration feedback, which was more than capable of effectively mapping this synergy between only two COM acceleration inputs and 16 muscle excitation outputs. With a prediction error <1e-3 for all outputs, the ANN was successfully driven by proportional feedback to modulate excitation levels for reducing upper extremity loading required to resist disturbances compared to the typical clinical case of maximal constant muscle excitation. COM acceleration feedback control markedly reduced upper extremity loading across all external disturbances for both two-arm and one-arm support conditions by 43% and during functional task performance by 51%. Disturbance rejection during one-arm support and functional task performance are conditions sets more pertinent to standing activities of daily living. Thus, future investigations may include optimizing the control system with one-arm support. However, results from this study demonstrate similar total UE loading with the controller active regardless of support condition, suggesting the robustness of controller action despite the nature of support.
This demonstrates the potential of COM acceleration feedback to provide a notable improvement in neuroprosthetic standing performance despite the limited number of paralyzed muscles available. The same muscles were required to both support the body against collapse and generate the additional moments required to reject perturbations. While basic upright support was achieved with optimal baseline stimulation levels generating necessary joint moments as reported in [30], gluteus maximus and vasti were nearly maximally activated to produce the necessary baseline hip and knee extension moments and could not be recruited to resist disturbances. Tibialis anterior was also omitted from the study. Yet, recruitment of the remaining 10 muscles was still sufficient to produce an effective balance control system in simulation, further highlighting the potential benefits of COM acceleration feedback.
The formulation of the controller presented in this study is based on proportional feedback driving an ANN that imposed a synergy to generate optimal changes in muscle activation to produce desired changes in COM acceleration and counter effects of postural disturbance about neutral, erect stance. Negative feedback was employed to recruit the muscles required to oppose the COM accelerations encountered during perturbation and recovery. We validated this construction by observing reductions in upper-body loading with simulated one-arm and two-arm support during a wide range of disturbance locations, amplitudes and directions, as well as during simulated functional tasks with our SCI-adjusted model. Furthermore, our able-bodied EMG data corroborated this approach and coincided with simulation results indicating that muscle groups are largely activated to counter the disturbances reflected in COM acceleration direction. Model-predicted COM accelerations were consistently observed to be in the opposite direction of the net action of the most active muscles during repeated disturbances. Notable exceptions (vasti and gluteus maximus) can be attributed primarily to muscle recruitment for other objectives, such as the necessary positional corrections to prevent frank system failure and outright falls. This underlies the notion that comprehensive standing is a complex, multi-sensory task [39] that employs joint-based feedback. Theoretically, some form of position or joint-based control would be required to replicate the intact balance control apparatus and achieve truly hands-free standing with FNS. However, coordination of muscle activity according to COM acceleration still seems to characterize much of the initial standing response to applied disturbances. More importantly, this synergy may be exploited for substantially extending and improving the functionality of standing neuroprostheses.
This approach for constructing a muscle-based acceleration synergy for neuroprosthetic standing was inspired from Kuo et al., [31] who developed an algorithm to generate “feasible acceleration sets” (FAS) composed of joint angular accelerations for all feasible normalized muscle activations subject to observed experimental constraints (e.g., knees-locked, heel and toe lift-off) of sagittal plane standing. That study used the FAS to identify which muscles, if strengthened, would produce the greatest increases in standing mobility. Our study created feasible, optimal activation patterns that could generate targeted changes in linear COM acceleration, the proposed sensor-based feedback variable for control of SCI standing.
Although this study supports the use of COM acceleration as a potentially effective feedback variable controlling standing with FNS, additional work is still required to implement such a control system clinically. The clinical viability of acceleration-based feedback control depends on performance in the presence of typical sources of feedback error (e.g., sensor placement, measurement accuracy, soft tissue effects). The model system presented serves as an appropriate test-bed for future study to systematically introduce feedback error and quantify its effects on performance. Most importantly, efficient techniques need to be developed to fit, tune and specify system parameters for a particular user in a clinical setting. Methods have been previously outlined for determining user-specific musculoskeletal and UE controller parameters to develop a model-based system for initial controller tuning and evaluation prior to laboratory implementation [9]. While this study employed a generic bipedal model of standing to conceptually validate the proposed control system producing a potentially substantial improvement in standing performance, creation of user-specific models would be necessary to develop model-based solutions for control systems for specific users. This would include scaling the muscle geometry, muscle force-generating capabilities, and length and mass-inertia properties of segments. Only an accurate description of those features would generate an optimal control solution that produces kinematic and kinetic responses in simulation that appropriately represent those expected to be observed during live laboratory performance.
The methods from [9] for creating user-specific control systems were suggested for a joint feedback system composed of proportional and derivative inputs from nine individual joints. Properly assessing performance to robustly tune a system with that many feedback gains during live conditions may be intractable. Both model and laboratory development of FNS control systems can be expedited with control structures containing fewer feedback variables. The control system examined in this study employs only two feedback variables (AP and ML COM acceleration) that would need to be measured and two feedback gains that would need to be tuned.
While a user-specific model-based solution could still be explored, a paradigm could also be devised to produce the data (equations 2, 3) used to construct the optimal synergy entirely from a live user. The paralyzed subject could be at neutral, erect stance in a walker while sufficient but minimal constant stimulation was applied as the “baseline activation”. Stimulation could then be discretely ramped from baseline levels for individual muscles (Wi) and the corresponding peak induced COM acceleration (aCOM,i) could be recorded. This peak would serve only as an estimate of the aCOM,i induced by a particular muscle group since it would occur across the excitation-activation coupling dynamics and small postural changes away from the neutral setpoint position may occur. However, the extent the assumption of linear superposition governing equations 2 and 3 breaks down should be investigated experimentally. Given that significant changes in acceleration can occur without large changes in configuration, it is realistic for the suggested methodology to produce a viable solution for neuroprosthetic standing. Finally, an instrumented walker employing load-sensitive handles could be used to tune the system online against disturbances applied by the perturbation system described in II.E, as well as provide a metric of goodness of fit for a clinical system.
V. Conclusions
Center of mass acceleration may be an advantageous alternative to joint kinematics as a feedback variable for control of FNS standing. This study suggests that even with control of only 16 SCI-paralyzed muscles available to provide basic standing support, significant improvement in disturbance rejection can still be achieved with COM acceleration feedback modulation of muscle excitation levels. Further study to demonstrate potential clinical viability is necessary such as evaluating performance robustness in the presence of expected feedback measurement errors. Ultimately, control systems should be developed according to user-specific characteristics for laboratory testing with live SCI subjects.
Acknowledgments
The authors would like to acknowledge support from the National Institutes of Health (NIH; #R01 NS040547-04A2) and the Motion Study Laboratory at the Louis Stokes Cleveland Veterans Affairs Medical Center.
FUNDING: National Institutes of Health (NIH; #R01 NS040547-04A2)
ABBREVIATIONS
- Ab
Abduction
- Ad
Adduction
- ANN
Artificial Neural Network
- AP
Anterior-Posterior
- COM
Center of Mass
- DOF
Degree of Freedom
- DF
Dorsiflexion
- EMG
Electromyography
- Ev
Eversion
- Ext
External Rotation
- FNS
Functional Neuromuscular Stimulation
- Int
Internal Rotation
- Inv
Inversion
- ML
Medial-Lateral
- PD
Proportional-Derivative
- PF
Plantarflexion
- PID
Proportional-Integral-Derivative
- SCI
Spinal Cord Injury
- UE
Upper Extremity
Footnotes
Content from [9]
Content from [9]
Content from [9]
Content from [9]
AUTHOR STATEMENT:
This work was conducted at the Motion Study Laboratory at the Louis Stokes Cleveland Department of Veterans Affairs Medical Center (VAMC) in conjunction with the Cleveland Functional Electrical Stimulation (FES) Center and Case Western Reserve University. The Cleveland FES Center aims to develop neuroprostheses to restore function following spinal cord injury. Research at the VAMC strives to improve quality of life of veterans. There are no conflicts of interest, financial or otherwise, to report.
Contributor Information
Raviraj Nataraj, Email: rxn25@case.edu, Louis Stokes VAMC, 10701 East Boulevard, Room C-15 (Motion Study Lab), Cleveland, OH, 44106, Phone: (216) 791-3800 ext 3838.
Musa L. Audu, Email: mxa93@case.edu, Louis Stokes VAMC, 10701 East Boulevard, Room C-15 (Motion Study Lab), Cleveland, OH, 44106, Phone: (216) 791-3800 ext 3821.
Robert F. Kirsch, Email: rfk3@case.edu, 10900 Euclid Avenue, Biomedical Engineering, Cleveland, OH, 44106, Phone: (216) 368-3158.
Ronald J. Triolo, Email: rxt24@case.edu, Louis Stokes VAMC, 10701 East Boulevard, Room C-15 (Motion Study Lab), Cleveland, OH, 44106, Phone: (216) 791-3800 ext 4138.
References
- 1.Triolo RJ, Bogie K. Lower extremity applications of functional neuromuscular stimulation after spinal cord injury. Topics in SCI Rehab. 1999;5:44–65. [Google Scholar]
- 2.Kralj A, Bajd T, Turk R. Electrical stimulation providing functional use of paraplegic patient muscles. Med Prog Technol. 1980;7:3–19. [PubMed] [Google Scholar]
- 3.Jaeger RJ. Design and simulation of closed-loop electrical stimulation orthoses for restoration of quiet standing in paraplegia. J Biomech. 1986;19:825–35. doi: 10.1016/0021-9290(86)90133-8. [DOI] [PubMed] [Google Scholar]
- 4.Moynahan M, Chizeck HJ. Characterization of paraplegic disturbance response during FNS standing. IEEE Trans Rehabil Eng. 1993;1:43–48. [Google Scholar]
- 5.Chizeck HJ, Kobetic R, Marsolais EB, Abbas JJ, Donner IH, Simon E. Control of functional neuromuscular stimulation systems for standing and locomotion in paraplegics. Proc IEEE. 1988;76:1155–65. [Google Scholar]
- 6.Abbas JJ, Chizeck HJ. Feedback control of coronal plane hip angle in paraplegic subjects using functional neuromuscular stimulation. IEEE Trans Biomed Eng. 1991;38:687–98. doi: 10.1109/10.83570. [DOI] [PubMed] [Google Scholar]
- 7.Hunt KJ, Gollee H, Jaime RP, Donaldson NN. Control of paraplegic ankle joint stiffness using FES while standing. Med Eng Phys. 2001;23:541–55. doi: 10.1016/s1350-4533(01)00089-3. [DOI] [PubMed] [Google Scholar]
- 8.Matjacic Z, Bajd T. Arm-free paraplegic standing. II. Experimental results. IEEE Rehab Eng. 1998;6:139–150. doi: 10.1109/86.681179. [DOI] [PubMed] [Google Scholar]
- 9.Nataraj R, Audu ML, Kirsch RF, Triolo RJ. Comprehensive Joint-Feedback Control of Standing for Standing by Functional Neuromuscular Stimulation following Spinal. IEEE Trans Neur Rehab Syst. 2010;18:646–657. doi: 10.1109/TNSRE.2010.2083693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayagoitia RE, Lotters JC, Veltink PH, Hermens H. Standing balance evaluation using a triaxial accelerometer. Gait and Posture. 2002;16:55–59. doi: 10.1016/s0966-6362(01)00199-0. [DOI] [PubMed] [Google Scholar]
- 11.Moe-Nilssen R, Helbostad JL. Trunk accelerometery as a measure of balance control during quiet standing. Gait Posture. 2002;16(1):60–8. doi: 10.1016/s0966-6362(01)00200-4. [DOI] [PubMed] [Google Scholar]
- 12.Betker AL, Moussavi Z, Szturm T. Center of Mass Approximation and Prediction as a Function of Body Acceleration. IEEE Trans Biomed Eng. 2006;53(4):686–693. doi: 10.1109/TBME.2006.870222. [DOI] [PubMed] [Google Scholar]
- 13.Pai YC, Patton J. Center of mass velocity-position predictions for balance control. J Biomech. 1997;30(4):347–354. doi: 10.1016/s0021-9290(96)00165-0. [DOI] [PubMed] [Google Scholar]
- 14.Horak FB, Nashner LM, Diener HC. Central programming of postural movements: adaptation to altered support-surface configurations. J Neurophysiol. 1986;55:1369–81. doi: 10.1152/jn.1986.55.6.1369. [DOI] [PubMed] [Google Scholar]
- 15.Krishnamoorthy V, Goodman S, Zatsiorsky V, Latash ML. Muscle synergies during shifts of the center of pressure by standing persons: identification of muscle modes. Biol Cybern. 2003;89:152–161. doi: 10.1007/s00422-003-0419-5. [DOI] [PubMed] [Google Scholar]
- 16.Winter DA. Biomechanics and Motor Control of Human Movement. 2. Toronto: John Wiley & Sons, Inc; 1990. [Google Scholar]
- 17.Zajac FE, Gordon ME. Determining muscle’s force and action in multi-articular movement. Exercise Sport Sci Review. 1989;17:187–230. [PubMed] [Google Scholar]
- 18.Zajac FE. Muscle and tendon: properties, models, scaling, and application to biomechanics and motor control. Crit Rev Biomed Eng. 1989;17:359–411. [PubMed] [Google Scholar]
- 19.Zhao W, Kirsch RF, Triolo RJ, Delp S. A bipedal, closed-chain dynamic model of the human lower extremities and pelvis for simulation-based development of standing and mobility neuroprostheses. Proc IEEE EMBS. 1998;5(28):2605–08. [Google Scholar]
- 20.Lambrecht JM, Audu ML, Triolo RJ, Kirsch RF. A musculoskeletal model of the trunk and hips for the development of a seated posture-control neuroprosthesis. J Rehabil Res Dev. 2009;46:515–528. doi: 10.1682/jrrd.2007.08.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amankwah K, Triolo RJ, Kirsch R. Effects of spinal cord injury on lower-limb passive joint moments revealed through a nonlinear viscoelastic model. J Rehabil Res Dev. 2004;41:15–32. doi: 10.1682/jrrd.2004.01.0015. [DOI] [PubMed] [Google Scholar]
- 22.Kim JY, Popovic MR, Mills JK. Optimal combination of minimum degrees of freedom to be actuated in the lower limbs to faciltate arm-free paraplegic standing. J Biomech Eng. 2007;129:838–48. doi: 10.1115/1.2800767. [DOI] [PubMed] [Google Scholar]
- 23.Bhadra N. Kilgore KL, Peckham PH. Implanted stimulators for restoration of function in spinal cord injury. Med Eng Phys. 2001;23:19–28. doi: 10.1016/s1350-4533(01)00012-1. [DOI] [PubMed] [Google Scholar]
- 24.Kobetic R, Marsolais EB. Synthesis of paraplegic gait with multichannel functional neuromuscular stimulation. IEEE Trans Biomed Eng. 1994;2:66–67. [Google Scholar]
- 25.Ogata K. Modern Control Engineering. 4. Prentice Hall; 2002. [Google Scholar]
- 26.Pai YC, Patton J. Static versus dynamic predictions of protective stepping following waist-pull perturbations in young and older adults. J Biomech. 1998;31:1111–18. doi: 10.1016/s0021-9290(98)00124-9. [DOI] [PubMed] [Google Scholar]
- 27.Kandel E, Schwartz JH, Jessell TM. Principles of Neural Science. 4. McGraw-Hill Medical; 2000. [Google Scholar]
- 28.Uhlir JP, Triolo RJ, Kobetic R. Performance of epimysial stimulating electrodes in the lower extremities of individuals with spinal cord injury. IEEE Trans Neural Syst Rehabil Eng. 2004;12:279–87. doi: 10.1109/TNSRE.2004.827224. [DOI] [PubMed] [Google Scholar]
- 29.Audu ML, Kirsch RF, Triolo RJ. Experimental verification of a computational technique for determining ground reactions in human bipedal stance. J Biomech. 2007;40:1115–24. doi: 10.1016/j.jbiomech.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 30.Kagaya H, Sharma M, Kobetic R. Ankle, knee, and hip moments during standing with and without joint contractures: simulation study for functional electrical stimulation. Am J Phys Med Rehabil. 1998;77:49–54. doi: 10.1097/00002060-199801000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Kuo AD, Zajac FE. Biomechanical analysis of muscle strength as a limiting factor in standing posture. J Biomech. 1993;26(suppl 1):137–150. doi: 10.1016/0021-9290(93)90085-s. [DOI] [PubMed] [Google Scholar]
- 32.Crowninshield RD, Brand RA. A physiologically-based criterion of muscle force prediction in locomotion. J Biomech. 1981;14:793–801. doi: 10.1016/0021-9290(81)90035-x. [DOI] [PubMed] [Google Scholar]
- 33.Haykin SS. Neural Networks: A Comprehensive Foundation. 2. Prentice Hall; 1999. [Google Scholar]
- 34.Hagan MT, Menhaj MB. Training feedforward networks with the Marquardt algorithm. IEEE Trans Neural Networks. 1994;5:989–93. doi: 10.1109/72.329697. [DOI] [PubMed] [Google Scholar]
- 35.Gray GA, Kolda TG. Algorithm 856: APPSPACK 4.0: Asynchronous Parallel Pattern Search for Derivative-Free Optimization. ACM Transactions on Mathematical Software. 2006;32:486–507. [Google Scholar]
- 36.Triolo RJ, Werner KN, Kirsch RF. Modeling the postural disturbances caused by upper extremity movements. IEEE Trans Neural Syst Rehabil Eng. 2001;9:137–44. doi: 10.1109/7333.928573. [DOI] [PubMed] [Google Scholar]
- 37.Rietdyk S, Patla AE, Winter DA, Ishac MG, Little CE. Balance recovery from medio-lateral perturbations of the upper body during standing. J Biomech. 1999;32 (11):1149–1158. doi: 10.1016/s0021-9290(99)00116-5. [DOI] [PubMed] [Google Scholar]
- 38.Winter DA. Stiffness Control of Balance in Quiet Standing. J Neurophysiol. 1998;80(3):1211–21. doi: 10.1152/jn.1998.80.3.1211. [DOI] [PubMed] [Google Scholar]
- 39.Allum JHJ, Honegger F. Interactions between vestibular and proprioceptive inputs triggering and modulating human balance-correcting responses differ across muscles. Exp Brain Res. 1994;121 (4):478–494. doi: 10.1007/s002210050484. [DOI] [PubMed] [Google Scholar]