Abstract
Prokaryotes are highly susceptible to exogenous copper and employ metalloregulatory proteins to control the intracellular concentration. CsoR (copper-sensitive operon repressor) is one such protein that represses transcription of a Cu(I)-effluxing ATPase in its apo form. Cu(I)-binding leads to transcriptional derepression and cellular copper resistance. Herein, we present substantially complete backbone (HN, N, C’, C , C ) resonance assignments of tetrameric (48 kD) Geobacillus thermodenitrificans (Gt) CsoR in its apo- and Cu(I)-saturated states. These data provide the first spectroscopic evidence that Cu(I)-binding induces an interruption in the long 2 helix of CsoR.
Keywords: metalloregulatory protein, allosteric regulation, CsoR, copper sensor, Geobacillus thermodenitrificans, thermophile
Biological Context
Severe bacterial infections such as those caused by multidrug-resistant Staphylococcus aureus (MRSA) are becoming more prevalent in hospitals and our current antibiotic regimens are becoming less effective at treatment. In humans, copper is an essential biomineral and functions as a cofactor in selected enzymes, but becomes toxic at high, unregulated concentrations. The toxicity of copper derives from the catalytic production of free radicals via Fenton chemistry and/or disruption and/or replacement of other metal cofactors resulting in pleomorphic metabolic defects, including inhibition of amino acid biosynthesis (Macomber and Imlay 2009). Mechanisms of copper homeostasis and resistance are genetically encoded in all organisms and occur through coordinated copper transport and efflux which collectively minimize deleterious cellular reactions of unregulated Cu(I). In contrast to eukaryotes, non-photosynthetic prokaryotes appear to lack a metabolic requirement for cytoplasmic copper; as a result, the bacterial response to copper toxicity is more of a defense mechanism (Dupont et al. 2011).
Metalloregulatory proteins control the expression of genes necessary to remove exogenous copper from the cell. CsoR (Liu et al. 2007), a metalloregulatory protein, found in a subset of Gram-positive organisms binds operator DNA known as cso boxes, which are upstream of the genes required for copper resistance. It has reduced affinity for operator DNA in the presence of copper and the subsequent dissociation results in the up-regulation of copper resistance proteins, including the Cu(I)-effluxing ATPase (Liu et al. 2007). CsoR was originally discovered in Mycobacterium tuberculosis (Mtb) and structurally characterized in the Cu(I)-bound state as a dimer of dimers helical bundle architecture (Liu et al. 2007). Recently, a metal-free apo-structure of CsoR from Streptomyces lividians was reported and shown to adopt of dimer-of-dimers structure, with each protomer containing three helical segments, as in Mtb CsoR (Dwarakanath et al. 2012). There exists no atomic-level structural description of a conformational change that must occur in a single CsoR in response to Cu(I) coordination that drives the dissociation from corresponding operator DNA.
Previous NMR investigations of CsoR from B. subtilis and M. tuberculosis have been hampered by very broad resonances indicative of high molecular weight complexes (data not shown). In this note, we employ a thermophilic CsoR from Geobacillus thermodenitrificans (Gt) to take advantage of faster correlation times obtainable at higher temperatures, thereby making multidimensional experiments feasible on these high molecular weight tetramers (45-50 kD). Here, we present nearly complete backbone (HN, N, C’, C , C ) resonance assignments of tetrameric (48 kD) Gt CsoR in its apo- and Cu(I)-saturated states, the analysis of which provides unambiguous evidence for a structural transition in the tetramer linked to Cu(I)-binding and negative allosteric regulation of DNA operator binding.
Methods and experiments
Protein expression and purification
The gene encoding the putative Gt CsoR (locus tag GTNG_1533) was PCR amplified from Geobacillus thermodenitrificans NG80-2 genomic DNA and subcloned into pET15b (Novagen) between the NcoI and BamHI sites to drive the expression of T2A Gt CsoR, taken as wild-type. The plasmid was transformed into Rosetta BL21(DE3) expression cells using ampicillin and chloramphenicol for selection. A series of starting cultures were utilized to inoculate a 70% D2O M9 culture supplemented with D-Glucose-13C6, C-D7 and 15N NH4Cl. The isotopically enriched media was placed at 37 °C shaking at 200 rpm and induced at an O.D. of 0.55 to a final IPTG concentration of 1.0 mM. The recombinant expression was continued for 3 h at 37 °C, afterwards the cells were collected by centrifugation for 20 min at 4000 rpm. The cell pellet was lysed in 100 mL of 10 mM MES, 120 mM NaCl, 5 mM TCEP, 5 mM EDTA by sonication for 9 min (10s on, 50s off) at 65% amplitude. The lysate was then placed in a 65 °C water bath for 30 min and subsequently spun at 9000 rpm for 25 min at 4 °C. Polyethyleneamine was added to the supernatant at a final concentration of 0.015% (v/v) and stirred at 4° for 30 min. After clarification of the supernatant the sample was precipitated with the addition of 20% and 80% NH4SO4, stirring for 30 min at 4 °C, with centrifugation at 9000 rpm for 25 min at 4 °C between steps. The pellet from the 80% NH4SO4 cut was dissolved with lysis buffer and dialyzed overnight at 4 °C in low salt buffer, 10 mM MES, 50 mM NaCl, 5 mM TCEP, 3 mM EDTA pH 6.0. The supernatant of the dialysis was purified over a 100 min gradient to 100% high salt buffer (10 mM MES, 1 M NaCl, 5 mM TCEP, 3mM EDTA pH 6.0) using a 6 mL SP column. CsoR-containing fractions were identified by SDS-PAGE and pooled for further purification by gel filtration (G75 Superdex) in 10 mM MES, 120 mM NaCl, 5 mM TCEP and 5 mM EDTA pH 6.0. Sample purity was assessed by SDS-PAGE and ESI-MS, and the CsoR fractions absent of impurities or bound Cu(I) were pooled and concentrated to generate the apo CsoR sample. Any fractions that were perceived to have bound Cu(I), indicative of a low 280 nm/260 nm ratio, were pooled for in vitro metallation with Cu(I). The samples were reduced, placed in an anaerobic chamber, buffer exchanged and incubated with one molar equivalent of Cu(I) from an anaerobically prepared CuCl stock. The protein concentration was measured by determining the concentration of free thiols and the Cu(I) concentration by atomic absorption spectroscopy. The observed molecular weight by ESI-MS was found to be 11926 D (11925 D with the N-terminal Met processed) for unlabeled CsoR. Gel filtration chromatography (G75 Superdex) is consistent with an tetrameric assembly state as are multiple sedimentation velocity (Optima XL-1 analytical ultracentrifuge) runs (10 mM MES, 200 mM NaCl, 5 mM TCEP pH 6.0) at 20 °C (55,000 rpm, monitored at 230 nm, 20 μM protomer) and 40 °C (60,000 rpm, monitored at 280 nm; 0.16-0.37 mM) which reveal a model-independent s20,w=3.4 (±0.1) S at both temperatures, a value consistent with another tetrameric (≈46 kD) B. subtilis CsoR in the apo-state (Ma et al. 2009).
NMR spectroscopy
Samples for NMR spectroscopy were prepared at 0.8 mM and 0.6 mM for apo and Cu(I) CsoR, respectively. The sample buffer was 10 mM MES, 120 mM NaCl, 5 mM TCEP, 5 mM EDTA, 20 mM Arginine, 20 mM Glutamate, pH 6.0. All NMR experiments were performed on a Varian DDR 800 MHz spectrometer fitted with a cryogenic probe system at 322 K in the METACyt Biomolecular NMR laboratory at Indiana University. The spectra were referenced to external DSS and NaN3 was used as a preservative. NMR data processing and analysis were performed using NMRPipe (Delaglio et al. 1995) and SPARKY (Goddard and Kneller). Sequential backbone resonance assignments were obtained using standard triple-resonance NMR spectroscopy: HNCA, HN(CO)CA, HNCACB, HN(COCA)CB , HNCO, and HN(CA)CO (Grzesiek and Bax 1992). All of the 2D & 3D pulse sequences used were acquired using TROSY-based spectral selection and suppression of side chain carboxamide couplings..
Assignments and data deposition
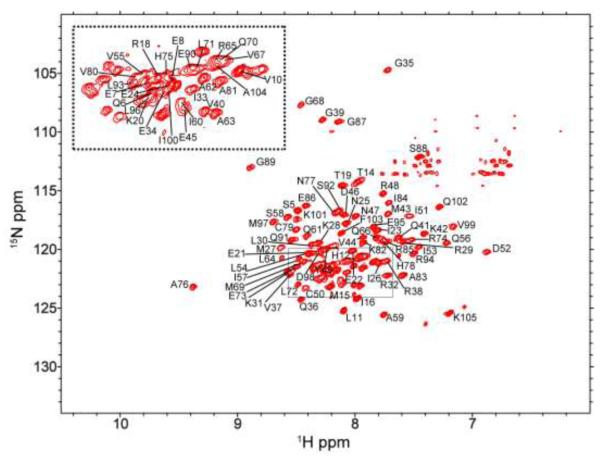
The 1H, 15N TROSY spectra of apo and Cu(I)-loaded CsoR are shown in Figs. 1 and 2, respectively. A suite of 13C-based multidimensional experiments were used to obtain substantially complete residue-specific HN, N, C’, C and C assignments obtained in both metal-free apo- and Cu(I)-bound conformational states. At least two backbone nuclei were specifically assigned for over 95% of the non-Pro residues (101 expected 1H-15N crosspeaks from 105 residues), with the extreme N-terminal residues 2-4 not assigned in either state. Both spectra are of comparable quality and the linewidths are suggestive of no substantial change in quaternary structure when CsoR binds Cu(I), as previously found for B. subtilis CsoR (Ma et al. 2009). However, the chemical shift dispersion of the metallated CsoR is detectably greater than that of apo-CsoR (compare Figs. 1 and 2), and the fact that the spectra are easily distinguished from one another is consistent with a significant conformational change upon metal coordination.
Fig. 1.
1H-15N TROSY spectrum of the apo Gt CsoR tetramer. Residue numbers are labeled on the crosspeaks. Crosspeaks from the middle of the spectrum are shown in the inserted box, left.
Fig. 2.
1H-15N TROSY spectrum of the Cu(I)-bound Gt CsoR tetramer. Residue numbers are labeled on the crosspeaks. Crosspeaks from the middle of the spectrum are shown in the inserted box, left.
A TALOS+ analysis of apo and Cu(I)-loaded Gt CsoR was used to determine backbone and dihedral angles (Fig. 3A and 3B) (Shen et al. 2009). Apo Gt CsoR consists of three uninterrupted helices as was recently shown crystallographically in Streptomyces lividians apo-CsoR (Dwarakanath et al. 2012) ( 1: E21-E45; 2: C50-R85; 3: E90-Q102). Although Cu(I)-loaded Gt CsoR consists of essentially the same three helices, there is a clear interruption or kink introduced into the 2 helix between the presumptive Cu(I) ligands H75 and C79, likely required to accommodate Cu(I) coordination ( 1: K20-E45; 2a: C50-A76; 2b:C79-R85; 3: G89-F103). The 2 helix was shown to terminate two residues following the His ligand (H61) in Cu(I)-bound Mtb CsoR (Liu et al, 2007). This change in secondary structure is likely coincident with a significant change in tertiary or quaternary structural packing given the large upfield shifts of A76 and A81 when apo-CsoR binds Cu(I). The large downfield shift of I16 upon Cu(I) binding (Fig. 2) may be reporting on local folding in this region of the molecule which sits immediately above the Cu(I)-binding site; the crystal structure of T. thermophilus apo-CsoR )Sakamoto et al., 2010) suggests some -strand structure in this region that might be stabilized by Cu(I) binding. Backbone (HN, N, C’, C , C ) resonance assignments for both apo- and Cu(I)-bound Gt CsoR have been deposited in the BioMagResBank database (http://www.bmrb.wisc.edu) under the accession numbers 18472 and 18470 for apo- and Cu(I)-CsoR, respectively.
Fig. 3.
TALOS+ derived backbone torsion angles and secondary 13C chemical shifts of apo-CsoR (A) and Cu(I)-CsoR (B). 13C shifts calculated by subtraction of published random coil values from the experimental 13C chemical shifts. Filled circles, phi angles; open circles, psi angles; filled diamonds, secondary 13C chemical shifts. A secondary structure schematic based on TALOS+ analysis is shown at the top panels A and B for the apo- and Cu(I)-loaded CsoR, respectively.
Acknowledgments
This work was supported by a grant from the NIH (GM042569) to D. P. G. We thank Dr. Dejian Ma for his help in acquiring NMR spectra and Dr. Chul Won Lee and Mr. Ben Kester for initial methods used to purify labeled Gt CsoR samples used for this study. We also acknowledge Dr. Todd Stone of the Physical Biochemistry Instrumentation Facility for his help in analyzing the sedimentation velocity data for apo-Gt CsoR.
References
- Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- Dupont CL, Grass G, Rensing C. Copper toxicity and the origin of bacterial resistance-new insights and applications. Metallomics. 2011;3:1109–1118. doi: 10.1039/c1mt00107h. [DOI] [PubMed] [Google Scholar]
- Dwarakanath S, Chaplin AK, Hough MA, Rigali S, Vijgenboom E, Worrall AR. Response to copper stress in Streptomyces lividans extends beyond genes under direct control of a copper-sensitive operon repressor protein (CsoR) J Biol Chem. 2012;287:17833–17847. doi: 10.1074/jbc.M112.352740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard TD, Kneller DG. SPARKY 3. University of California; San Francisco: [Google Scholar]
- Grzesiek S, Bax A. Improved 3D Triple-Resonance NMR Techniques Applied to a 31 kDa Protein. J Magn Res. 1992;96:432–440. [Google Scholar]
- Liu T, Ramesh A, Ma Z, Ward S, Zhang L, George GN, Talaat AM, Sacchettini JC, Giedroc DP. CsoR is a novel Mycobacterium tuberculosis copper sensing transcriptional regulator. Nat Chem Biol. 2007;3:60–68. doi: 10.1038/nchembio844. [DOI] [PubMed] [Google Scholar]
- Ma Z, Cowart DM, Scott RA, Giedroc DP. Molecular insights into the metal selectivity of the copper(I)-sensing repressor CsoR from Bacillus subtilis. Biochemistry. 2009;48:3325–3334. doi: 10.1021/bi900115w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macomber L, Imlay JA. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci USA. 2009;106:8344–8349. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Agari Y, Agari K, Kuramitsu S, Shinkai A. Structural and functional characterization of the transcriptional repressor CsoR from Thermus thermophilus HB8. Microbiology. 2010;156:1993–2005. doi: 10.1099/mic.0.037382-0. [DOI] [PubMed] [Google Scholar]
- Shen Y, Delaglio F, Cornilescu G, Bax A. TALOS+: a hybrid method for predicting Protein backbone torsion angles from NMR chemical shifts. J Biomol NMR. 2009;44:213–223. doi: 10.1007/s10858-009-9333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]