Abstract
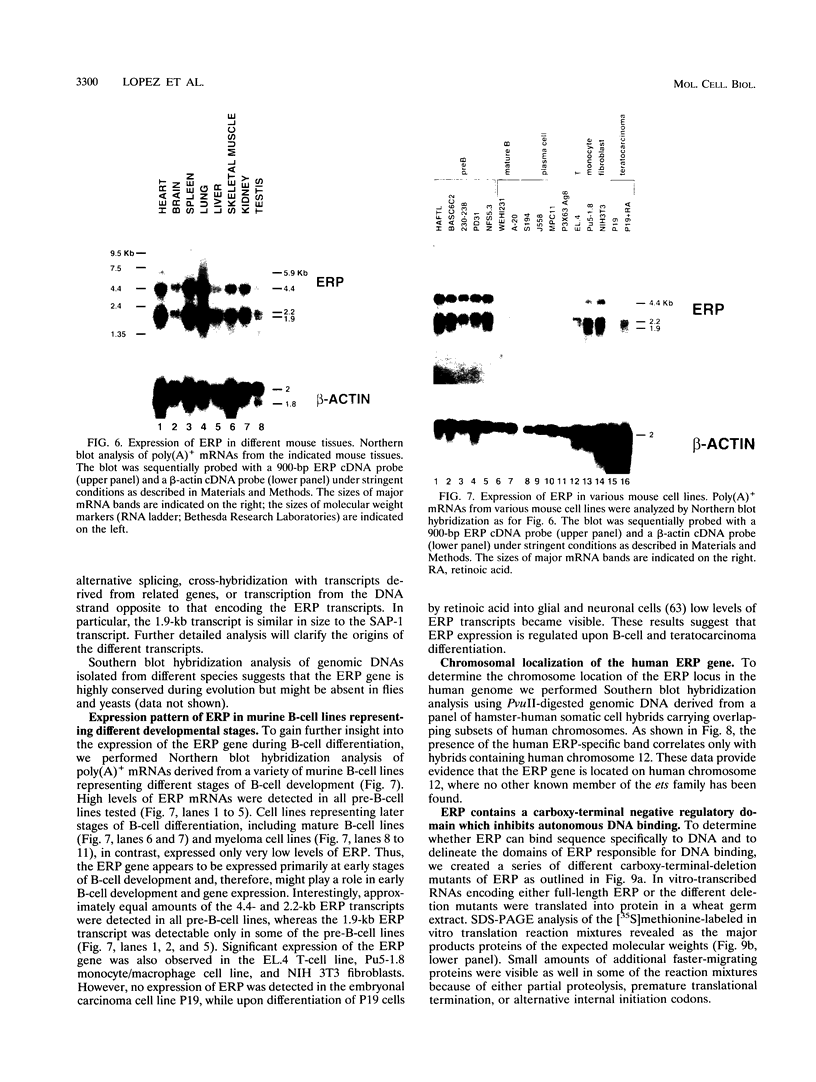
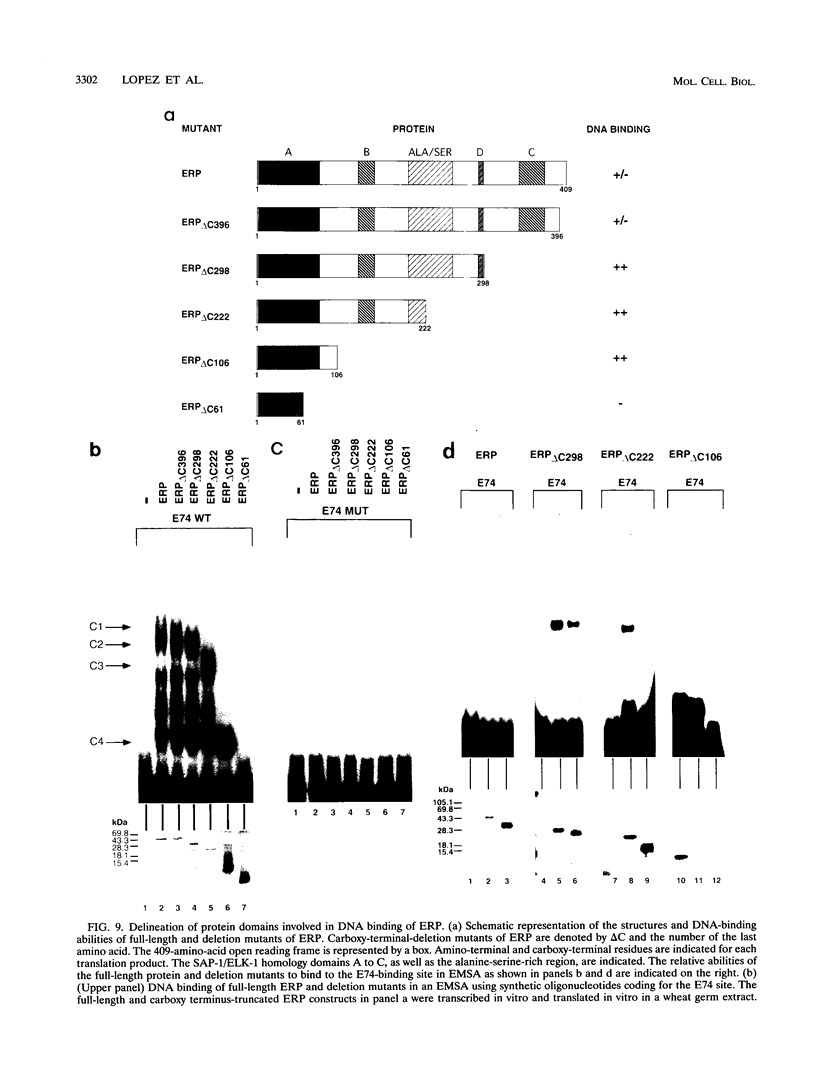
The ets gene family encodes a group of proteins which function as transcription factors under physiological conditions and, if aberrantly expressed, can cause cellular transformation. We have recently identified two regulatory elements in the murine immunoglobulin heavy-chain (IgH) enhancer, pi and microB, which exhibit striking similarity to binding sites for ets-related proteins. To identify ets-related transcriptional regulators expressed in pre-B lymphocytes that may interact with either the pi or the microB site, we have used a PCR approach with degenerate oligonucleotides encoding conserved sequences in all members of the ets family. We have cloned the gene for a new ets-related transcription factor, ERP (ets-related protein), from the murine pre-B cell line BASC 6C2 and from mouse lung tissue. The ERP protein contains a region of high homology with the ETS DNA-binding domain common to all members of the ets transcription factor/oncoprotein family. Three additional smaller regions show homology to the ELK-1 and SAP-1 genes, a subgroup of the ets gene family that interacts with the serum response factor. Full-length ERP expresses only negligible DNA-binding activity by itself. Removal of the carboxy terminus enables ERP to interact with a variety of ets-binding sites including the E74 site, the IgH enhancer pi site, and the lck promoter ets site, suggesting a carboxy-terminal negative regulatory domain. At least three ERP-related transcripts are expressed in a variety of tissues. However, within the B-cell lineage, ERP is highly expressed primarily at early stages of B-lymphocyte development, and expression declines drastically upon B-cell maturation, correlating with the enhancer activity of the IgH pi site. These data suggest that ERP might play a role in B-cell development and in IgH gene regulation.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharon T., Schneider R. J. Selective destabilization of short-lived mRNAs with the granulocyte-macrophage colony-stimulating factor AU-rich 3' noncoding region is mediated by a cotranslational mechanism. Mol Cell Biol. 1993 Mar;13(3):1971–1980. doi: 10.1128/mcb.13.3.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez E., Northwood I. C., Gonzalez F. A., Latour D. A., Seth A., Abate C., Curran T., Davis R. J. Pro-Leu-Ser/Thr-Pro is a consensus primary sequence for substrate protein phosphorylation. Characterization of the phosphorylation of c-myc and c-jun proteins by an epidermal growth factor receptor threonine 669 protein kinase. J Biol Chem. 1991 Aug 15;266(23):15277–15285. [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-kappa B transcription factor. Cell. 1988 Apr 22;53(2):211–217. doi: 10.1016/0092-8674(88)90382-0. [DOI] [PubMed] [Google Scholar]
- Bauer S. R., Kudo A., Melchers F. Structure and pre-B lymphocyte restricted expression of the VpreB in humans and conservation of its structure in other mammalian species. EMBO J. 1988 Jan;7(1):111–116. doi: 10.1002/j.1460-2075.1988.tb02789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello M. J., Salagnon N., Rey J. A., Guichaoua M. R., Bergé-Lefranc J. L., Jordan B. R., Luciani J. M. Precise in situ localization of NCAM, ETS1, and D11S29 on human meiotic chromosomes. Cytogenet Cell Genet. 1989;52(1-2):7–10. doi: 10.1159/000132828. [DOI] [PubMed] [Google Scholar]
- Boulukos K. E., Pognonec P., Begue A., Galibert F., Gesquière J. C., Stéhelin D., Ghysdael J. Identification in chickens of an evolutionarily conserved cellular ets-2 gene (c-ets-2) encoding nuclear proteins related to the products of the c-ets proto-oncogene. EMBO J. 1988 Mar;7(3):697–705. doi: 10.1002/j.1460-2075.1988.tb02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. J., Smeal T., Defize L. H., Angel P., Woodgett J. R., Karin M., Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991 Feb 8;64(3):573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- Brown T. A., McKnight S. L. Specificities of protein-protein and protein-DNA interaction of GABP alpha and two newly defined ets-related proteins. Genes Dev. 1992 Dec;6(12B):2502–2512. doi: 10.1101/gad.6.12b.2502. [DOI] [PubMed] [Google Scholar]
- Burtis K. C., Thummel C. S., Jones C. W., Karim F. D., Hogness D. S. The Drosophila 74EF early puff contains E74, a complex ecdysone-inducible gene that encodes two ets-related proteins. Cell. 1990 Apr 6;61(1):85–99. doi: 10.1016/0092-8674(90)90217-3. [DOI] [PubMed] [Google Scholar]
- Chen J. H. Cloning, sequencing, and expression of mouse c-ets-1 cDNA in baculovirus expression system. Oncogene Res. 1990;5(4):277–285. [PubMed] [Google Scholar]
- Chen T., Bunting M., Karim F. D., Thummel C. S. Isolation and characterization of five Drosophila genes that encode an ets-related DNA binding domain. Dev Biol. 1992 May;151(1):176–191. doi: 10.1016/0012-1606(92)90225-6. [DOI] [PubMed] [Google Scholar]
- Dalton S., Treisman R. Characterization of SAP-1, a protein recruited by serum response factor to the c-fos serum response element. Cell. 1992 Feb 7;68(3):597–612. doi: 10.1016/0092-8674(92)90194-h. [DOI] [PubMed] [Google Scholar]
- Dariavach P., Williams G. T., Campbell K., Pettersson S., Neuberger M. S. The mouse IgH 3'-enhancer. Eur J Immunol. 1991 Jun;21(6):1499–1504. doi: 10.1002/eji.1830210625. [DOI] [PubMed] [Google Scholar]
- Delattre O., Zucman J., Plougastel B., Desmaze C., Melot T., Peter M., Kovar H., Joubert I., de Jong P., Rouleau G. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992 Sep 10;359(6391):162–165. doi: 10.1038/359162a0. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldhaus A. L., Mbangkollo D., Arvin K. L., Klug C. A., Singh H. BLyF, a novel cell-type- and stage-specific regulator of the B-lymphocyte gene mb-1. Mol Cell Biol. 1992 Mar;12(3):1126–1133. doi: 10.1128/mcb.12.3.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher C. L., Ghysdael J., Cambier J. C. Ligation of membrane Ig leads to calcium-mediated phosphorylation of the proto-oncogene product, Ets-1. J Immunol. 1991 Mar 15;146(6):1743–1749. [PubMed] [Google Scholar]
- Gegonne A., Punyammalee B., Rabault B., Bosselut R., Seneca S., Crabeel M., Ghysdael J. Analysis of the DNA binding and transcriptional activation properties of the Ets1 oncoprotein. New Biol. 1992 May;4(5):512–519. [PubMed] [Google Scholar]
- Gitlin S. D., Bosselut R., Gégonne A., Ghysdael J., Brady J. N. Sequence-specific interaction of the Ets1 protein with the long terminal repeat of the human T-lymphotropic virus type I. J Virol. 1991 Oct;65(10):5513–5523. doi: 10.1128/jvi.65.10.5513-5523.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant P. A., Arulampalam V., Ahrlund-Richter L., Pettersson S. Identification of Ets-like lymphoid specific elements within the immunoglobulin heavy chain 3' enhancer. Nucleic Acids Res. 1992 Sep 11;20(17):4401–4408. doi: 10.1093/nar/20.17.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther C. V., Nye J. A., Bryner R. S., Graves B. J. Sequence-specific DNA binding of the proto-oncoprotein ets-1 defines a transcriptional activator sequence within the long terminal repeat of the Moloney murine sarcoma virus. Genes Dev. 1990 Apr;4(4):667–679. doi: 10.1101/gad.4.4.667. [DOI] [PubMed] [Google Scholar]
- Hagman J., Grosschedl R. An inhibitory carboxyl-terminal domain in Ets-1 and Ets-2 mediates differential binding of ETS family factors to promoter sequences of the mb-1 gene. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8889–8893. doi: 10.1073/pnas.89.19.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipskind R. A., Rao V. N., Mueller C. G., Reddy E. S., Nordheim A. Ets-related protein Elk-1 is homologous to the c-fos regulatory factor p62TCF. Nature. 1991 Dec 19;354(6354):531–534. doi: 10.1038/354531a0. [DOI] [PubMed] [Google Scholar]
- Ho I. C., Bhat N. K., Gottschalk L. R., Lindsten T., Thompson C. B., Papas T. S., Leiden J. M. Sequence-specific binding of human Ets-1 to the T cell receptor alpha gene enhancer. Science. 1990 Nov 9;250(4982):814–818. doi: 10.1126/science.2237431. [DOI] [PubMed] [Google Scholar]
- Holmes K. L., Pierce J. H., Davidson W. F., Morse H. C., 3rd Murine hematopoietic cells with pre-B or pre-B/myeloid characteristics are generated by in vitro transformation with retroviruses containing fes, ras, abl, and src oncogenes. J Exp Med. 1986 Aug 1;164(2):443–457. doi: 10.1084/jem.164.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janknecht R., Nordheim A. Elk-1 protein domains required for direct and SRF-assisted DNA-binding. Nucleic Acids Res. 1992 Jul 11;20(13):3317–3324. doi: 10.1093/nar/20.13.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemsz M. J., Maki R. A., Papayannopoulou T., Moore J., Hromas R. Characterization of the ets oncogene family member, fli-1. J Biol Chem. 1993 Mar 15;268(8):5769–5773. [PubMed] [Google Scholar]
- Klemsz M. J., McKercher S. R., Celada A., Van Beveren C., Maki R. A. The macrophage and B cell-specific transcription factor PU.1 is related to the ets oncogene. Cell. 1990 Apr 6;61(1):113–124. doi: 10.1016/0092-8674(90)90219-5. [DOI] [PubMed] [Google Scholar]
- Korsmeyer S. J. Chromosomal translocations in lymphoid malignancies reveal novel proto-oncogenes. Annu Rev Immunol. 1992;10:785–807. doi: 10.1146/annurev.iy.10.040192.004033. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo A., Sakaguchi N., Melchers F. Organization of the murine Ig-related lambda 5 gene transcribed selectively in pre-B lymphocytes. EMBO J. 1987 Jan;6(1):103–107. doi: 10.1002/j.1460-2075.1987.tb04725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzel E. A., Mulligan J. A., Sommercorn J., Krebs E. G. Substrate specificity determinants for casein kinase II as deduced from studies with synthetic peptides. J Biol Chem. 1987 Jul 5;262(19):9136–9140. [PubMed] [Google Scholar]
- LaMarco K., Thompson C. C., Byers B. P., Walton E. M., McKnight S. L. Identification of Ets- and notch-related subunits in GA binding protein. Science. 1991 Aug 16;253(5021):789–792. doi: 10.1126/science.1876836. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai Z. C., Rubin G. M. Negative control of photoreceptor development in Drosophila by the product of the yan gene, an ETS domain protein. Cell. 1992 Aug 21;70(4):609–620. doi: 10.1016/0092-8674(92)90430-k. [DOI] [PubMed] [Google Scholar]
- Leduc I., Preud'homme J. L., Cogné M. Structure and expression of the mb-1 transcript in human lymphoid cells. Clin Exp Immunol. 1992 Oct;90(1):141–146. doi: 10.1111/j.1365-2249.1992.tb05846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiden J. M. Transcriptional regulation during T-cell development: the alpha TCR gene as a molecular model. Immunol Today. 1992 Jan;13(1):22–30. doi: 10.1016/0167-5699(92)90200-q. [DOI] [PubMed] [Google Scholar]
- Leiden J. M., Wang C. Y., Petryniak B., Markovitz D. M., Nabel G. J., Thompson C. B. A novel Ets-related transcription factor, Elf-1, binds to human immunodeficiency virus type 2 regulatory elements that are required for inducible trans activation in T cells. J Virol. 1992 Oct;66(10):5890–5897. doi: 10.1128/jvi.66.10.5890-5897.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M., Pierce J. W., Baltimore D. Protein-binding sites in Ig gene enhancers determine transcriptional activity and inducibility. Science. 1987 Jun 19;236(4808):1573–1577. doi: 10.1126/science.3109035. [DOI] [PubMed] [Google Scholar]
- Leprince D., Gesquiere J. C., Stehelin D. The chicken cellular progenitor of the v-ets oncogene, p68c-ets-1, is a nuclear DNA-binding protein not expressed in lymphoid cells of the spleen. Oncogene Res. 1990;5(4):255–265. [PubMed] [Google Scholar]
- Leung S., McCracken S., Ghysdael J., Miyamoto N. G. Requirement of an ETS-binding element for transcription of the human lck type I promoter. Oncogene. 1993 Apr;8(4):989–997. [PubMed] [Google Scholar]
- Libermann T. A., Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol. 1990 May;10(5):2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libermann T. A., Baltimore D. Pi, a pre-B-cell-specific enhancer element in the immunoglobulin heavy-chain enhancer. Mol Cell Biol. 1993 Oct;13(10):5957–5969. doi: 10.1128/mcb.13.10.5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libermann T. A., Friesel R., Jaye M., Lyall R. M., Westermark B., Drohan W., Schmidt A., Maciag T., Schlessinger J. An angiogenic growth factor is expressed in human glioma cells. EMBO J. 1987 Jun;6(6):1627–1632. doi: 10.1002/j.1460-2075.1987.tb02410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libermann T. A., Lenardo M., Baltimore D. Involvement of a second lymphoid-specific enhancer element in the regulation of immunoglobulin heavy-chain gene expression. Mol Cell Biol. 1990 Jun;10(6):3155–3162. doi: 10.1128/mcb.10.6.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht J. D., Grossel M. J., Figge J., Hansen U. M. Drosophila Krüppel protein is a transcriptional repressor. Nature. 1990 Jul 5;346(6279):76–79. doi: 10.1038/346076a0. [DOI] [PubMed] [Google Scholar]
- Lieberson R., Giannini S. L., Birshtein B. K., Eckhardt L. A. An enhancer at the 3' end of the mouse immunoglobulin heavy chain locus. Nucleic Acids Res. 1991 Feb 25;19(4):933–937. doi: 10.1093/nar/19.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim F., Kraut N., Framptom J., Graf T. DNA binding by c-Ets-1, but not v-Ets, is repressed by an intramolecular mechanism. EMBO J. 1992 Feb;11(2):643–652. doi: 10.1002/j.1460-2075.1992.tb05096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo K., Landau N. R., Smale S. T. LyF-1, a transcriptional regulator that interacts with a novel class of promoters for lymphocyte-specific genes. Mol Cell Biol. 1991 Oct;11(10):5229–5243. doi: 10.1128/mcb.11.10.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher B., Christenson E., Litchfield D. W., Krebs E. G., Eisenman R. N. Myb DNA binding inhibited by phosphorylation at a site deleted during oncogenic activation. Nature. 1990 Apr 5;344(6266):517–522. doi: 10.1038/344517a0. [DOI] [PubMed] [Google Scholar]
- Macleod K., Leprince D., Stehelin D. The ets gene family. Trends Biochem Sci. 1992 Jul;17(7):251–256. doi: 10.1016/0968-0004(92)90404-w. [DOI] [PubMed] [Google Scholar]
- Marais R., Wynne J., Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993 Apr 23;73(2):381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- Markovitz D. M., Smith M. J., Hilfinger J., Hannibal M. C., Petryniak B., Nabel G. J. Activation of the human immunodeficiency virus type 2 enhancer is dependent on purine box and kappa B regulatory elements. J Virol. 1992 Sep;66(9):5479–5484. doi: 10.1128/jvi.66.9.5479-5484.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelsen B., Kadesch T., Sen R. Complex regulation of the immunoglobulin mu heavy-chain gene enhancer: microB, a new determinant of enhancer function. Mol Cell Biol. 1990 Jun;10(6):3145–3154. doi: 10.1128/mcb.10.6.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelsen B., Tian G., Erman B., Gregoire J., Maki R., Graves B., Sen R. Regulation of lymphoid-specific immunoglobulin mu heavy chain gene enhancer by ETS-domain proteins. Science. 1993 Jul 2;261(5117):82–86. doi: 10.1126/science.8316859. [DOI] [PubMed] [Google Scholar]
- Nerlov C., Rørth P., Blasi F., Johnsen M. Essential AP-1 and PEA3 binding elements in the human urokinase enhancer display cell type-specific activity. Oncogene. 1991 Sep;6(9):1583–1592. [PubMed] [Google Scholar]
- Nye J. A., Petersen J. M., Gunther C. V., Jonsen M. D., Graves B. J. Interaction of murine ets-1 with GGA-binding sites establishes the ETS domain as a new DNA-binding motif. Genes Dev. 1992 Jun;6(6):975–990. doi: 10.1101/gad.6.6.975. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Okazawa H., Okuda A., Sakai M., Muramatsu M., Hamada H. A novel octamer binding transcription factor is differentially expressed in mouse embryonic cells. Cell. 1990 Feb 9;60(3):461–472. doi: 10.1016/0092-8674(90)90597-8. [DOI] [PubMed] [Google Scholar]
- Pongubala J. M., Nagulapalli S., Klemsz M. J., McKercher S. R., Maki R. A., Atchison M. L. PU.1 recruits a second nuclear factor to a site important for immunoglobulin kappa 3' enhancer activity. Mol Cell Biol. 1992 Jan;12(1):368–378. doi: 10.1128/mcb.12.1.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad D. D., Rao V. N., Reddy E. S. Structure and expression of human Fli-1 gene. Cancer Res. 1992 Oct 15;52(20):5833–5837. [PubMed] [Google Scholar]
- Pribyl L. J., Watson D. K., Schulz R. A., Papas T. S. D-elg, a member of the Drosophila ets gene family: sequence, expression and evolutionary comparison. Oncogene. 1991 Jul;6(7):1175–1183. [PubMed] [Google Scholar]
- Raney A. K., Easton A. J., Milich D. R., McLachlan A. Promoter-specific transactivation of hepatitis B virus transcription by a glutamine- and proline-rich domain of hepatocyte nuclear factor 1. J Virol. 1991 Nov;65(11):5774–5781. doi: 10.1128/jvi.65.11.5774-5781.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao V. N., Huebner K., Isobe M., ar-Rushdi A., Croce C. M., Reddy E. S. elk, tissue-specific ets-related genes on chromosomes X and 14 near translocation breakpoints. Science. 1989 Apr 7;244(4900):66–70. doi: 10.1126/science.2539641. [DOI] [PubMed] [Google Scholar]
- Rao V. N., Modi W. S., Drabkin H. D., Patterson D., O'Brien S. J., Papas T. S., Reddy E. S. The human erg gene maps to chromosome 21, band q22: relationship to the 8; 21 translocation of acute myelogenous leukemia. Oncogene. 1988 Nov;3(5):497–500. [PubMed] [Google Scholar]
- Rao V. N., Reddy E. S. A divergent ets-related protein, elk-1, recognizes similar c-ets-1 proto-oncogene target sequences and acts as a transcriptional activator. Oncogene. 1992 Jan;7(1):65–70. [PubMed] [Google Scholar]
- Rao V. N., Reddy E. S. elk-1 domains responsible for autonomous DNA binding, SRE:SRF interaction and negative regulation of DNA binding. Oncogene. 1992 Nov;7(11):2335–2340. [PubMed] [Google Scholar]
- Ray D., Bosselut R., Ghysdael J., Mattei M. G., Tavitian A., Moreau-Gachelin F. Characterization of Spi-B, a transcription factor related to the putative oncoprotein Spi-1/PU.1. Mol Cell Biol. 1992 Oct;12(10):4297–4304. doi: 10.1128/mcb.12.10.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond V., Atwater J. A., Verma I. M. Removal of an mRNA destabilizing element correlates with the increased oncogenicity of proto-oncogene fos. Oncogene Res. 1989;5(1):1–12. [PubMed] [Google Scholar]
- Reddy E. S., Rao V. N., Papas T. S. The erg gene: a human gene related to the ets oncogene. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6131–6135. doi: 10.1073/pnas.84.17.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy E. S., Rao V. N. Structure, expression and alternative splicing of the human c-ets-1 proto-oncogene. Oncogene Res. 1988;3(3):239–246. [PubMed] [Google Scholar]
- Reddy E. S., Rao V. N. erg, an ets-related gene, codes for sequence-specific transcriptional activators. Oncogene. 1991 Dec;6(12):2285–2289. [PubMed] [Google Scholar]
- Richards M. L., Katz D. H., Liu F. T. Complete genomic sequence of the murine low affinity Fc receptor for IgE. Demonstration of alternative transcripts and conserved sequence elements. J Immunol. 1991 Aug 1;147(3):1067–1074. [PubMed] [Google Scholar]
- Riley L. K., Morrow J. K., Danton M. J., Coleman M. S. Human terminal deoxyribonucleotidyltransferase: molecular cloning and structural analysis of the gene and 5' flanking region. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2489–2493. doi: 10.1073/pnas.85.8.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimokh R., Rouault J. P., Wahbi K., Gadoux M., Lafage M., Archimbaud E., Charrin C., Gentilhomme O., Germain D., Samarut J. A chromosome 12 coding region is juxtaposed to the MYC protooncogene locus in a t(8;12)(q24;q22) translocation in a case of B-cell chronic lymphocytic leukemia. Genes Chromosomes Cancer. 1991 Jan;3(1):24–36. doi: 10.1002/gcc.2870030106. [DOI] [PubMed] [Google Scholar]
- Rivera R. R., Stuiver M. H., Steenbergen R., Murre C. Ets proteins: new factors that regulate immunoglobulin heavy-chain gene expression. Mol Cell Biol. 1993 Nov;13(11):7163–7169. doi: 10.1128/mcb.13.11.7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanghera J. S., Hall F. L., Warburton D., Campbell D., Pelech S. L. Identification of epidermal growth factor Thr-669 phosphorylation site peptide kinases as distinct MAP kinases and p34cdc2. Biochim Biophys Acta. 1992 Jun 29;1135(3):335–342. doi: 10.1016/0167-4889(92)90240-c. [DOI] [PubMed] [Google Scholar]
- Savant-Bhonsale S., Cleveland D. W. Evidence for instability of mRNAs containing AUUUA motifs mediated through translation-dependent assembly of a > 20S degradation complex. Genes Dev. 1992 Oct;6(10):1927–1939. doi: 10.1101/gad.6.10.1927. [DOI] [PubMed] [Google Scholar]
- Schlissel M. S., Baltimore D. Activation of immunoglobulin kappa gene rearrangement correlates with induction of germline kappa gene transcription. Cell. 1989 Sep 8;58(5):1001–1007. doi: 10.1016/0092-8674(89)90951-3. [DOI] [PubMed] [Google Scholar]
- Seipel K., Georgiev O., Schaffner W. Different activation domains stimulate transcription from remote ('enhancer') and proximal ('promoter') positions. EMBO J. 1992 Dec;11(13):4961–4968. doi: 10.1002/j.1460-2075.1992.tb05603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A., Ascione R., Fisher R. J., Mavrothalassitis G. J., Bhat N. K., Papas T. S. The ets gene family. Cell Growth Differ. 1992 May;3(5):327–334. [PubMed] [Google Scholar]
- Shimizu K., Ichikawa H., Tojo A., Kaneko Y., Maseki N., Hayashi Y., Ohira M., Asano S., Ohki M. An ets-related gene, ERG, is rearranged in human myeloid leukemia with t(16;21) chromosomal translocation. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10280–10284. doi: 10.1073/pnas.90.21.10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon E., Borrow J., Goddard A. D. Chromosome aberrations and cancer. Science. 1991 Nov 22;254(5035):1153–1160. doi: 10.1126/science.1957167. [DOI] [PubMed] [Google Scholar]
- Thompson C. B., Wang C. Y., Ho I. C., Bohjanen P. R., Petryniak B., June C. H., Miesfeldt S., Zhang L., Nabel G. J., Karpinski B. cis-acting sequences required for inducible interleukin-2 enhancer function bind a novel Ets-related protein, Elf-1. Mol Cell Biol. 1992 Mar;12(3):1043–1053. doi: 10.1128/mcb.12.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. C., Brown T. A., McKnight S. L. Convergence of Ets- and notch-related structural motifs in a heteromeric DNA binding complex. Science. 1991 Aug 16;253(5021):762–768. doi: 10.1126/science.1876833. [DOI] [PubMed] [Google Scholar]
- Treisman R., Marais R., Wynne J. Spatial flexibility in ternary complexes between SRF and its accessory proteins. EMBO J. 1992 Dec;11(12):4631–4640. doi: 10.1002/j.1460-2075.1992.tb05565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urness L. D., Thummel C. S. Molecular interactions within the ecdysone regulatory hierarchy: DNA binding properties of the Drosophila ecdysone-inducible E74A protein. Cell. 1990 Oct 5;63(1):47–61. doi: 10.1016/0092-8674(90)90287-o. [DOI] [PubMed] [Google Scholar]
- Varticovski L., Chahwala S. B., Whitman M., Cantley L., Schindler D., Chow E. P., Sinclair L. K., Pepinsky R. B. Location of sites in human lipocortin I that are phosphorylated by protein tyrosine kinases and protein kinases A and C. Biochemistry. 1988 May 17;27(10):3682–3690. doi: 10.1021/bi00410a024. [DOI] [PubMed] [Google Scholar]
- Visvader J., Begley C. G. Helix-loop-helix genes translocated in lymphoid leukemia. Trends Biochem Sci. 1991 Sep;16(9):330–333. doi: 10.1016/0968-0004(91)90137-k. [DOI] [PubMed] [Google Scholar]
- Wang C. Y., Petryniak B., Ho I. C., Thompson C. B., Leiden J. M. Evolutionarily conserved Ets family members display distinct DNA binding specificities. J Exp Med. 1992 May 1;175(5):1391–1399. doi: 10.1084/jem.175.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B., Wasylyk C., Flores P., Begue A., Leprince D., Stehelin D. The c-ets proto-oncogenes encode transcription factors that cooperate with c-Fos and c-Jun for transcriptional activation. Nature. 1990 Jul 12;346(6280):191–193. doi: 10.1038/346191a0. [DOI] [PubMed] [Google Scholar]
- Wasylyk C., Gutman A., Nicholson R., Wasylyk B. The c-Ets oncoprotein activates the stromelysin promoter through the same elements as several non-nuclear oncoproteins. EMBO J. 1991 May;10(5):1127–1134. doi: 10.1002/j.1460-2075.1991.tb08053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk C., Kerckaert J. P., Wasylyk B. A novel modulator domain of Ets transcription factors. Genes Dev. 1992 Jun;6(6):965–974. doi: 10.1101/gad.6.6.965. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Sawada J., Yano K., Yamaguchi K., Goto M., Handa H. cDNA cloning of transcription factor E4TF1 subunits with Ets and notch motifs. Mol Cell Biol. 1993 Mar;13(3):1385–1391. doi: 10.1128/mcb.13.3.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. K., McWilliams M. J., Lapis P., Lautenberger J. A., Schweinfest C. W., Papas T. S. Mammalian ets-1 and ets-2 genes encode highly conserved proteins. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7862–7866. doi: 10.1073/pnas.85.21.7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. K., Sacchi N., McWilliams-Smith M. J., O'Brien S. J., Papas T. S. The avian and mammalian ets genes: molecular characterization, chromosome mapping, and implication in human leukemia. Anticancer Res. 1986 Jul-Aug;6(4):631–636. [PubMed] [Google Scholar]
- Williams T., Tjian R. Analysis of the DNA-binding and activation properties of the human transcription factor AP-2. Genes Dev. 1991 Apr;5(4):670–682. doi: 10.1101/gad.5.4.670. [DOI] [PubMed] [Google Scholar]
- Woods D. B., Ghysdael J., Owen M. J. Identification of nucleotide preferences in DNA sequences recognised specifically by c-Ets-1 protein. Nucleic Acids Res. 1992 Feb 25;20(4):699–704. doi: 10.1093/nar/20.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin J. H., Cowie A., Lachance P., Hassell J. A. Molecular cloning and characterization of PEA3, a new member of the Ets oncogene family that is differentially expressed in mouse embryonic cells. Genes Dev. 1992 Mar;6(3):481–496. doi: 10.1101/gad.6.3.481. [DOI] [PubMed] [Google Scholar]