Summary
Background and objectives
B cell significance in ANCA disease pathogenesis is underscored by the finding that ANCA alone can cause disease in mouse models and by the effectiveness of rituximab as therapy in ANCA-small vessel vasculitis (ANCA-SVV). To avoid infections and adverse events from therapy, clinicians require improved markers of disease activity and impending relapse to guide immunosuppression strategies after rituximab treatment.
Design, setting, participants, & measurements
The B cell phenotype was investigated in patients with active ANCA-SVV and in remission. From 2003 to 2009, 54 patients were followed longitudinally for 4–99 months and compared with 68 healthy controls. In a subset of 19 patients, the B cell immunophenotype was examined in samples after rituximab therapy.
Results
Patients with active ANCA-SVV had lower %CD5+ B cells, whereas %CD5+ B cells from patients in remission were indistinguishable from healthy controls. After rituximab, median time to relapse was 31 months in patients maintaining normalized %CD5+ B cells, with or without maintenance immunosuppression. Among patients whose B cells repopulated with low %CD5+ B cells or had a sharply declining %CD5+ B cells, those who were on low or no maintenance immunosuppression relapsed sooner (median 17 months) than patients who were maintained on high levels of oral maintenance immunosuppression (29 months; P=0.002).
Conclusions
The %CD5+ B cells, as a component of the human B regulatory cell phenotype, is a useful indicator of disease activity, remission, and future relapse, and thus may guide remission maintenance therapy after rituximab treatment.
Introduction
ANCA-small vessel vasculitis (ANCA-SVV) is a severe relapsing disease wherein B cells produce autoantibodies directed against myeloperoxidase (MPO) (1) or proteinase 3 (PR3) (1,2). These autoantibodies can cause disease in mouse models (3–5). Recently, rituximab (a B cell–depleting mAb) has been shown to be effective in treating ANCA-SVV, suggesting that B cells play an important role in the pathophysiology of this disease (6,7). We predicted that B cell phenotype might be used as an indicator of disease activity, response to treatment, or future relapse. CD5 mutes B cell signaling and maintains immune tolerance via anergy (8–12). Recently, human B regulatory (Breg) cells characterized as CD24hi and either CD38hi (13) or CD27+ (14) were described. These cells are also noted to be CD5+ (13). We investigated CD5+ B cells in patients during the course of disease activity and with response to rituximab therapy.
We report a B cell population that partially overlaps with the immunophenotype for regulatory B cells and correlates with disease activity in patients with ANCA-SVV. To further evaluate the relationship of CD5+ B cells and states of remission and relapse in ANCA-SVV, we examined peripheral blood samples from patients who received rituximab therapy and underwent B cell depletion. We hypothesized that patients who repopulated with normalized %CD5+ B cells after rituximab would have a more sustained remission than patients who repopulated with low %CD5+ B cells.
Materials and Methods
Patient and Healthy Control Samples
We performed flow cytometry analysis of lymphocyte samples from 54 patients with ANCA-SVV and 68 healthy controls between the years 2003 and 2009. Informed consent was obtained in accordance with our institutional review board’s guidelines for human participants. Peripheral blood samples were collected from patients positive for MPO-ANCA and/or PR3-ANCA by either indirect immunofluorescence or antigen-specific ELISA. Patients with Churg-Strauss syndrome or anti-glomerular basement membrane or overlap ANCA/anti-glomerular basement membrane disease were excluded. Forty-nine of 54 patients had biopsy-proven ear, nose, and throat, pulmonary, renal, or dermatologic small vessel vasculitis. Clinical and serological data were gathered during routine clinic visits at the time of blood draw for B cell analysis. Patients with end stage kidney disease were excluded from this study unless there were overt extrarenal manifestations of vasculitis.
Patient Groups
Vasculitis disease activity was measured using the Birmingham Vasculitis Activity Score (BVAS) (15). Patients with a BVAS ≥1 were considered to have active disease. When possible, “active” samples were obtained at disease onset; otherwise, the sample corresponding to the highest BVAS score was used in these analyses. Samples were classified as “remission” if patients were in remission for 3 months before and after the collection date. Active versus remission samples were compared in rituximab-naive patients.
When available, blood samples were evaluated before and after rituximab treatment. We examined the last sample obtained before rituximab treatment and samples obtained after rituximab treatment in which the %CD19+ B cells were ≥1%. For post-rituximab evaluation, patients were separated into three groups. Patients whose %CD5+ B cells measured at >30% (“normal” based on the mean of healthy controls) at the time of B cell repopulation and in the samples after B cell repopulation were labeled group 1 regardless of remission maintenance therapy dose. Patients whose %CD5+ B cells measured ≤30% at the time of B cell repopulation, or decreased to ≤30% within 12 months, were subdivided based on the dose of mycophenolate mofetil (MMF) received after rituximab treatment. Patients who had low-dose MMF (≤1 g/d) were labeled group 2, whereas those maintained on higher doses of MMF (>1 g/d) after rituximab infusion were labeled group 3. Only two of our patients were taking any steroids in addition to the MMF dose stated for maintenance therapy after rituximab infusion. One of our group 2 patients was taking 100 mg/d cyclosporine and 6 mg/d prednisone instead of MMF. One of our group 3 patients (on 2 g/d of MMF) was also taking 10 mg prednisone every other day after B cell recovery through time of flare. Because there were only two patients taking prednisone as part of their maintenance therapy and this dose was quite minimal, we did not consider the prednisone dose in our division of patients with low %CD5+ B cells into low and high immunosuppression subgroups (groups 2 and 3).
We performed a sensitivity analysis by regrouping the patients based on CD5+ B cells at the time of B cell repopulation only, without considering the subsequent trend of CD5+ B cells, and then reanalyzing the data as done for the primary analysis.
Cell Preparation and Cell Surface Staining
PBMCs were purified from heparinized peripheral blood samples by centrifugation in cell preparation tubes (Becton Dickinson and Company, Franklin Lakes, NJ). Cells were washed in PBS, resuspended in Hank’s buffered salt solution (2% FCS, 0.1% sodium azide) and stained with CD19-APC (HIB19) in combination with two of the following either FITC- or PE-fluorescently labeled antibodies to CD21 (B-ly4), CD24 (ML5), CD27 (M-T271), CD38 (HIT2), CD5 (UCHT2), IgM (G20–127), or IgD (IA6–2) (BD Pharmingen, San Diego, CA). After fixation with 1% paraformaldehyde, cells were analyzed using a FACSCalibur flow cytometer. B cells were gated based on CD19+ staining. Data were analyzed with Summit (DakoCytomation, Carpinteria, CA) or FlowJo (Treestar, Ashland, OR) software.
Statistical Analyses
Mean ± SD or median and interquartile range (IQR) were used to describe demographic and clinical characteristics as appropriate. Wilcoxon rank-sum or Kruskal–Wallis tests were used to compare groups for continuous variables, and Fisher’s exact tests were used for categorical variables. We used the paired Wilcoxon signed rank test to evaluate the paired difference of B cell phenotypes in the subgroups. P values reported with a two-side P value of ≤0.05 indicate a significant difference. Analyses were conducted using SAS 9.1 software (SAS Institute, Cary, NC).
Results
The %CD5+ B Cells Is Reduced in Patients with Active Disease and Before Relapse
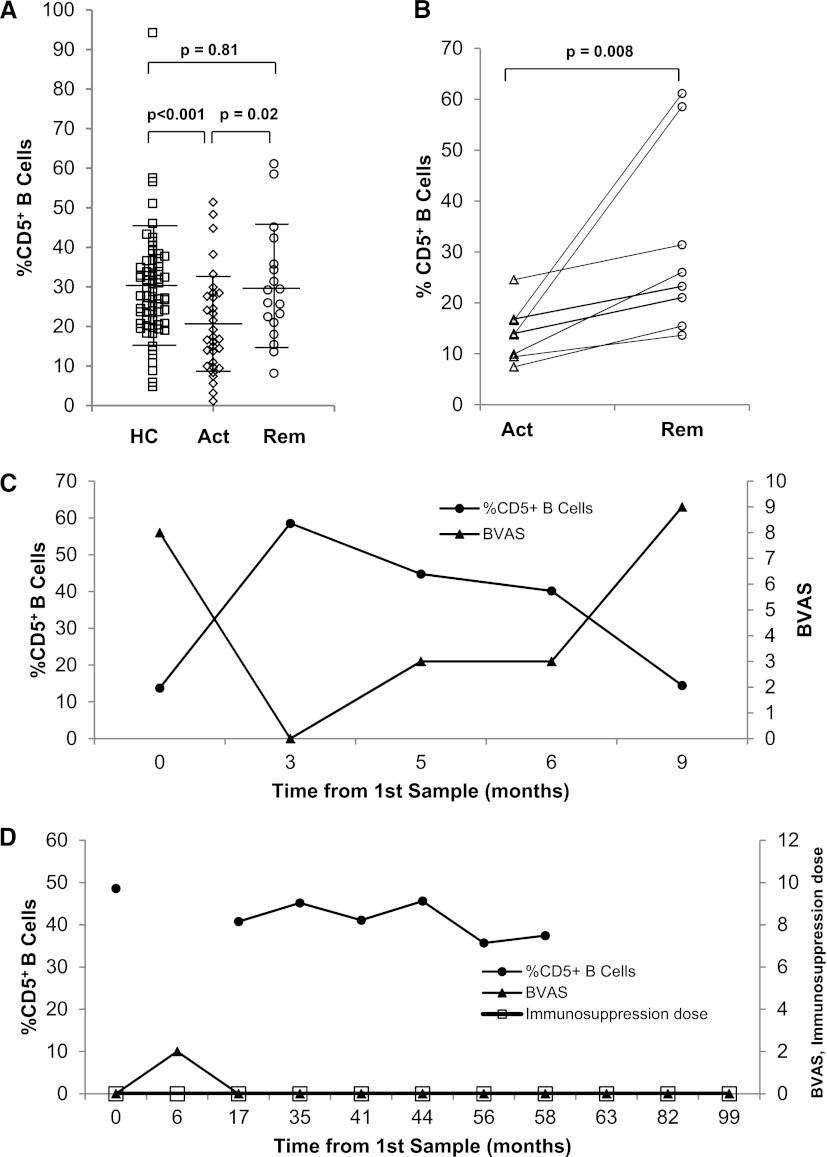
We first examined %CD5+ B cell expression in rituximab-naïve ANCA-SVV patients. Samples were evaluated at the time of either active disease (BVAS ≥1, n=24) or remission (BVAS=0, n=19) (Table 1). There were no significant differences between active disease patients compared with those in remission with respect to age, sex, ethnicity, ANCA type, disease category, organ involvement, or peak creatinine at disease onset (Table 1). Patients with active disease had significantly lower %CD5+ B cells (median 17%; IQR, 10, 28) than those in remission (26%; IQR, 21, 36; P=0.02) and healthy controls (28%; IQR, 21, 35; P<0.001) (Figure 1A, Table 1). The %CD5+ B cells during remission did not differ significantly from the percentage found in healthy controls. Although patients were significantly older than healthy controls, the %CD5+ B cells did not correlate with age in healthy controls, patients with active disease or patients in remission (data not shown). In patients for whom active and remission samples were available, the %CD5+ B cells increased from a median of 14% (IQR, 10, 17) in active disease to a median of 25% (IQR, 17, 45; P=0.008) as patients entered remission (Figure 1B). When %CD5+ B cells were compared with disease activity over time, downward trends in CD5 were associated with relapse (representative Figure 1C). An example of a patient who maintained >30% of CD5+ B cells and remained in remission without maintenance immunosuppression with a persistently high MPO-ANCA titer for 82 months is shown in Figure 1D.
Table 1.
Characteristics of patient groups and healthy controls
| Characteristic | Active n=24 | Remission n=19 | Healthy Controls n=68 | P Valuea |
|---|---|---|---|---|
| Age | 58 (48,68)c | 58 (38,66)c | 34 (25,46)b | <0.001 |
| Sex | 0.32 | |||
| Female | 11 (46%) | 13 (68%) | 41 (60%) | |
| Race | 0.76 | |||
| White | 19 (79%) | 14 (74%) | 48 (71%) | |
| ANCA | N/A | 0.07 | ||
| MPO | 8 (33%) | 12 (63%) | ||
| PR3 | 16 (67%) | 7 (37%) | ||
| Disease | N/A | 0.23 | ||
| GPA | 10 (42%) | 8 (42%) | ||
| MPA | 8 (33%) | 10 (53%) | ||
| Renal limited | 6 (25%) | 1 (5%) | ||
| Organ involvement | N/A | |||
| Upper respiratory | 12 (50%) | 14 (74%) | 0.13 | |
| Pulmonary | 14 (58%) | 11 (58%) | >0.99 | |
| Renal | 15 (94%) | 14 (93%) | >0.99 | |
| Peak serum creatinine at disease onset (mg/dl) | 3 (1,5) | 3 (1,5) | N/A | 0.97 |
| BVAS | 12 (7,16) | 0 (0,0) | N/A | <0.001 |
| %CD5+ B cells | 17 (11,28)b | 26 (21,36)c | 28 (21,35)c | 0.003 |
| ANCA titer (U/ml)d | 43 (98) | 19 (67) | N/A | 0.36 |
| MPO-ANCA titer (U/ml) | 50 (19,71) | 19 (8,37) | N/A | 0.11 |
| PR3-ANCA titer (U/ml) | 102 (50,162) | 117 (76,121) | N/A | 0.94 |
Data are summarized as n (%) or median with interquartile range. B cell data are reported as a percentage of CD19+ B cells. MPO, myeloperoxidase; PR3, proteinase 3; GPA, granulomatosis with polyangiitis; MPA, microscopic polyangiitis; BVAS, Birmingham Vasculitis Activity Score.
P values were calculated by Kruskal-Wallis test for comparison in three groups and Wilcoxon two-sample test for two groups.
Different superscript letters indicate a statistically significant difference between groups after a Bonferroni correction (P < 0.02).
ANCA titer indicates the MPO-ANCA titer for MPO-ANCA patients or the PR3-ANCA titer for PR-3 patients combined together as a group for all patients in either remission or active disease. ANCA titers were determined by the McLendon Clinical Laboratories at the University of North Carolina using ELISA kits specific for either MPO or PR3 (Inova Diagnostics, San Diego, CA). Negative titers are ≤ 20 U/ml.
Figure 1.
The %CD5+ B cells decreases in active ANCA disease and rebounds with remission. (A) Shown are the %CD5+ B cells in healthy controls (□, n=68), patients with active disease (◊, n=24), and patients in remission (○, n=19). Error bars represent the mean ± SD. The %CD5+ B cells is lower in patients with active disease (P<0.001) and returns to levels similar to healthy controls during remission of disease (P=0.81). (B) Paired active and remission samples from eight patients demonstrate the increase in %CD5+ B cells observed as an individual transitions from active disease to remission (P=0.01). (C and D) The relationship between %CD5+ B cells (●) on the left axis and BVAS (▴) on the right axis over time is depicted; the immunosuppression dose (grams per day) is indicated on the right axis (D). A reciprocal pattern of %CD5+ B cells and BVAS is observed in a patient (C) who is active at time 0, enters remission at 3 months as %CD5+ B cells reach normal levels, and then relapses at 9 months after a steady decline in %CD5+ B cells. A representative example of a patient who maintained a normal %CD5+ B cells over 82 months and remained in remission off therapy with a persistently high MPO-ANCA titer during this period is shown in D. HC, healthy controls; Act, active disease; Rem, remission; BVAS, Birmingham Vasculitis Activity Score; MPO, myeloperoxidase (MPO).
B Cell Phenotypes after Rituximab Therapy
To further elucidate the relationship between %CD5+ B cells and disease activity, we studied a subset of 19 patients who received rituximab (Table 2). The %CD5+ B cells were measured following B cell repopulation after rituximab. Group 1 (patients who repopulated with >30% CD5+ B cells) was diverse with regard to MMF dose; there were three patients on no immunosuppression, two patients on low immunosuppression, and two patients on high immunosuppression with a mean dose of 0.75 ±0.8 g/d (Table 2). By definition, patients who repopulated with ≤30% CD5+ B cells and were maintained on ≤1 g/d MMF (group 2) were prescribed 75% less MMF (mean 0.43±0.5 g/d) than group 3 patients who also repopulated with ≤30% CD5+ B cells but were maintained on >1 g/d MMF (mean 1.95±0.7 g/d) (P=0.01). On average, group 2 and group 1 were similar with regard to immunosuppression dose (P=0.4). All patients treated with oral remission maintenance therapy after rituximab infusion were prescribed MMF with the exception of two patients who received low-dose cyclosporine (<1 mg/kg per day) in addition to MMF. Patient characteristics were similar across the three groups (Table 2).
Table 2.
Comparison of patient groups after treatment with rituximab
| Characteristic | Group 1 (n=7) | Group 2 (n=7) | Group 3 (n=5) | P Valuec |
| Repopulation with normal %CD5+ B cells | Repopulation with low % CD5+ B cells (≤30%), low remission maintenance medicationa | Repopulation with low %CD5+ cells (≤30%), high remission maintenance medicationb | ||
| Age | 59 (32,61) | 52 (45,59) | 51 (38,58) | 0.93 |
| Sex | 0.03 | |||
| Female | 1 (14%) | 6 (86%) | 2 (40%) | |
| Race | 0.80 | |||
| White | 6 (86%) | 6 (86%) | 4 (80) | |
| ANCA | 0.18 | |||
| MPO | 3 (43%) | 0 (0%) | 2 (40%) | |
| PR3 | 4 (57%) | 6 (86%) | 3 (60%) | |
| PR3 and MPO | 0 (0%) | 1 (14%) | 0 (0%) | |
| Disease | 0.70 | |||
| GPA | 5 (71%) | 4 (57%) | 4 (80%) | |
| MPA | 1 (14%) | 3 (43%) | 1 (20%) | |
| ANCA GN (renal limited) | 1 (14%) | 0 (0%) | 0 (0%) | |
| Organ involvement | ||||
| Upper Respiratory | 4 (57%) | 5 (72%) | 5 (100%) | 0.35 |
| Pulmonary | 5 (71%) | 7 (100%) | 3 (60%) | 0.25 |
| Renal | 5 (83%) | 4 (100) | 4 (80%) | >0.99 |
| Peak serum creatinine at disease onset (mg/dl) | 1.7 (1.0,2.9) | 2.5 (1.8,2.9) | 1.6 (1.2,1.8) | 0.28 |
| %CD5+ B cells at time of B cell repopulation | 57 (48,70)d | 18 (11,31)e | 23 (13,53)d,e | 0.02 |
| %CD5+ B cells at last sample available prior to flare | 34 (27,41) | 16 (15,18) | 4 (4,16) | 0.06 |
| Dose of MMF for remission maintenance (g/day) | 1.00 (0,1.25)d,e | 0 (0,1.0)d | 2.0 (1.5,2.0)e | 0.007 |
| Time to relapse from rituximab (months) | 31 (25,48)d | 17 (12,20)e | 29 (29,35)d | 0.002 |
| Time to relapse from B cell repopulation (months) | 22 (17,36)d | 7 (3,11)e | 22 (20,27)d | 0.002 |
| Total B cell number (x104/ml blood) | 5.7 (2.4,15.0) | 1.7 (1.0,3.2) | 3.9 (2.4,4.5) | 0.15 |
| ANCA titer (U/ml)f | 39 (10,95) | 52 (5,71) | 8 (6,24) | 0.28 |
Values for variables examined in patient groups after rituximab therapy are reported as n (%) or median (interquartile range). MPO, myeloperoxidase; PR3, proteinase 3; GPA, granulomatosis with polyangiitis; MPA, microscopic polyangiitis; MMF, mycophenolate mofetil.
One group 2 patient was on cyclosporine (50 mg twice daily) and prednisone (6 mg/day).
One group 3 patient was on MMF (1 g twice daily) and prednisone (10 mg every other day) after rituximab therapy concurrent with seven monthly intravenous doses of cyclophosphamide.
P values were calculated by Fisher exact test for categorical variables and Kruskal-Walls Test for continuous variables.
Different superscript letters indicate a statistically significant difference between groups after a Bonferroni correction (P <0.02).
ANCA titer indicates the MPO-ANCA titer for MPO-ANCA patients or the PR3-ANCA titer for PR-3 patients combined together as a group for all patients in either group 1, 2 or 3. ANCA titers were determined by the McLendon Clinical Laboratories at the University of North Carolina using ELISA kits specific for either MPO or PR3 (Inova Diagnostics, San Diego, CA). Negative titers are ≤ 20 U/ml.
Group 1 had a significantly higher %CD5+ B cells (median 57%; IQR, 48, 70) at the time of B cell repopulation than group 2 (18%; IQR, 11, 31; P=0.003) (Table 2). Group 3 had a similarly low %CD5+ B cells (23%; IQR 13, 53) but did not reach statistical significance due to the small number of patients. The median %CD5+ B cells at the last sample available before flare for group 1 was 34% CD5+ B cells (IQR, 27, 41). The median %CD5+ B cells at the time proximal to flare was 16% (IQR, 15,18) and 4% (IQR, 4,16) for groups 2 and 3, respectively. Time to relapse after rituximab infusion was significantly shorter when CD5 was ≤30% at the time of B cell repopulation (group 2) (P=0.002; Table 2). In patients who had CD5 levels >30% at the time of B cell repopulation and remained >30% for all subsequent samples evaluated (group 1), but similarly low levels of oral remission maintenance therapy, time to flare was 18 months longer on average than group 2 (Table 2). Group 3 patients had similarly low CD5 levels to group 2 patients (P=0.52), but were maintained on significantly higher doses of MMF (P=0.01). Their time to flare after rituximab infusion was on average 20 months longer than group 2 (P=0.01; Table 2). Time to flare from B cell repopulation was also significantly different between group 2 patients and either patients whose %CD5+ B cells remained >30% after B cell repopulation maintained on similarly low remission maintenance therapy (P=0.002, group 1) or when oral remission maintenance therapy was maintained at significantly higher doses (group 3) (P=0.01, Table 2).
Sensitivity Analyses
Sensitivity analyses were performed to evaluate whether the association between %CD5+ B cells and time to relapse held up after regrouping patients based strictly on %CD5+ B cells at the time of B cell recovery (Supplemental Table 1). Patients having >30% CD5+ B cells at the time of B cell repopulation became group 1S (n=10) regardless of whether the %CD5+ decreased below 30% subsequently. Group 2S (n=6) had ≤30% CD5+ B cells at B cell repopulation and were on ≤1 g of MMF daily (mean 0.33±0.5 g/d). Group 3S (n=3) had ≤30% CD5+ B cells at B cell repopulation and were on >1 g of MMF daily (mean 2±1 g/d) (P=0.04 compared with group 2S). By definition, group 1S repopulated with higher %CD5+ B cells (median 55%; IQR, 48, 70) compared with both group 2S (17%; IQR, 11, 30) and group 3S (13%; IQR, 12, 23) (P=0.001) after rituximab. The time to flare after rituximab therapy for group 2S was significantly shorter (median 16 months; IQR, 12, 18) compared with both group 1S (28 months; IQR, 25, 34) and group 3S (35 months; IQR, 29, 65) (P=0.002). The %CD5+ B cells at the time of documented flare did not differ for group 1S and group 2S (median 26% and 16%, respectively; P=0.18), whereas the %CD5+ B cells were lower when relapses occurred in group 3S (4%; P=0.05).
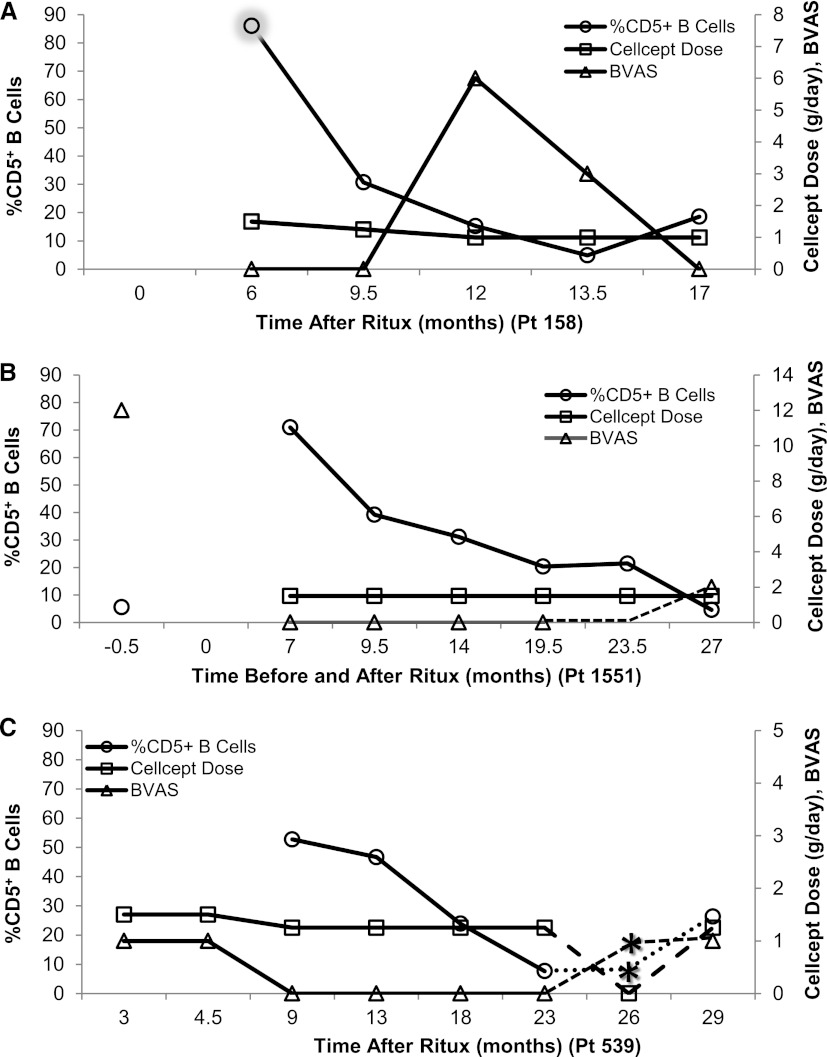
To evaluate %CD5+ B cells with respect to clinical disease activity in patients treated with rituximab, %CD5+ B cells were plotted against BVAS and MMF dose. Three examples depict the consistent decline in %CD5+ B cells that we observed before disease relapse (Figure 2, A–C). Time to relapse appears delayed if higher levels of remission maintenance therapy were given when CD5 levels were <30%.
Figure 2.
Decrease in %CD5+ B cells is associated with an increase in disease activity. Examples of the longitudinal relationship between %CD5+ B cells (○) on the left axis compared with BVAS (∆) and CellCept (MMF) dose (□) on the right axis over time before and/or after rituximab are depicted (A– C). (A) Patient 158 (group 2), who had 85% CD5+ B cells before full B cell recovery (<1% B cells at 6 m, shadowed circle), showed a precipitous drop in CD5 during the next 3–6 months after B cell recovery. Because this patient appeared to be in clinical remission, the CellCept dose was decreased during this time period and the patient flared 12 months after rituximab treatment. (B) Patient 1551 (group 3) had a BVAS of 12 and 5.6% CD5+ B cells before rituximab treatment. Although the %CD5+ cells is initially normal at B cell repopulation, it steadily declines over the next 2.5–20 months without overt clinical activity in the context of high immunosuppression until month 27. (C) Another group 3 patient, 539, had a decrease in %CD5+ cells from 9 to 23 months after rituximab therapy with “no signs of active disease” at months 18 and 23. Upon self-discontinuation of CellCept while %CD5+ B cells were below normal, the patient flared before the clinic visit at 29 months. The %CD5+ B cells, BVAS, and CellCept dose during the time period between 23 and 29 months are depicted by dashed lines to indicate inferred information. The %CD5+ B cells are assumed to be the same as the previous sample; the BVAS is assumed to be at least equal to the subsequent sample. Asterisks indicate the approximate time of flare gleaned from clinic notes for this time period. BVAS, Birmingham Vasculitis Activity Score; MMF, mycophenolate mofetil.
Other B cell populations—including naïve, switched, and nonswitched memory, IgD,CD27-double negative, and pre-germinal center founder (Bm2’3δ) cells—are different in ANCA patients compared with controls but do not correlate with disease activity (Supplemental Table 2). CD21 differs between active disease and remission (P<0.001) but is not clearly associated with time to relapse.
CD5 as a Surrogate Marker for Putative B Regulatory Cells
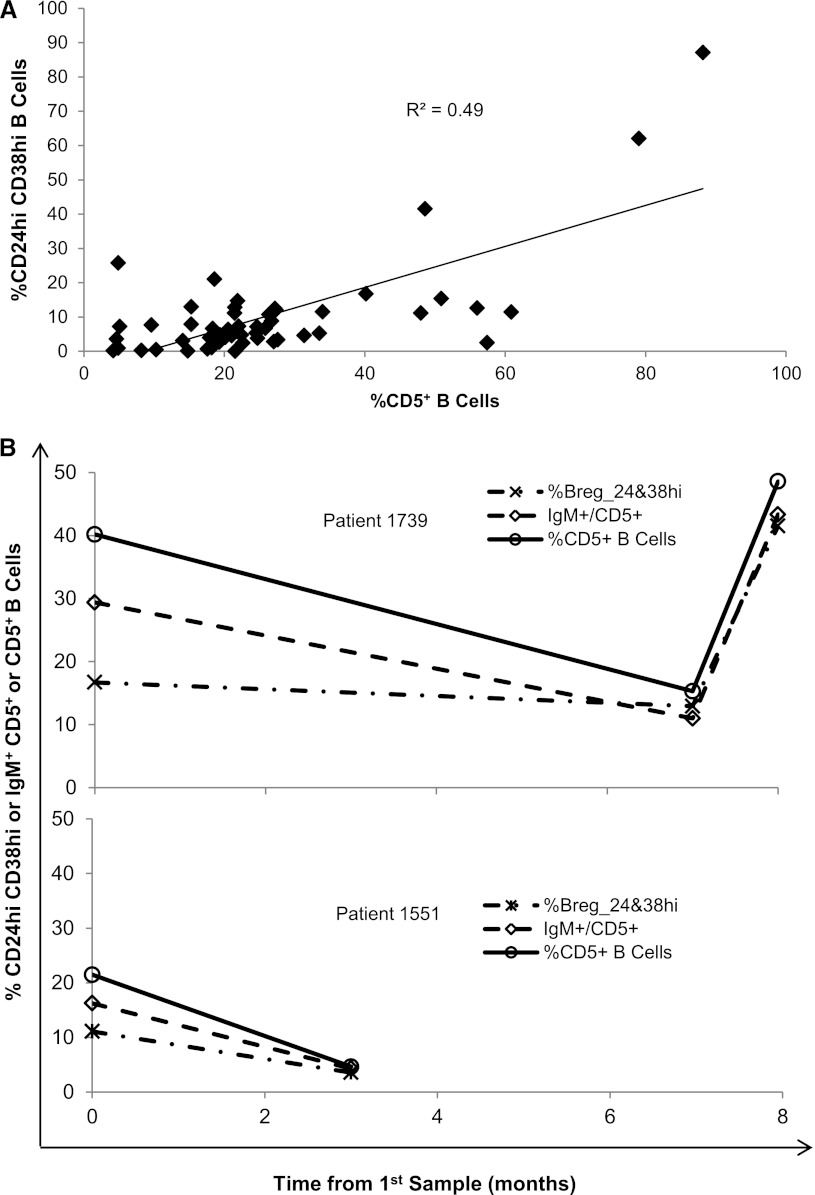
Because of its role as a negative regulator of B cell receptor signaling and its inclusion in the immunophenotype reported for Breg cells, we compared CD5+ B cells with other phenotypes reported for Breg cells. CD5+ B cells correlate well with the CD24hiCD38hi population of Breg cells that has been shown to secrete IL-10 (R2=0.50; Figure 3A) in all samples for which both stains were available (21 healthy controls, 17 with active disease, 13 in remission). When flow cytometric data were available for all three stain sets (CD24hiCD38hi, IgM+CD5+, and CD5+) these B cell populations correlated well over time (representative patients shown in Figure 3B).
Figure 3.
The %CD5+ B cells reflect putative B regulatory cells. (A) The %CD5+ B cells correlates with the percentage of B regulatory cells identified as CD24hiCD38hi B cells (R2=0.50). This correlation includes all samples for which both CD19+CD5+ and CD19+CD24hiCD38hi data were available (21 healthy controls, 17 with active disease, 13 in remission). (B) The correlation between percentages of CD24hiCD38hi (dash-dot line), IgM+CD5+ (dash-dash line), and CD5+ B cells (solid line) are shown for two representative patients for whom all three stain sets were available.
Discussion
The last 2 decades have witnessed a marked improvement in the induction treatment of patients with ANCA vasculitis, with remission rates around 80% (16–18). A major remaining challenge in the long-term management of patients pertains to the prevention and treatment of relapses. The risk of relapse is not uniform for all patients with ANCA vasculitis. PR3-ANCA (compared with MPO-ANCA), lung disease, upper respiratory tract disease, a clinical diagnosis of granulomatosis with polyangiitis (compared with microscopic polyangiitis or renal limited disease), cardiovascular involvement, and a lack of renal impairment (creatinine <200 µmol/L) have been reported as risk factors for relapse (19–21). Nevertheless, no clinical or serologic measure is currently available that allows effective disease monitoring and distinguishes patients in long-term stable remission from those at imminent risk of relapse (22–26). Such a tool would allow physicians to better tailor the duration and intensity of immunosuppressive therapy based on the individual patient’s needs. Our goal was to evaluate whether certain B cell subpopulations could be used to assess immunologic disease activity or a patient’s risk of relapse. Although limited to a small number of patients, we determined that a low percentage (≤30%) of circulating CD5+B cells correlates with disease activity and a shorter time to relapse. Patients in remission had %CD5+ B cells similar to healthy controls and significantly higher than patients with active disease. After rituximab therapy, low or declining %CD5+ B cells was associated with a shorter time to disease relapse among patients on no or low-dose maintenance therapy with MMF. The use of full-dose MMF was associated with a longer time to relapse in the setting of a low %CD5+ B cells. Additional data will be required to definitively address the correlation of %CD5+B cells with sustained remission.
If our findings are confirmed in a larger population, then the clinical implications of our results may pertain to the decision to use maintenance immunosuppression after rituximab therapy and its timing. Our data suggest that patients whose %CD5+ B cells remain low or decline after a period of normalization after rituximab therapy would be at higher risk of subsequent relapse and would likely benefit from maintenance immunosuppression. Conversely, such immunotherapy could be avoided in patients who maintain a normal %CD5+ B cells.
Our results are consistent with current knowledge of B cell subtypes and function. Breg cells, defined by their ability to suppress INF-γ and TNF-α expression in T cells via expression of IL-10, have been described as having a CD24hiCD38hi phenotype (13). Breg cells were also reported to be CD5+IgM+/hiIgD+/hiCD10low/+CD27negCD1dhi, although consensus on their immunophenotype is not yet fully established (14). We propose that the CD5 marker is an acceptable measure of Breg cells based on our data demonstrating a high correlation with CD24hiCD38hi and IgM+CD5+ subpopulations. CD5 is reported to induce IL-10 expression and promote cell survival in human B cells (27), human chronic lymphocytic leukemia B cells (28), and mice (29). In mice, CD5+CD1d+ B cells secrete IL-10 and have a regulatory function evidenced by their inhibition of INF-γ and TNF-α expression in T cells (30). Our results add to accumulating evidence that a paucity of, or nonfunctional, Breg cells are associated with increased disease activity in autoimmune disease (13,14,31). Years ago, when dogma was that CD5+ B cells were increased in autoimmune disease (32), patients with active Kawasaki disease were reported to have a decreased %CD5+ B cells (33). These findings, as well as our results, raise the possibility that a robust Breg subpopulation could be a goal of immunotherapy, as well as a means of monitoring its efficacy. This hypothesis would best be tested prospectively as part of a clinical trial.
Other B cell populations—including naïve, switched, and nonswitched memory, IgD,CD27-double negative, and pre-germinal center founder (Bm2’3δ) cells—have been reported to correlate with response to rituximab therapy in SLE and rheumatoid arthritis (34–36). Neither these B cell populations nor ANCA titer correlated with disease activity or time to flare after rituximab therapy in our patient cohort.
Patients in our study were treated with rituximab for induction of remission after a clinical relapse (to avoid repeat exposure to cyclophosphamide) or because of persistent disease activity despite cyclophosphamide and corticosteroids. Although B cell phenotype data emanate from rituximab-treated patients, they may not be restricted to this form of therapy. Indeed, treatment with cyclophosphamide results in peripheral B cell depletion, albeit more slowly and to a lesser magnitude than with rituximab (6). Studies are ongoing to assess whether similar effects on the CD5+ B cell subpopulation are detectable with cyclophosphamide-based therapies.
The optimal choice and duration of maintenance therapy is the subject of current clinical investigations. In this study, the choice of MMF as maintenance therapy after rituximab was not predetermined by protocol, and antedates the published results on the International Mycophenolate Mofetil Protocol to Reduce Outbreaks of Vasculitides study in which azathioprine was associated with a statistically significant decrease in the rate of relapses compared with MMF (24). The demonstrated efficacy of rituximab in treating active ANCA-SVV has raised the question as to its possible role in maintenance therapy, given at regular intervals regardless of clinical signs of disease activity (37). It will be interesting to test the validity of our hypothesis in a setting where a robust CD5+ Breg population may be suppressed by a regimen of prolonged B cell depletion. It is possible that a state of immune tolerance may require the presence of robust Breg and/or Treg populations, which would be prevented by sustained B cell depletion.
There are limitations to our study. The relatively small sample size of patients with longitudinal data limits our ability to evaluate the correlation between %CD5+ B cells and time to relapse while correcting for other risk factors such as PR3-ANCA, organ involvement, or disease phenotype. Although we attempted to obtain patient samples every 3 months, the timing of our blood collections was not standardized. Samples were obtained from patients whenever they presented for care.
A future research direction will be to validate our findings in a larger cohort of patients treated with either rituximab or cyclophosphamide-based regimens, while formally assessing the time to relapse from the time of decline in %CD5+ B cells. We aim to study the relationship between CD5 levels, IL-10–expressing Breg cells, and disease activity in ANCA-SVV. The expression of an alternatively spliced variant of CD5 resulting in reduced membrane expression of CD5 through methylation changes driven by IL-6 was recently described (38), which may regulate the function of Breg cells. The effect, if any, of the CD5 splice variant on disease activity or response to therapy will be interesting to evaluate.
In summary, we identified a CD5+ B cell subpopulation as a potential immunologic marker of sustained remission when robust, or a harbinger of subsequent relapse when low or declining. These findings may offer a clinical tool to monitor disease activity and modulate maintenance immunotherapy.
Disclosures
None.
Supplementary Material
Acknowledgments
We appreciate the help of the following past members of the UNC Kidney Center: Pamela Sullivan and Julie Hamra for help in consenting patients and obtaining blood samples and Joe Piscitello for technical assistance. We thank Ahinee Amamoo and Hyunsook Chin for preliminary statistical analysis and Carmen Mendoza for help with figure preparation. This work was supported by Program Project Grant P01-DK5-30834 from the National Institute for Diabetes and Digestive and Kidney Diseases of the National Institutes of Health and the Vasculitis Foundation.
Portions of this work were previously presented in poster form at the 2007 and 2008 annual meetings of the American Society of Nephrology, held November 2–5, 2007, in San Francisco, California, and November 6–9, 2008, in Philadelphia, Pennsylvania, respectively.
Footnotes
Present address for Dr. Nirmal B. Khandoobhai: Wake Forest Baptist Health, Winston Salem, North Carolina.
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03950412/-/DCSupplemental.
References
- 1.Falk RJ, Jennette JC: Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med 318: 1651–1657, 1988 [DOI] [PubMed] [Google Scholar]
- 2.Jennette JC, Falk RJ: Small-vessel vasculitis. N Engl J Med 337: 1512–1523, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y, Maeda N, Falk RJ, Jennette JC: Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest 110: 955–963, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Little MA, Al-Ani B, Ren S, Al-Nuaimi H, Leite M, Jr, Alpers CE, Savage CO, Duffield JS: Anti-proteinase 3 anti-neutrophil cytoplasm autoantibodies recapitulate systemic vasculitis in mice with a humanized immune system. PLoS ONE 7: e28626, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McQueen F: A B cell explanation for autoimmune disease: The forbidden clone returns. Postgrad Med J 88: 226–233, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, Kallenberg CG, St Clair EW, Turkiewicz A, Tchao NK, Webber L, Ding L, Sejismundo LP, Mieras K, Weitzenkamp D, Ikle D, Seyfert-Margolis V, Mueller M, Brunetta P, Allen NB, Fervenza FC, Geetha D, Keogh KA, Kissin EY, Monach PA, Peikert T, Stegeman C, Ytterberg SR, Specks U, RAVE-ITN Research Group : Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 363: 221–232, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones RB, Tervaert JW, Hauser T, Luqmani R, Morgan MD, Peh CA, Savage CO, Segelmark M, Tesar V, van Paassen P, Walsh D, Walsh M, Westman K, Jayne DR, European Vasculitis Study Group : Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 363: 211–220, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Berland R, Wortis HH: Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol 20: 253–300, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Soldevila G, Raman C, Lozano F: The immunomodulatory properties of the CD5 lymphocyte receptor in health and disease. Curr Opin Immunol 23: 310–318, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Youinou P, Renaudineau Y: The paradox of CD5-expressing B cells in systemic lupus erythematosus. Autoimmun Rev 7: 149–154, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Youinou P, Renaudineau Y: The antiphospholipid syndrome as a model for B cell-induced autoimmune diseases. Thromb Res 114: 363–369, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Hippen KL, Tze LE, Behrens TW: CD5 maintains tolerance in anergic B cells. J Exp Med 191: 883–890, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blair PA, Noreña LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C: CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity 32: 129–140, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM, Williams AD, Hall RP, St Clair EW, Tedder TF: Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 117: 530–541, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luqmani RA, Bacon PA, Moots RJ, Janssen BA, Pall A, Emery P, Savage C, Adu D: Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM 87: 671–678, 1994 [PubMed] [Google Scholar]
- 16.Novack SN, Pearson CM: Cyclophosphamide therapy in Wegener’s granulomatosis. N Engl J Med 284: 938–942, 1971 [DOI] [PubMed] [Google Scholar]
- 17.Nachman PH, Hogan SL, Jennette JC, Falk RJ: Treatment response and relapse in antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol 7: 33–39, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Holle JU, Gross WL, Latza U, Nölle B, Ambrosch P, Heller M, Fertmann R, Reinhold-Keller E: Improved outcome in 445 patients with Wegener’s granulomatosis in a German vasculitis center over four decades. Arthritis Rheum 63: 257–266, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Pagnoux C, Hogan SL, Chin H, Jennette JC, Falk RJ, Guillevin L, Nachman PH: Predictors of treatment resistance and relapse in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis: Comparison of two independent cohorts. Arthritis Rheum 58: 2908–2918, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierrot-Deseilligny Despujol C, Pouchot J, Pagnoux C, Coste J, Guillevin L: Predictors at diagnosis of a first Wegener’s granulomatosis relapse after obtaining complete remission. Rheumatology (Oxford) 49: 2181–2190, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Walsh M, Flossmann O, Berden A, Westman K, Höglund P, Stegeman C, Jayne D, European Vasculitis Study Group : Risk factors for relapse of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 64: 542–548, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Tomasson G, Grayson PC, Mahr AD, Lavalley M, Merkel PA: Value of ANCA measurements during remission to predict a relapse of ANCA-associated vasculitis—a meta-analysis. Rheumatology (Oxford) 51: 100–109, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kälsch AI, Csernok E, Münch D, Birck R, Yard BA, Gross W, Kälsch T, Schmitt WH: Use of highly sensitive C-reactive protein for followup of Wegener’s granulomatosis. J Rheumatol 37: 2319–2325, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Finkielman JD, Merkel PA, Schroeder D, Hoffman GS, Spiera R, St Clair EW, Davis JC, Jr, McCune WJ, Lears AK, Ytterberg SR, Hummel AM, Viss MA, Peikert T, Stone JH, Specks U, WGET Research Group : Antiproteinase 3 antineutrophil cytoplasmic antibodies and disease activity in Wegener granulomatosis. Ann Intern Med 147: 611–619, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Monach PA, Tomasson G, Specks U, Stone JH, Cuthbertson D, Krischer J, Ding L, Fervenza FC, Fessler BJ, Hoffman GS, Ikle D, Kallenberg CG, Langford CA, Mueller M, Seo P, St Clair EW, Spiera R, Tchao N, Ytterberg SR, Gu YZ, Snyder RD, Merkel PA: Circulating markers of vascular injury and angiogenesis in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 63: 3988–3997, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomasson G, Lavalley M, Tanriverdi K, Finkielman JD, Davis JC, Jr, Hoffman GS, McCune WJ, St Clair EW, Specks U, Spiera R, Stone JH, Freedman JE, Merkel PA, Wegener’s Granulomatosis Etanercept Trial (WGET) Research Group : Relationship between markers of platelet activation and inflammation with disease activity in Wegener’s granulomatosis. J Rheumatol 38: 1048–1054, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gary-Gouy H, Harriague J, Bismuth G, Platzer C, Schmitt C, Dalloul AH: Human CD5 promotes B-cell survival through stimulation of autocrine IL-10 production. Blood 100: 4537–4543, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Garaud S, Morva A, Lemoine S, Hillion S, Bordron A, Pers JO, Berthou C, Mageed RA, Renaudineau Y, Youinou P: CD5 promotes IL-10 production in chronic lymphocytic leukemia B cells through STAT3 and NFAT2 activation. J Immunol 186: 4835–4844, 2011 [DOI] [PubMed] [Google Scholar]
- 29.O’Garra A, Chang R, Go N, Hastings R, Haughton G, Howard M: Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur J Immunol 22: 711–717, 1992 [DOI] [PubMed] [Google Scholar]
- 30.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF: A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 28: 639–650, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Knippenberg S, Peelen E, Smolders J, Thewissen M, Menheere P, Cohen Tervaert JW, Hupperts R, Damoiseaux J: Reduction in IL-10 producing B cells (Breg) in multiple sclerosis is accompanied by a reduced naïve/memory Breg ratio during a relapse but not in remission. J Neuroimmunol 239: 80–86, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Youinou P, Mackenzie LE, Lamour A, Mageed RA, Lydyard PM: Human CD5-positive B cells in lymphoid malignancy and connective tissue diseases. Eur J Clin Invest 23: 139–150, 1993 [DOI] [PubMed] [Google Scholar]
- 33.Kim HS, Noh GW, Kim DS, Lee KY, Lee HS, Lee HK, Lee SI: Decreased CD5+ B cells during the acute phase of Kawasaki disease. Yonsei Med J 37: 52–58, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Anolik JH, Friedberg JW, Zheng B, Barnard J, Owen T, Cushing E, Kelly J, Milner EC, Fisher RI, Sanz I: B cell reconstitution after rituximab treatment of lymphoma recapitulates B cell ontogeny. Clin Immunol 122: 139–145, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JC: Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum 54: 613–620, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Roll P, Dörner T, Tony HP: Anti-CD20 therapy in patients with rheumatoid arthritis: Predictors of response and B cell subset regeneration after repeated treatment. Arthritis Rheum 58: 1566–1575, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Rhee EP, Laliberte KA, Niles JL: Rituximab as maintenance therapy for anti-neutrophil cytoplasmic antibody-associated vasculitis. Clin J Am Soc Nephrol 5: 1394–1400, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garaud S, Le Dantec C, de Mendoza AR, Mageed RA, Youinou P, Renaudineau Y: IL-10 production by B cells expressing CD5 with the alternative exon 1B. Ann N Y Acad Sci 1173: 280–285, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.