Summary
Background and objectives
Adiponectin (ADPN), an adipose tissue–derived hormone, has protective properties with respect to atherogenesis, inflammation, and energy homeostasis. Its beneficial role has not been consistent in patients with CKD or those undergoing dialysis.
Design, setting, participants, & measurements
This study examined the association of plasma ADPN levels in 987 prevalent kidney transplant recipients (mean age ± SD, 51.0±12.8 years; estimated GFR, 52.8±21.9 ml/min per 1.73 m2; median time since transplant, 78 months) on all-cause mortality and death-censored graft failure. Patients were enrolled between February and August 2007 and were followed for a median of 51 months (interquartile range, 49–53 months). Using Cox proportional hazard models, the association of log-transformed plasma adiponectin was studied, with and without adjustment for demographic variables, baseline GFR, markers of inflammation, and cardiovascular risk factors.
Results
At baseline, patients in the lowest ADPN tertile were significantly more likely to be male; to be smokers; to have a higher baseline GFR, lower systolic BP, and lower HDL cholesterol level; and to have higher body mass index, abdominal circumference, C-reactive protein level, and total cholesterol level. The adjusted hazard ratio for death with elevated plasma ADPN (per natural log) was 1.44, and there was no significant interaction with any relevant cardiovascular risk subgroups (i.e., advanced age; diabetes; or elevated body mass index, waist circumference, C-reactive protein, or Framingham risk score). The hazard for death-censored graft failure was nonsignificant at 1.03.
Conclusion
Elevated ADPN levels are associated with higher risk for death but not allograft failure in prevalent kidney transplant recipients.
Introduction
Cardiovascular disease is a major cause of death in patients after kidney transplantation. The role of nontraditional risk factors in kidney transplant recipients is not well known. Adiponectin (ADPN) is a 30-kDa adipose tissue–derived hormone that has been linked to antiinflammatory and antiatherogenic properties, and it plays an essential role in energy homeostasis (1). Serum levels of ADPN are inversely related to adipose mass, with lower levels present in obese individuals. In the general population, elevated ADPN levels are associated with cardioprotective and antiarteriosclerotic properties. Prospective studies in the general population have demonstrated that higher ADPN levels are associated with a lower risk for myocardial infarction in men, including those with diabetes (2,3).
This association has not been consistent in populations with CKD. Renal clearance is an important factor in determining serum ADPN levels, and those with CKD or ESRD exhibit markedly higher levels of ADPN. Paradoxically, elevated ADPN levels are associated with increased cardiovascular disease and mortality in patients with CKD and those undergoing hemodialysis (4–6). The high levels of ADPN in those receiving hemodialysis decline after kidney transplantation, but they do not return to normal levels (7).
Whether ADPN is associated with a higher risk of death in patients after kidney transplantation remains poorly understood. We tested the hypothesis that restoring kidney function by kidney transplantation would reestablish the protective role of ADPN. We examine the association of elevated ADPN levels on all-cause mortality and death-censored graft survival in a stable, prevalent kidney transplant population.
Materials and Methods
Study Design and Population
All prevalent kidney transplant recipients 18 years of age or older followed at a single transplant clinic at Semmelweis University in Budapest, Hungary, were invited to participate in this observational, cohort study. Exclusion criteria were acute rejection within the last 4 weeks, current hospitalization, transplantation in the previous 3 months, acute infection, or active bleeding. Of the 993 patients enrolled, 987 (99%) had baseline blood samples that were available for ADPN assay. All patients were recruited between February 2007 and August 2007, and the study methods have been previously described (8).
The study was approved by the institutional ethics committee, and all patients provided written and verbal informed consent.
Exposure and Outcomes
All laboratory data were measured at the baseline visit in a fasting state and included blood hemoglobin, serum C-reactive protein (CRP) and creatinine, and serum albumin levels. Serum samples were also collected at the time of the baseline assessment and stored at −70°C for future use. Serum ADPN concentration was measured using immunoassay kits based on solid-phase sandwich ELISA (R&D Systems, Minneapolis, MN).
Data regarding demographic and medical history, including age, sex, cause of CKD, and comorbid conditions, were collected at baseline. Transplant-related data included immunosuppressive treatment, time elapsed since the date of transplantation, dialysis vintage (length of time on dialysis), type of allograft (deceased or live donor), history of acute rejection(s) after transplantation, HLA mismatch, panel reactive antibody titer, cold ischemia time, donor age and sex, and history of delayed graft function.
BP measurements were recorded as the average of three readings during the same clinic visit. Serum creatinine was measured annually for 2 years after study enrollment. Estimated GFR (eGFR) was calculated using the CKD-Epidemiology study equation.
The modified Charlson Comorbidity Index (CCI) score was also determined for each patient. Previous studies have shown that CCI score predicts poor survival in kidney transplant recipients. The modified CCI is a weighted scoring system based on the presence or absence of each of 17 variables. Because one of the variables is the presence of moderate-to-severe renal disease, the modified CCI score in our cohort ranged from 2 to a possible maximum of 33. Classification of the metabolic syndrome was defined according to the 2004 National Heart, Lung, and Blood Institute/American Heart Association Conference Proceedings (9). A malnutrition-inflammation (MIS) was calculated for each patient on the basis of a previously published MIS for patients undergoing maintenance hemodialysis. The MIS has 10 components, each with 4 levels of severity ranging from 0 (normal) to 3 (severely abnormal). The sum of all 10 MIS components ranges from 0 (normal) to 30 (severely malnourished). The components of the MIS were related to the patients’ medical history (change in weight in the last 3–6 months, dietary intake, gastrointestinal symptoms, and functional capacity), physical examination according to Subjective Global Nutritional Assessment criteria (decreased fat stores and loss of subcutaneous fat, signs of muscle wasting), body mass index (BMI), and laboratory measures (albumin and total iron-binding capacity). In contrast to the original MIS, we did not include dialysis vintage in the score; rather, we modeled this variable independently (8,10).
Standard maintenance immunosuppressive therapy at our institution consisted of prednisolone, with cyclosporine A microemulsion formulation or tacrolimus, combined with mycophenolate mofetil or azathioprine or sirolimus.
The first outcome of interest was all-cause mortality, which included all deaths with a functioning graft and deaths occurring after a return to dialysis. Deaths were verified with data from the Hungarian Central Office of Administrative and Electronic Public Services, the government agency maintaining official vital status records. The cause of death was not available for this study. A secondary outcome was death-censored graft failure. This outcome was classified as the need for long-term dialysis, and the time of initiation of dialysis was deemed to be the time of graft failure.
Statistical Analyses
We used descriptive statistics to compare clinical and biochemical characteristics across tertiles of baseline serum ADPN. Data were summarized using proportions, means ± SD or medians (interquartile range) as appropriate. Categorical variables were analyzed with chi-squared test, and continuous variables were compared using t test or the Mann-Whitney U test, Kruskal-Wallis test, or ANOVA as appropriate. Two-sided tests were used and the threshold for statistical significance was P=0.05.
Serum levels of ADPN were nonnormally distributed in the population; therefore, natural log-transformed ADPN levels were used in all analyses. The association between baseline ADPN levels and all-cause mortality or death-censored graft failure was assessed using Cox proportional regression analysis. Hazard ratios were expressed per each natural log of ADPN level as well as by ADPN tertile, with the lowest tertile as the reference range. We examined for any violations of the proportional hazards assumption using Schoenfeld residuals. Patients were censored at the time of death or at the end of the follow-up period (July 31, 2011). We used a complete-case analysis approach because there was less than 1% missing data for any variable at baseline.
In univariate analyses, we looked at the association of ADPN (log-transformed) with mortality across various subgroups (age, sex, diabetes, time since kidney transplantation, eGFR categories, higher BMI and waist circumference groups, and higher CRP level and Framingham risk score). We then developed multivariable Cox proportional hazard models initially adjusting for age and sex; followed by time-varying eGFR, serum albumin, and CRP; and finally a fully adjusted model that included the CCI score, MIS, abdominal circumference, baseline systolic BP, and time since kidney transplantation.
We also examined the possible nonlinear relationship between serum ADPN and the hazard ratio of mortality nonparametrically with restricted cubic splines (11). This model also adjusted for all the same covariates listed above for our standard Cox model. Tests for nonlinearity used the likelihood ratio test, comparing the model with only the linear term to the model with the linear and the cubic spline terms. Because our study involved a prevalent kidney transplant population, we also performed a left-truncated analysis as a sensitivity analysis.
To address the issue of changing renal function being associated with mortality, we used Cox models using time-updated eGFR in our primary analysis. We also examined the association of ADPN with death with a functioning graft (see Supplementary Appendix). This outcome censors for graft failure, which can be considered a proxy for declining kidney function. Finally, we used PROC GLM to examine the between- and within-group effects of eGFR by ADPN tertile, as well as the interaction of ADPN and time as they relate to changing GFR (see Supplementary Appendix).
Statistical analyses were performed using SAS software, version 9.2 (SAS Institute Inc., Cary, NC), and some of the figures were generated using Stata software, version 11.1 (Stata Corp., College Station, TX).
Results
Baseline characteristics for all the study patients are shown in Table 1, stratified by tertile of ADPN. Deceased donor transplants represented 96% of the study population. Those in the lowest ADPN tertile represented an increased prevalence of men, smokers, patients with diabetes, those with higher C reactive protein level, higher BMI and abdominal circumference, and lower HDL cholesterol level. This group also represented patients with lower systolic BP and total cholesterol levels and lower MIS, suggesting a mixed cardiovascular risk profile. The median eGFR was lowest in patients in the highest ADPN tertile. Although remaining in a relatively normal range, hemoglobin, serum albumin, and calcium levels tended to be lower whereas phosphorus level was higher in the highest ADPN tertile. The highest ADPN tertile represented a higher use of oral prednisone, cyclosporine rather than tacrolimus, and sirolimus as maintenance immunosuppression. Delayed graft function did not differ across ADPN tertiles.
Table 1.
Baseline characteristics by adiponectin tertile
| Characteristic | Adiponectin Tertile 1 (n=328) | Adiponectin Tertile 2 (n=330) | Adiponectin Tertile 3 (n=329) | P Value |
|---|---|---|---|---|
| Median adiponectin level (μg/ml) | 5.0 (3.7–6.2) | 10.1 (3.7–6.2) | 22.0 (17.2–32.3) | |
| Demographic factors | ||||
| Age (yr) | 49.7±12.5 | 51.7±12.9 | 51.6±12.8 | 0.07 |
| Men (%) | 73 | 58 | 42 | <0.001 |
| Current smoker (%) | 23 | 19 | 15 | 0.03 |
| Cardiovascular and metabolic risk factors | ||||
| Diabetes (%) | 26 | 18 | 20 | 0.04 |
| Myocardial infarction (%) | 8 | 9 | 9 | 0.85 |
| Congestive heart failure (%) | 13 | 16 | 13 | 0.40 |
| Cerebrovascular accident (%) | 4 | 3 | 3 | 0.75 |
| Charlson Comorbidity Index score | 2 (2–3) | 2 (2–4) | 2 (2–4) | 0.07 |
| Malnutrition-inflammation score | 3 (1–4) | 3 (2–5) | 4 (2–6) | <0.001 |
| Systolic BP (mmHg) | 139.3±18.4 | 141.2±18.9 | 145.6±20.3 | 0.001 |
| Diastolic BP (mmHg) | 83.3±11.3 | 83.7±11.2 | 85.0±13.4 | 0.14 |
| Body mass index (kg/m2) | 28.0±4.6 | 27.1±5.1 | 25.9±4.7 | <0.001 |
| Abdominal circumference (cm) | 102.8±13.0 | 99.5±14.9 | 94.3±14.0 | <0.001 |
| Cholesterol (mmol/L) | 5.36±1.19 | 5.42±1.15 | 5.75±1.44 | 0.001 |
| LDL cholesterol (mmol/L) | 120.3±33.6 | 122.2±34.4 | 123.3±39.1 | 0.53 |
| HDL cholesterol (mmol/L) | 44.9±11.2 | 50.7±14.7 | 58.0±20.9 | <0.001 |
| Metabolic syndrome (%) | 75 | 59 | 58 | <0.001 |
| C-reactive protein (mg/L) | 3.7 (1.9–7.3) | 3.4 (1.4–7.2) | 2.7 (1.2–5.8) | 0.008 |
| Kidney and transplant factors | ||||
| Estimated GFR (ml/min per 1.73 m2) | 56.7 (43.0–71.4) | 51.4 (40.0–67.4) | 44.1 (28.0–61.1) | <0.001 |
| Cause of ESRD (%) | 0.35 | |||
| Diabetes | 3 | 4 | 6 | |
| Hypertension | 5 | 8 | 6 | |
| GN | 24 | 23 | 21 | |
| Polycystic kidney disease | 20 | 17 | 18 | |
| Other/unknown | 48 | 48 | 49 | |
| Median time since transplant (mo) | 76 (47–121) | 74 (39–116) | 86 (43–124) | 0.25 |
| Median time on dialysis (mo) | 19 (9–37) | 18 (9–37) | 23 (11–40) | 0.12 |
| Delayed graft function (%) | 31 | 24 | 23 | 0.09 |
| Acute rejection (%) | 16 | 12 | 18 | 0.39 |
| HLA mismatch (%) | 0.81 | |||
| 0 | 1 | 1 | 1 | |
| 1–2 | 30 | 29 | 25 | |
| 3–4 | 64 | 65 | 68 | |
| 5–6 | 5 | 5 | 5 | |
| Immunosuppression | ||||
| Steroids | 78 | 78 | 86 | 0.03 |
| Cyclosporine | 46 | 48 | 55 | 0.06 |
| Tacrolimus | 46 | 45 | 33 | 0.003 |
| Mycophenolate mofetil | 80 | 79 | 75 | 0.26 |
| Sirolimus | 5 | 7 | 11 | 0.03 |
| Laboratory | ||||
| Hemoglobin (g/L) | 14.0±1.5 | 13.5±1.6 | 12.9±1.8 | <0.001 |
| Serum albumin (g/L) | 4.1±0.4 | 4.1±0.4 | 3.9±0.5 | <0.001 |
| White blood cell (103 cells/mm3) | 8.3±2.4 | 7.7±2.0 | 7.7±2.4 | 0.006 |
| Calcium (g/L) | 9.44±0.56 | 9.52±0.60 | 9.36±0.68 | 0.003 |
| Phosphorus (g/L) | 3.22±1.02 | 3.28±0.77 | 3.50±0.87 | 0.001 |
Values expressed with a plus/minus sign are the mean ± SD or medians with interquartile ranges in brackets. GFR was calculated per CKD-Epidemiology study equation.
ADPN and All-Cause Mortality
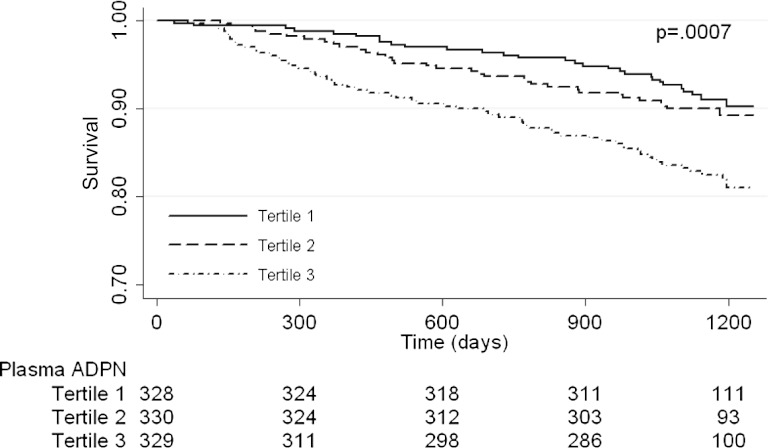
Patients were followed for a median of 51 months (interquartile range, 49–53 months). There were 122 deaths from all causes during the observation period, of which 98 were deaths with a functioning graft. Figure 1 depicts the Kaplan-Meier survival plot for the unadjusted all-cause mortality by ADPN tertiles. Mortality was significantly higher among individuals in the highest ADPN tertile. When examined as a continuous measure, the levels of ADPN (per natural log) were also positively associated with all-cause mortality, as shown in Table 2.
Figure 1.
Kaplan-Meier plot for the proportion of patients surviving over time by serum adiponectin (ADPN) tertile. Values below plot represent at-risk individuals at different time points. Log-rank P=0.0007 for all-cause mortality across adiponectin tertiles.
Table 2.
Association of serum adiponectin levels with all-cause mortality, with estimated GFR used as a time-varying covariate
| Variable | Median ADPN Level (Range) | Hazard Ratio (95% Confidence Interval) | |||
|---|---|---|---|---|---|
| Unadjusted | Model 1a | Model 2b | Model 3c,d | ||
| Overall population | |||||
| Per natural log-transformed ADPN | 10.1 (0.04–127.1) | 1.76 (1.41–2.20) | 1.79 (1.43–2.26) | 1.62 (1.19–2.21) | 1.72 (1.22–2.44) |
| ADPN tertile | |||||
| 1 | 5.0 (0.04–7.4) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| 2 | 10.1 (7.4–14.1) | 1.19 (0.72–1.95) | 1.13 (0.69–1.87) | 0.92 (0.46–1.85) | 1.11 (0.55–2.26) |
| 3 | 22.0 (14.1–127.1) | 2.17 (1.39–3.38) | 2.27 (1.43–3.60) | 1.77 (0.93–3.40) | 2.10 (1.05–4.22) |
ADPN (μg/ml), adiponectin.
Model 1 adjusts for age and sex.
Model 2 adjusts for covariates in model 1 plus time-varying estimated GFR, serum albumin, and log-transformed C-reactive protein.
Model 3 adjusts for covariates in model 2 plus Charlson Comorbidity Index score, abdominal circumference, systolic BP, time since kidney transplantation, and malnutrition-inflammation score.
Data available for n=952.
Other variables positively associated with time to death in univariate analyses were patient age, male sex, lower eGFR, serum albumin level, time since kidney transplant, systolic BP, CRP (natural log-transformed), MIS, and the CCI score. When adjusted for these potential confounders, ADPN remained independently associated with all-cause mortality, with a hazard ratio of 1.44 (95% confidence interval, 1.13–1.85). Similarly, when examined by ADPN tertiles, patients in the highest tertile had an adjusted mortality of 1.80 (95% confidence interval, 1.09–2.96) compared with those in the lowest ADPN tertile.
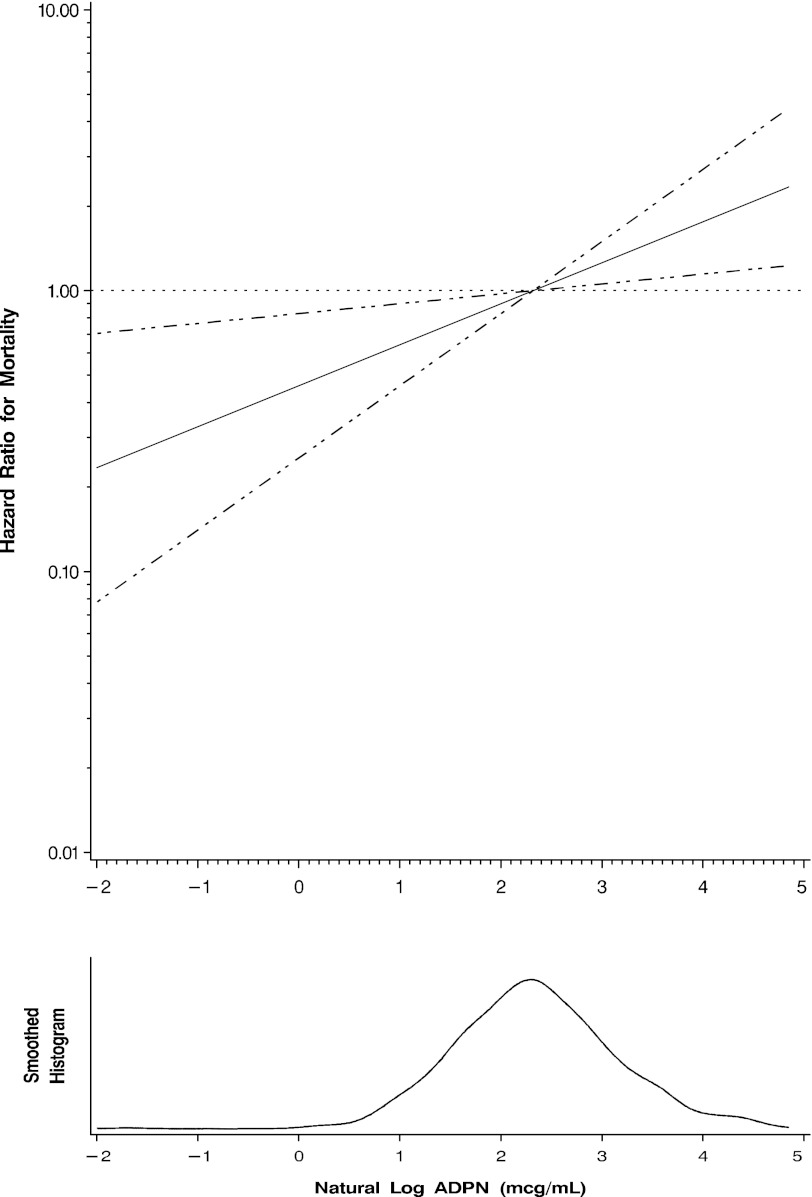
The univariate association of ADPN levels (natural log-transformed) with mortality across varying subgroups is shown in Figure 2. There was no significant interaction by age, sex, diabetes status, BMI, waist circumference, CRP, or Framingham risk score. Figure 3 displays the adjusted hazard ratio for all-cause mortality across the range of serum ADPN level (natural log-transformed) using a cubic spline function. The model is adjusted for age, sex, eGFR, serum albumin, log-transformed CRP, abdominal circumference, systolic BP, CCI score, and time since kidney transplantation. Our left-truncated analysis model did not provide differing results from our conventional Cox models (not shown).
Figure 2.
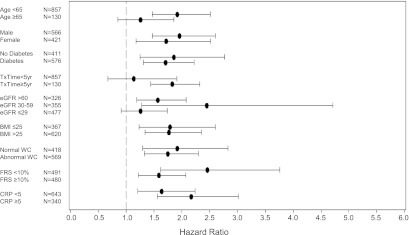
Hazard ratio with 95% confidence intervals for the association of adiponectin level (natural log-transformed) with all-cause mortality, stratified by subgroups. BMI, body mass index (kg/m2); CRP: C-reactive protein (mg/L); eGFR, estimated GFR calculated by the CKD-Epidemiology study equation (ml/min per 1.73 m2); FRS, Framingham risk score for 10-year coronary heart disease risk; TxTime, years since kidney transplantation; WC, waist circumference (normal, <102 cm for men or <88 cm for women).
Figure 3.
Estimated adjusted hazard ratio (solid line) with 95% confidence intervals (dashed lines) for the association of serum adiponectin level and all-cause mortality. The median adiponectin (ADPN) level was used as a reference point for the calculation of all hazard ratios. Nonlinear relationships were first examined using restricted cubic splines (knots=3; P=0.88 for the test of nonlinearity; P<0.001 for the test of linear relation). The model is adjusted for age, sex, estimated GFR, serum albumin, natural log-transformed C-reactive protein, abdominal circumference, systolic BP, Charlson Comorbidity Index score, malnutrition-inflammation score, and time since kidney transplantation.
ADPN and Death-Censored Graft Failure
During the observation period, there were 141 kidney graft failures, defined as a return to long-term dialysis. In univariate analysis, higher ADPN level predicted death-censored graft failure (hazard ratio, 1.83; 95% confidence interval, 1.48–2.26 per one log). However, this association was abrogated after adjustment for age, sex, and eGFR and remained nonsignificant in a fully adjusted model (Supplementary Table 1).
Discussion
In our study of prevalent kidney transplant recipients, higher serum ADPN levels were associated with increased hazard for all-cause mortality. Despite successful kidney transplantation and stable clinical status, there remains a paradoxical association of ADPN and mortality, similar to that seen in other groups of patients with CKD or those undergoing hemodialysis. We did not find any association between serum ADPN levels and kidney allograft failure.
Lower ADPN levels have been associated with type 2 diabetes mellitus, hypertension, and coronary artery disease in the general, non-CKD population. There are several possible explanations for the elevated ADPN levels in kidney transplant recipients. Hyperadiponectinemia may be a result of a compensatory response to ongoing metabolic or vascular insults. In this paradigm, ADPN may indeed be beneficial in lessening atherosclerotic lesions, such as opposing the expression of endothelial adhesion molecules (vascular cell adhesion molecule-1, intercellular adhesion molecule-1, E-selectin), whereas the adverse risk would be mediated by the underlying arteriosclerotic or inflammatory processes. In this case, the higher ADPN level would be a marker of the underlying pathophysiologic processes, and this would explain the association with adverse outcome. Another explanation may be a state of ADPN resistance in those with CKD or those who have undergone transplantation, whether by dysfunction or downregulation at the level of its receptor or even post-translational protein modification (12,13). However, it has also been demonstrated that high ADPN levels are associated with, or may even induce, protein-energy wasting (5,14). This observation may be one of the key explanations to account for its adverse risk in patients with CKD. Similarly, higher serum ADPN levels in kidney transplant recipients may identify those with ongoing states of body fat loss or malnutrition. Whether the relationship of ADPN with mortality can be explained by malnutrition remains to be formally studied, but we could not statistically account for this association after adjusting for MIS, serum albumin, BMI, abdominal circumference, or even CRP.
The LANDMARK 2 (Longitudinal Assessment of Numerous Discrete Modifications of Atherosclerotic Risk Factors in Kidney disease 2) study examined ADPN levels in 137 prevalent kidney transplant recipients (15). Using baseline data, they reported that higher ADPN levels were associated with lower prevalence of cardiovascular disease, although they did not find any association of ADPN with GFR in their cohort and they did not examine any relationships with future outcomes. With our larger sample size and longitudinal, rather than cross-sectional, approach, we find higher ADPN to be associated with higher risk of adverse outcomes. We did find that ADPN was associated with a mixed cardiac risk profile. At baseline, lower BMI, higher HDL cholesterol level, and lower CRP level were associated with higher ADPN. This relationship has been reported in other cross-sectional transplant studies; nevertheless, this may reflect survival bias when studying a prevalent transplant population (16). Several cardiovascular risk factors (higher LDL cholesterol level, higher BP), however, were also associated with higher ADPN level in our cohort, highlighting the complex relationship it may have with conventional cardiac risk factors.
Higher serum adiponectin levels have also been associated with CKD progression. The Mild to Moderate Kidney Disease study prospectively followed 177 patients during a 7-year period and found higher ADPN levels to be associated with progression (doubling of creatinine or reaching kidney failure) in men but not in women (17). In our study, we did not find a similar association with graft failure, irrespective of sex. We did not track GFR decline; however, given our substantial number of events of graft failure (n=141), we cannot ascribe a significant role to ADPN on influencing rapid progression to kidney failure in this population.
Previous studies have shown that higher ADPN levels are protective against hypertension and new-onset diabetes after transplantation (18,19). We cannot comment on incident diabetes or the BP evolution in our study population; however, our study did not find any suggestion of improved overall patient outcome in those in the highest ADPN range.
Limitations of this study include the prevalent nature of the population, which captures patients who survived the early transplant period and were more likely to have achieved stable allograft function. Our single-center study used only a single, baseline measure of ADPN and did not account for possible variability in ADPN over time. We did not examine the specific causes of death, particularly cardiovascular disease events, although we validated deaths with national registry data; this minimizes concerns of any informative censoring. We also did not have data on proteinuria, which is an established risk factor for adverse patient and renal outcomes in other CKD populations. There is also a likelihood of unmeasured or residual confounding in any observational study such as ours. We used total ADPN levels, although the biologic activity of ADPN may be better captured as a ratio of its different isoforms. Nevertheless, we had the strength of a large, well characterized cohort that is probably similar to kidney transplant populations at similar academic centers.
Compared with patients undergoing dialysis, kidney transplantation offers patients a partial return to native kidney function. Serum ADPN levels decline after renal transplantation, but they continue to remain elevated relative to those of normal controls. In our study we found the relationship between ADPN and mortality to remain consistent to that observed in other CKD groups, rather than returning to that of the general population. We suggest that our observational study supports future mechanistic studies to examine the relationship of ADPN with cardiovascular risk and mortality. We suggest that attention should focus on the determinants of sustained ADPN elevation after transplantation, independent of kidney function. Given the high cardiovascular burden of kidney transplant recipients, ADPN may serve as a valuable marker for mortality and a possible target for therapeutic intervention.
Disclosures
None.
Supplementary Material
Acknowledgments
This study was supported by grants from the Egeszsegugyi Tudomanyos Tanacs (206/09), the Hungarian Kidney Foundation, Hungarian Society of Hypertension, Hungarian Society of Nephrology, and the Foundation for Prevention in Medicine. M.Z.M. is recipient of the Hungarian Eötvös Scholarship (MÖB/77-2/2012). A.U. is recipient of the Hungarian Eötvös Scholarship (MÖB/77-3/2012).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.04370512/-/DCSupplemental.
References
- 1.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF: A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 270: 26746–26749, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB: Plasma adiponectin levels and risk of myocardial infarction in men. JAMA 291: 1730–1737, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Schulze MB, Shai I, Rimm EB, Li T, Rifai N, Hu FB: Adiponectin and future coronary heart disease events among men with type 2 diabetes. Diabetes 54: 534–539, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Menon V, Li L, Wang X, Greene T, Balakrishnan V, Madero M, Pereira AA, Beck GJ, Kusek JW, Collins AJ, Levey AS, Sarnak MJ: Adiponectin and mortality in patients with chronic kidney disease. J Am Soc Nephrol 17: 2599–2606, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Stenvinkel P: Adiponectin in chronic kidney disease: A complex and context sensitive clinical situation. J Ren Nutr 21: 82–86, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Lo MM, Mitsnefes M: Adiponectin, cardiovascular disease, chronic kidney disease: Emerging data on complex interactions. Pediatr Nephrol 27: 521–527, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Chudek J, Adamczak M, Karkoszka H, Budziński G, Ignacy W, Funahashi T, Matsuzawa Y, Cierpka L, Kokot F, Wiecek A: Plasma adiponectin concentration before and after successful kidney transplantation. Transplant Proc 35: 2186–2189, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Molnar MZ, Keszei A, Czira ME, Rudas A, Ujszaszi A, Haromszeki B, Kosa JP, Lakatos P, Sarvary E, Beko G, Fornadi K, Kiss I, Remport A, Novak M, Kalantar-Zadeh K, Kovesdy CP, Mucsi I: Evaluation of the malnutrition-inflammation score in kidney transplant recipients. Am J Kidney Dis 56: 102–111, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Grundy SM, Brewer HBJ, Jr, Cleeman JI, Smith SCJ, Jr, Lenfant C, National Heart, Lung, and Blood Institute. American Heart Association : Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol 24: e13–e18, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH: A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis 38: 1251–1263, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Durrleman S, Simon R: Flexible regression models with cubic splines. Stat Med 8: 551–561, 1989 [DOI] [PubMed] [Google Scholar]
- 12.Kadowaki T, Yamauchi T: Adiponectin and adiponectin receptors. Endocr Rev 26: 439–451, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Xu A, Knight C, Xu LY, Cooper GJ: Hydroxylation and glycosylation of the four conserved lysine residues in the collagenous domain of adiponectin. Potential role in the modulation of its insulin-sensitizing activity. J Biol Chem 277: 19521–19529, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Park SH, Carrero JJ, Lindholm B, Stenvinkel P: Adiponectin in chronic kidney disease has an opposite impact on protein-energy wasting and cardiovascular risk: Two sides of the same coin. Clin Nephrol 72: 87–96, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Kaisar MO, Armstrong K, Hawley C, Campbell S, Mudge D, Johnson DW, Prins JB, Isbel NM: Adiponectin is associated with cardiovascular disease in male renal transplant recipients: Baseline results from the LANDMARK 2 study. BMC Nephrol 10: 29, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bakkaloglu SA, Soylemezoglu O, Buyan N, Oktar SO, Funahashi T, Pasaoglu H, Elhan AH, Peru H, Hasanoglu E: Adiponectin levels and arteriosclerotic risk factors in pediatric renal transplant recipients. Pediatr Transplant 10: 187–192, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Kollerits B, Fliser D, Heid IM, Ritz E, Kronenberg F, MMKD Study Group : Gender-specific association of adiponectin as a predictor of progression of chronic kidney disease: The Mild to Moderate Kidney Disease Study. Kidney Int 71: 1279–1286, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Sethna CB, Leonard MB, Gallagher PR, Meyers KE: Serum adiponectin levels and ambulatory blood pressure monitoring in pediatric renal transplant recipients. Transplantation 88: 1030–1037, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Bayés B, Granada ML, Pastor MC, Lauzurica R, Salinas I, Sanmartí A, Espinal A, Serra A, Navarro M, Bonal J, Romero R: Obesity, adiponectin and inflammation as predictors of new-onset diabetes mellitus after kidney transplantation. Am J Transplant 7: 416–422, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.