Summary
Background and objectives
This study aimed to compare the longitudinal performance of the malnutrition-inflammation score (MIS) and the geriatric nutritional risk index (GNRI), two nutritional scores for patients on maintenance hemodialysis.
Design, setting, participants, & measurements
Nutritional scores, dietary intake, biochemical markers, and body composition analysis were performed at baseline and at 6, 12, and 18 months after enrollment (which took place from January through December 2006) on 75 prevalent hemodialysis patients (43% women, mean age 64.8±11.9 years). The patients underwent simultaneous MIS and GNRI assessments calculated by two independent examiners from baseline. The study period was 46.8±16.4 months.
Results
GNRI had higher interobserver agreement (weighted κ-score 0.98) than MIS (weighted κ-score 0.62). Longitudinally, a 1-unit increase in MIS was associated with a 0.41 kcal/kg per day reduction in daily energy intake (P<0.001) and with a 0.014 g/kg per day reduction in nPNA (P=0.02). GNRI did not correlate with the change over time of dietary intake. Longitudinal changes of both scores were associated with appropriate changes over time in levels of nutritional biomarkers, inflammation (IL-6), and body composition parameters. Both scores expressed significant associations with prospective hospitalization, whereas only MIS was associated with mortality in this cohort. The multivariate Cox proportional hazard ratio was 1.15 for death for each 1-unit increase in the MIS (95% confidence interval, 1.03–1.3; P=0.02).
Conclusions
Both MIS and GNRI are valid tools for longitudinal assessment of hemodialysis patients’ nutritional status. MIS has lower interobserver reproducibility than GNRI; however, MIS is more comprehensive than GNRI.
Introduction
Protein-energy wasting is related to an increased morbidity and mortality in patients receiving maintenance hemodialysis treatment for end stage kidney disease (1,2). Simple prediction scores could help identify hemodialysis patients at high risk for protein-energy wasting. Many nutritional screening tools have been developed for general purposes and were further adapted for hemodialysis patients (3–6) for nutritional assessment. The subjective global assessment (SGA) method is a validated clinical tool for screening nutritional risk in hemodialysis patients (5). Another method, the malnutrition–inflammation score (MIS), was proposed by Kalantar-Zadeh et al. as a nutritional screening tool for hemodialysis patients (7,8). MIS has been validated as a better nutritional indicator than SGA (7) and was reported to correlate with morbidity, mortality, various nutritional variables, inflammation (7,8), quality of life (9), anemia, and erythropoietin hyporesponsiveness in hemodialysis patient maintenance (10). Still, MIS is based on the SGA and therefore requires a subjective assessment. There are, however, some simple fully objective nutritional screening scores that can be used in hemodialysis patients for nutritional risk assessment (6,11–13). Of the objective nutritional assessments, the geriatric nutritional risk index (GNRI) is recommended as the simplest and most accurate risk index. It was compared with the MIS (6) and was validated as a significant predictor of mortality in a study on Japanese chronic hemodialysis patients (11).
Reports were published on the reliability and concurrent validity for the cross-sectional assessment of the nutritional status of hemodialysis patients by both MIS (7–9) and GNRI (6,11). To the best of our knowledge, no longitudinal performance of most of the nutritional scores was investigated in hemodialysis patients. In studying the comparative longitudinal validity of different scoring systems, we focused mainly on MIS because it is the most validated nutritional score and on GNRI as on the simplest fully objective score. We examined the longitudinal performance of these scoring systems to determine how the changes over time in MIS and GNRI reflect the changes in the nutritional parameters burden in prevalent hemodialysis patients.
Materials and Methods
Patients
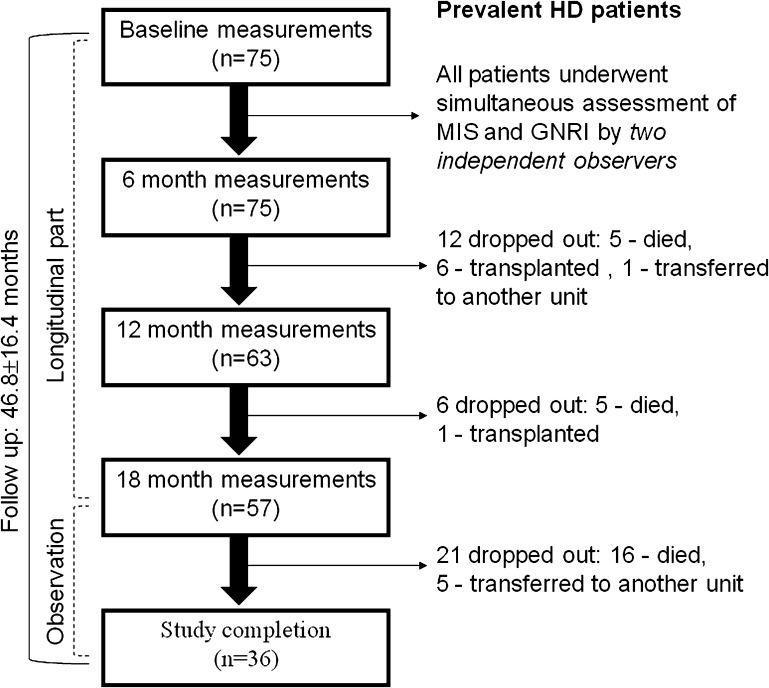
This prospective observational study was approved by the Ethics Committee of Assaf Harofeh Medical Center (affiliated with the Sackler Faculty of Medicine, Tel Aviv University, Zerifin, Israel). Informed consent was obtained before conducting any of the trial-related activities. Patients were eligible for this study if they had been on hemodialysis therapy for at least 3 months and were aged ≥18 years. A flow chart of the study is presented in Figure 1. This study included 75 patients (43 men and 32 women) with a mean age of 64.8±11.9 years, who were receiving maintenance hemodialysis treatment at our outpatient hemodialysis clinic and were recruited from January through December 2006. Baseline study measurements were performed at enrollment and the other measurements were taken at 6, 12, and 18 months from enrollment. In addition, intraobserver and interobserver agreement were assessed for nutritional scores (MIS and GNRI) at baseline measurements. To calculate interobserver error for both scores studied, the patients underwent simultaneous assessment of MIS and GNRI (in a fixed order, first MIS and then GNRI) calculated by two examiners independently (A.A. and H.K.). The intraobserver variability of both nutritional scores was assessed by a blinded re-evaluation by each observer within an interval of 7–10 days between the examinations. All of the following longitudinal measurements were performed by the same observer (A.A.) blinded to any information about disease status and nutritional management of the patients throughout the study period. After finishing the longitudinal measurements, we continued clinical observation on our cohort during 2 additional years. Thus, the total study period extended 46.8±16.4 months (interquartile range, 33.0–61.0 months). During this period, 26 patients (34.7%) died (the main causes of death were cardiovascular [12 of 26 patients; 46.2%] and sepsis [10 of 26 patients; 38.5%]), 7 patients (9.3%) underwent kidney transplants, and 6 patients (8%) transferred to other hemodialysis units. Thus, 13 patients were removed from the study from the time of their transplantation or from when they transferred to another hemodialysis unit. All patients underwent regular dialysis (80.0% of patients had an arteriovenous fistula) for 4–5 hours three times per week.
Figure 1.
Flow diagram of the study. HD, hemodialysis; MIS, malnutrition-inflammation score; GNRI, geriatric nutritional risk index.
Every study participant received nutritional counseling based on an individualized plan of care updated every 3 months. Individuals who were unable to meet their protein and energy requirements with food intake, according to Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines, received nutrition support including oral nutritional supplements (according to accepted standards of care in our institution).
MIS
The MIS has been described in detail in several previous studies (7,8). It consists of 4 sections—including medical history (change in dry weight, dietary intake, gastrointestinal symptoms, functional capacity, and comorbidity), physical examination, body mass index (BMI), and laboratory values—and 10 components. Each MIS component has four levels of severity from 0 (normal) to 3 (very severe). The sum of all 10 components results in an overall score ranging from 0 (normal) to 30 (severely malnourished).
GNRI
The GNRI was calculated from the patient’s serum albumin and body weight by using the equation developed by Bouillanne et al. (4) by modifying the nutritional risk index for elderly patients as follows:
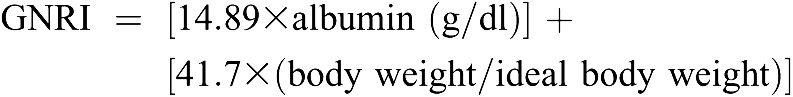 |
Ideal weight in this study was calculated from the Lorentz equations for men and women differently, as in the original GNRI equation (4). Recently, GNRI <90 was demonstrated as an indicator of poor nutritional status and as a significant predictor of mortality in 490 Japanese chronic hemodialysis patients (11).
Hospitalization
Hospitalization data were obtained for all 75 hemodialysis patients. Hospitalization was defined as any hospital admission that included at least one overnight stay in the hospital. The sum of all hospitalization days of a given patient during the study period was defined as the hospitalization days. The hospitalization frequency was the total number of hospital admissions. The access-related hospitalizations were not included in hospitalization data.
Dietary Intake
A continuous 3-day dietary history (including a dialysis day, a weekend day, and a nondialysis day) was recorded in a self-completed food diary. The methods used for collecting the dietary recalls were the same as those recently described by Bross et al. (14). Dietary energy and protein intake were calculated and normalized for adjusted body weight as accepted (15).
Dietary protein intake was also estimated by calculating normalized protein nitrogen appearance (nPNA) from the patient’s urea generation rate by using urea kinetics modeling, the single-pool model (16).
Anthropometric Measurements
BMI, triceps skinfold thickness, mid-arm circumference, and calculated mid-arm muscle circumference (MAMC) were measured for the anthropometric variables. The midweek, postdialysis weight was used for evaluation of BMI according to KDOQI guideline recommendations. MAMC was estimated as follows:
 |
TSF indicates triceps skinfold thickness. MAMC was recently validated as a correlate of lean body mass and associated with survival advantage in hemodialysis patients, especially in those with lower BMI (17).
Body Composition Analyses
Body composition was determined by body impedance analysis (BIA) (NutriGuard–M; Data-Input, Frankfurt, Germany). On the day of blood collection, patients underwent a BIA measurement at approximately 30 minutes postdialysis. BIA electrodes were placed on the same body side used for anthropometric measurements. The multifrequency technique was used. BIA was recently validated as reliable test for the estimation of total body fat percentage in maintenance hemodialysis patients (18). Fat free mass was calculated by using the approach of Kyle et al. (19).
Laboratory Evaluation
Blood samples were taken from nonfasting individuals before a midweek hemodialysis session. Complete blood count, creatinine, urea, albumin, transferrin, and total cholesterol were measured by routine laboratory methods. IL-6 levels were measured in plasma samples using commercially available ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s protocol.
Statistical Analyses
Data are expressed as mean ± SD, median and interquartile range (Q1 to Q3) for variables that did not follow a normal distribution, or frequencies for categorical variables. To assess agreement between the two examiners on the basis of MIS and GNRI, as well as to assess for an intraobserver agreement, we calculated the percentage of agreement between measurements and weighted κ, using linear weights. A weighted κ score was determined by using the result of the weighted agreement and weighted expected agreement (20).
Correlations were assessed using Pearson correlation coefficients or Spearman rank order correlation coefficients, in the cases of skewed distribution of data.
Repeated-measures ANOVA was performed by using the mixed model. Base models were adjusted for age, sex, diabetes status, dialysis vintage, and history of cardiovascular diseases. F tests were used to assess the significance of the fixed effects. To assess the risk of first hospitalization or death for MIS or GNRI, we obtained hazard ratios (HRs) with 95% confidence intervals (95% CIs) using Cox proportional hazard models. All covariates (except for categorical ones) were used as continuous variables. MIS and GNRI were included as continuous variables as well. In addition, to clearly show the effect size, we constructed models with subgroups with MIS or GNRI levels greater versus less than their median values.
All statistical analyses were performed using SPSS software (version 16.0; SPSS Inc, Chicago, IL).
Results
The baseline characteristics of the cohort are shown in Table 1.
Table 1.
Baseline demographics and clinical features of the study population (n=75)
| Variable | Value |
|---|---|
| Demographic and clinical characteristics | |
| Age (yr) | 64.8±11.9 |
| Sex (men/women) | 57/43 |
| Dialysis vintage (mo) | 41.0 (25.0–69.0) |
| Diabetes mellitus | 46.7 |
| History of cardiovascular disease | 50.7 |
| Kt/V | 1.38±0.26 |
| Access (fistula/graft/catheter) | 80/15/5 |
| Medications | |
| Aspirin | 68 |
| ACEi/ARB | 40 |
| Statin | 31 |
| EPO dosage (×103 U/wk) | 10.0 (5.0–15.0) |
| Dietary intake | |
| Energy intake (kcal/kg per day) | 22.0±5.9 |
| Protein intake (g/kg per day) | 0.93±0.24 |
| nPNA (g/kg per day) | 1.05±0.28 |
| Blood analysis | |
| Albumin (g/dl) | 3.94±0.32 |
| Creatinine (mg/dl) | 8.1±2.6 |
| Cholesterol (mg/dl) | 149.9±32.3 |
| Transferrin (mg/dl) | 166.4±36.7 |
| TLC (×103/ml) | 1.53±0.39 |
| Hemoglobin (g/dl) | 11.5±1.0 |
| IL-6 (pg/ml) | 8.2 (4.9–16.2) |
| Anthropometric measurements | |
| BMI (kg/m2) | 28.4±5.7 |
| TSF (mm) | 17.3±6.9 |
| MAC (cm) | 28.5±3.6 |
| MAMC (cm) | 23.1±2.4 |
| Bioimpedance analysis | |
| TBW (kg) | 36.0±7.18 |
| ECW (kg) | 14.2±4.09 |
| ECW/TBW | 0.39±0.05 |
| FM (kg) | 25.2±10.3 |
| Body fat (%) | 33.3±8.8 |
| FFM (kg) | 46.3±9.0 |
| Phase angle (deg) | 4.96±1.1 |
| Nutritional scores | |
| MIS | 7.2±3.6 |
| GNRI | 110.2±17.4 |
Continuous variables are expressed as mean ± SD or median with interquartile range (Q1 to Q3) for non-normally distributed data, and categorical variables are expressed as a percentage. ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; EPO, erythropoietin; nPNA, normalized protein nitrogen appearance; TLC, total lymphocyte count; BMI, body mass index; TSF, triceps skinfold thickness; MAC, mid-arm circumference; MAMC, mid-arm muscle circumference calculated; TBW, total body water; ECW, extracellular water; ECW/TBW, extracellular water/total body water ratio; FM, fat mass; FFM, fat free mass; MIS, malnutrition-inflammation score; GNRI, geriatric nutritional risk index.
On evaluating interobserver reproducibility, MIS exhibited substantial agreement (weighted κ=0.62) and GNRI showed almost-perfect agreement (weighted κ=0.98) as would be expected (Table 2). The κ scores for intraobserver reproducibility were consistent with almost-perfect agreement for both nutritional scores (weighted κ=0.77 for MIS and weighted κ=0.82 for GNRI). Thus, in evaluating reproducibility, GNRI has an advantage over MIS, especially in interobserver agreement.
Table 2.
Interobserver and intraobserver agreement (percentage agreement) and reliability (κ scores) for MIS and GNRI
| Variable | MIS | GNRI |
|---|---|---|
| Interobserver reproducibility | ||
| Agreement | 84 | 99 |
| Weighted κ score | 0.62 (0.52–0.72) | 0.98 (0.97–0.99) |
| Intraobserver reproducibility | ||
| Observer 1 | ||
| Agreement | 94 | 99 |
| Weighted κ score | 0.77 (0.53–0.99) | 0.82 (0.76–0.88) |
| Observer 2 | ||
| Agreement | 92 | 98 |
| Weighted κ score | 0.76 (0.60–0.91) | 0.88 (0.83–0.93) |
Data are presented as percentages or weighted κ scores (95% confidence intervals). MIS, malnutrition-inflammation score; GNRI, geriatric nutritional risk index.
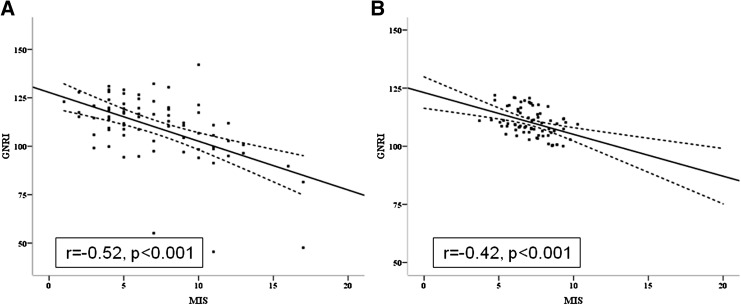
The correlation between MIS and GNRI at baseline (obtained by the measurements of observer 1) was strong and negative (Figure 2) in both the univariate analysis (r=−0.52; P<0.001) and the adjusted analysis (adjusted for age, sex, presence of diabetes mellitus, dialysis vintage, and history of cardiovascular disease; r=−0.42; P<0.001).
Figure 2.
Correlations of both MIS and GNRI of the study population at baseline. (A) Unadjusted correlations (r=−0.52; P<0.001) and (B) correlations after adjustments for age, sex, diabetes mellitus status, dialysis vintage, and history of cardiovascular disease (r=−0.42; P<0.001). The solid line represents a regression line, whereas the dashed lines above and below the solid line represent the 95% confidence interval. MIS, malnutrition-inflammation score; GNRI, geriatric nutritional risk index.
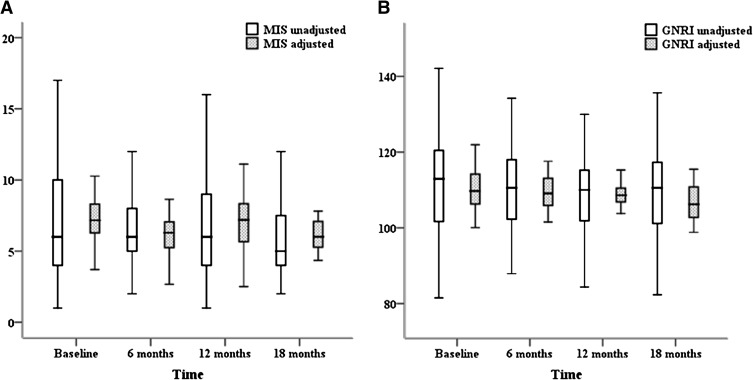
During the longitudinal part of the study, MIS exhibited a trend of decreasing with time, with a linear estimate of −0.22±0.14 per 1.5 years (P=0.13) in univariate analysis and −0.24±0.14 per 1.5 years (P=0.09) after adjustments for age, sex, diabetes mellitus status, dialysis vintage, and history of cardiovascular disease. A statistically significant decrease of GNRI with time was seen in both the unadjusted model (linear estimate: −1.12±0.50 per 1.5 years; P=0.03) and the adjusted model (adjusted for the same confounders as for MIS; linear estimate: −1.11±0.50 per 1.5 years; P=0.03) (Figure 3). Linear mixed models were used to study the associations of longitudinal MIS (Table 3) and GNRI (Table 4) changes with changes in nutritional parameters (slopes) over 18 months, including fixed parameters such as age, sex, diabetes status, dialysis vintage, and previous cardiovascular events. Longitudinally, a 1-unit increase in MIS over time, controlling for fixed factors, was associated with an 0.41 kcal/kg per day reduction in daily energy intake (P<0.001) and with an 0.014 g/kg per day reduction in nPNA (P=0.02). Longitudinal changes in GNRI did not correlate with the changes over time in parameters of dietary intake. Longitudinal changes of both scores were associated with the appropriate changes over time in the levels of most of the nutritional laboratory biomarkers and the parameters of body composition (see Tables 3 and 4). Analogous associations were observed between longitudinal changes in both scores and IL-6 levels. On average, IL-6 was higher by 1.39 pg/ml for every 1-unit increase in MIS (P=0.003) and lower by 0.62 pg/ml for every 1-unit increase in GNRI (P<0.001). As shown in Tables 3 and 4, longitudinal changes in hydration status parameters resulted in statistically significant results in the case of GNRI. Therefore, we examined whether controlling for the extracellular water/total body water ratio confounded the association of longitudinal change of MIS and GNRI with changes in dependent variables over time. After the extracellular water/total body water ratio has been added to the regression model, the prediction ability of the scores to detect changes in nutritional and inflammatory parameters remained unchanged (data not shown) except for longitudinal changes in fat free mass that turned to be significantly predicted by MIS changes over time (linear estimate: −0.15 kg [95% CI, −0.24 to −0.07; P<0.001] per 1.5 years). Thus, compared with the concurrent validity, MIS has an advantage over GNRI because changes in MIS over time reflected changes in all areas of nutritional status including dietary intake.
Figure 3.
Box and whisker plots of MIS and GNRI measurements at baseline (month 0) and at 6-month intervals in the study population. (A) MIS shows a trend for decrease with time. (B) GNRI decreases significantly with time in both univariate and multivariate analyses. MIS, malnutrition-inflammation score; GNRI, geriatric nutritional risk index.
Table 3.
Regression coefficients with 95% confidence intervals for the association of longitudinal MIS changes with the rates of changes in nutritional parameters (slopes) over 18 months
| Variable | Change per 1-Unit Increase in MIS | 95% Confidence Interval | ||
|---|---|---|---|---|
| Lower | Upper | P | ||
| Dietary intake | ||||
| DEI (kcal/kg per day) | −0.41 | −0.63 | −0.19 | <0.001 |
| DPI (g/kg per day) | −0.009 | −0.02 | 0.001 | 0.08 |
| nPNA (g/kg per day) | −0.014 | −0.024 | −0.003 | 0.02 |
| Biochemical markers | ||||
| Creatinine (mg/dl) | −0.12 | −0.17 | −0.06 | <0.001 |
| Cholesterol (mg/dl) | −1.37 | −0.81 | −2.74 | 0.05 |
| TLC (×103/ml) | 5.67 | −12.96 | 24.30 | 0.55 |
| IL-6 (pg/ml) | 1.39 | 0.49 | 2.28 | 0.003 |
| EPO dosage (×103 U/wk) | 52.65 | −258.84 | 364.13 | 0.74 |
| Anthropometric measurements | ||||
| TSF (mm) | −0.11 | −0.30 | 0.08 | 0.27 |
| MAC (cm) | −0.11 | −0.20 | −0.03 | 0.01 |
| MAMC (cm) | −0.17 | −0.28 | −0.06 | 0.002 |
| Bioimpedance analysis | ||||
| TBW (kg) | −0.04 | −0.16 | 0.08 | 0.50 |
| ECW (kg) | −0.01 | −0.09 | 0.07 | 0.80 |
| ECW/TBW | −0.0001 | −0.0009 | 0.001 | 0.98 |
| FM (kg) | −0.34 | −0.52 | −0.16 | <0.001 |
| Body fat (%) | −0.37 | −0.58 | −0.17 | <0.001 |
| FFM (kg) | −0.09 | −0.20 | 0.02 | 0.09 |
| Phase angle (deg) | −0.06 | −0.09 | −0.03 | <0.001 |
All nutritional variables presented above were modeled separately as dependent variables, whereas independent variables included fixed factors (such as age, sex, diabetes status, dialysis vintage, and past cardiovascular disease) and MIS as a continuous variable. The model takes into account every calculation of MIS and presents nutritional variables at each time point separately for each patient. Regression coefficients indicate mean longitudinal change in outcome variables associated with a 1-unit longitudinal increase in MIS, controlling for fixed factors (as mentioned above). MIS, malnutrition-inflammation score; DEI, daily energy intake; DPI, daily protein intake; nPNA, normalized protein nitrogen appearance; TLC, total lymphocyte count; EPO, erythropoietin; TSF, triceps skinfold thickness; MAC, mid-arm circumference; MAMC, mid-arm muscle circumference calculated; TBW, total body water; ECW, extracellular water; ECW/TBW, extracellular water /total body water ratio; FM, fat mass; FFM, fat free mass.
Table 4.
Regression coefficients with 95% confidence intervals for the association of longitudinal GNRI changes with the rates of changes in nutritional parameters (slopes) over 18 months
| Variable | Change per 1-Unit Increase in GNRI | 95% Confidence Interval | ||
|---|---|---|---|---|
| Lower | Upper | P | ||
| Dietary intake | ||||
| DEI (kcal/kg per day) | −0.02 | −0.07 | 0.03 | 0.38 |
| DPI (g/kg per day) | −0.0001 | −0.002 | 0.002 | 0.94 |
| nPNA (g/kg per day) | 0.0007 | −0.002 | 0.003 | 0.56 |
| Biochemical markers | ||||
| Transferrin (mg/dl) | 0.59 | 0.31 | 0.87 | <0.001 |
| Creatinine (mg/dl) | 0.02 | 0.009 | 0.04 | 0.002 |
| Cholesterol (mg/dl) | 0.46 | 0.12 | 0.81 | 0.01 |
| TLC (x103/ml) | 2.17 | −2.94 | 7.27 | 0.40 |
| IL-6 (pg/ml) | −0.62 | −0.87 | −0.37 | <0.001 |
| EPO dosage (×103 U/wk) | −11.80 | −77.97 | 54.02 | 0.72 |
| Anthropometric measurements | ||||
| TSF (mm) | 0.06 | 0.02 | 0.10 | 0.01 |
| MAC (cm) | 0.04 | 0.02 | 0.06 | <0.001 |
| MAMC (cm) | 0.05 | 0.03 | 0.08 | <0.001 |
| Bioimpedance analysis | ||||
| TBW (kg) | 0.03 | −0.0005 | 0.07 | 0.05 |
| ECW (kg) | 0.03 | 0.002 | 0.05 | 0.04 |
| ECW/TBW | 0.0003 | 0.00004 | 0.0006 | 0.05 |
| FM (kg) | 0.21 | 0.16 | 0.26 | <0.001 |
| Body fat (%) | 0.21 | 0.15 | 0.27 | <0.001 |
| FFM (kg) | 0.06 | 0.03 | 0.09 | 0.001 |
| Phase angle (deg) | 0.02 | 0.007 | 0.02 | <0.001 |
All nutritional variables presented above were modeled separately as dependent variables, whereas independent variables included fixed factors (such as age, sex, diabetes status, dialysis vintage, and past cardiovascular disease), and GNRI as a continuous variable. The model takes into account every calculation of GNRI and presents nutritional variables at each time point separately for each patient. Regression coefficients indicate mean longitudinal change in outcome variables associated with a 1-unit longitudinal increase in GNRI, controlling for fixed factors (as mentioned above). GNRI, geriatric nutritional risk index; DEI, daily energy intake; DPI, daily protein intake; nPNA, normalized protein nitrogen appearance; TLC, total lymphocyte count; EPO, erythropoietin; TSF, triceps skinfold thickness; MAC, mid-arm circumference; MAMC, mid-arm muscle circumference calculated; TBW, total body water; ECW, extracellular water; ECW/TBW, extracellular water/total body water ratio; FM, fat mass; FFM, fat free mass.
Both MIS and GNRI were significantly correlated with prospective hospitalization, hospital length of stay (r=0.37, P=0.001; and r=−0.39, P=0.001 for MIS and GNRI, respectively), and hospitalization frequency (r=0.31, P<0.001; and r=−0.41, P<0.001 for MIS and GNRI, respectively). Table 5 lists the HRs and 95% CIs of deaths and first hospitalizations using the Cox proportional hazard models based on initial values at the start of the prospective cohort and the time to death or first hospitalization, respectively. MIS, but not GNRI, showed a strong association with prospective mortality in both univariate and multivariate analyses. The adjusted HR for death for each 1-unit increase in MIS was 1.15 (95% CI, 1.03–1.3; P=0.02), whereas the HR for death for subgroups with MIS >6 increased to 2.90 (95% CI, 1.17–7.16; P=0.02) compared with subgroups who had MIS of <6. Both MIS and GNRI demonstrated a significant association with first hospitalization (Table 5). Thus, compared with predictive validity, MIS again has an advantage over GNRI because it predicts both morbidity and mortality in our study population.
Table 5.
Mortality and hospitalization HRs associated with MIS and GNRI using univariate and multivariate Cox regression analysis in 75 prevalent dialysis patients
| Variable | Units of Increase (↑) | Unadjusted Cox Regression | Multivariate Cox Regressiona | ||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| All-cause mortality | |||||
| MIS | 1 unit ↑ | 1.12 (1.01–1.24) | 0.03 | 1.15 (1.03–1.28) | 0.02 |
| ≤6 | 1.0 (ref) | 1.0 (ref) | |||
| >6 | 3.11 (1.31–7.37) | 0.01 | 2.90 (1.17–7.16) | 0.02 | |
| GNRI | 1 unit ↑ | 0.99 (0.970–1.01) | 0.24 | 1.01 (0.97–1.01) | 0.72 |
| ≤113 | 1.0 (ref) | 1.0 (ref) | |||
| >113 | 0.55 (0.21–1.45) | 0.22 | 0.77 (0.27–2.19) | 0.63 | |
| First hospitalization | |||||
| MIS | 1 unit ↑ | 1.14 (1.06–1.22) | <0.001 | 1.17 (1.08–1.28) | <0.001 |
| ≤6 | 1.0 (ref) | 1.0 (ref) | |||
| >6 | 2.39 (1.42–4.02) | 0.001 | 2.31 (1.34–3.98) | 0.003 | |
| GNRI | 1 unit ↑ | 0.98 (0.97–0.99) | 0.002 | 0.97 (0.96–0.99) | 0.001 |
| ≤113 | 1.0 (ref) | 1.0 (ref) | |||
| >113 | 0.44 (0.24–0.80) | 0.01 | 0.48 (0.25–0.92) | 0.03 | |
HR, hazard ratio; MIS, malnutrition-inflammation score; GNRI, geriatric nutritional risk index; CI, confidence interval.
Adjusted for age, sex, diabetes mellitus status, dialysis vintage and history of cardiovascular disease.
Discussion
The nutritional score validation for longitudinal observation should provide the following conditions: its changes over time must detect the longitudinal changes in dietary intake, biochemical markers of nutrition and inflammation, and body composition (concurrent validity). It also must be a good predictor of clinical outcome (predictive validity) and must be easily reproducible (intrarater and interrater reliability). The results of this study indicate that MIS had an almost-perfect intrarater reliability, substantial interrater reliability, and good concurrent and predictive validity. Although GNRI had an almost-perfect intrarater and interrater reliability, it had a lower concurrent validity compared with MIS and a poor predictive validity in our cohort. At the same time, the MIS requires assessment by a well trained staff and is much more time-consuming than the GNRI, which is calculated by a very simple equation (Table 6).
Table 6.
A comparative validation of MIS and GNRI in the study population
| Variable | MIS | GNRI |
|---|---|---|
| Reproducibility | ||
| Interrater agreement | ∼ | + |
| Intrarater agreement | + | + |
| Simplicity | ||
| Assessment by a well trained staff is necessary | + | — |
| Time-consuming | + | — |
| Concurrent validity | ||
| Dietary intake | + | — |
| Laboratory markers | + | + |
| Body composition | + | + |
| Predictive validity | ||
| Morbidity | + | + |
| Mortality | + | — |
MIS, malnutrition-inflammation score; GNRI, geriatric nutritional risk index. +, yes; −, no; ∼, moderate.
This study is the first longitudinal validation of the MIS and GNRI in maintenance hemodialysis patients. Our study showed the same weighted κ scores for interrater agreement of MIS as did Cooper et al. (5) studying validity of SGA. This is not surprising, because the MIS system is based on SGA. Visser et al. (21) and Kalantar-Zadeh et al. (22) reported a higher interrater reliability, whereas Steiber et al. reported (23) a lower interrater reliability of SGA. Differences in study design included use of different scales of SGA, a different number of observers, and in-person versus web-based training of observers. All of these may explain the discrepancies in the results. Only two published studies measured intrarater reliability of SGA in dialysis patients (21,23). The results of our study demonstrated high intraobserver reliability of MIS and our results were closer to the SGA results reported by Visser et al. (21). The almost-perfect intrarater and interrater reliability of GNRI is related to the objective nature of the score. Because GNRI scores are based on weight, height, and albumin measurements, we realized that the discrepancies between the measurements reflect only the changes in weight measurement. However, we introduced κ scores for GNRI in order to quantitatively compare MIS and GNRI in terms of intraobserver and interobserver variability.
Many different cross-sectional studies examined the concurrent and predictive validity of MIS (7–10) and GNRI (6,11) in hemodialysis patients. Given the estimates of the regression coefficients in Tables 3 and 4, it appears that changes of these scores over time were associated with corresponding changes over time in markers of dietary intake (examined by both dietary recall and nPNA calculation), laboratory markers of nutrition and body composition parameters in the case of MIS (Table 3), and only with laboratory and body composition parameters in the case of GNRI (Table 4). With regard to dietary intake, information about dietary recall results in different GNRI categories was not included in the previous cross-sectional studies that validated GNRI in both elderly (4) and hemodialysis patients (6,11). In addition, serial measurements of GNRI were not sensitive in detecting changes in the nutritional status, including dietary intake, in 106 peritoneal dialysis patients (24). With regard to significant associations of MIS changes with dietary intake changes in our study, mathematical coupling should contribute to this association because dietary intake is a component of the MIS.
One must also consider whether regression to the mean was relevant to the association of changes in longitudinal scores with changes in nutritional parameters (slopes) over time. High correlation ratios between the baseline and follow-up measurements of MIS and GNRI on the one hand, and the use of the linear mixed model for longitudinal data analysis that models the covariance matrix adjusting each participant’s follow-up measurement according to his or her baseline measurement on the other hand, allow for the assumption that the effect of regression to the mean was not high in our study.
In this study, MIS was found to be associated with prospective hospitalization and all-cause mortality. This confirms the results of previous studies in this field (7–9). As opposed to the results of study by Kobayashi et al. (11), GNRI did not appear to be a useful predictor of all-cause mortality in our cohort. The discrepancy between our results and theirs may be due to differences in the cohorts. The population presented by Kobayashi et al. (11) had a lower BMI. This is reported to be associated with the much lower morbidity rates in the Japanese population, in whom appropriate BMI and obesity criteria are different than that of Caucasians (25). In addition, the lower sample size of our study precluded us from achieving a significant association between cumulative survival and GNRI in our cohort. Of interest, the observations that both MIS and GNRI change with time are strongly associated with longitudinal changes of IL-6 levels in our cohort. Because IL-6 is the best indicator of an inflammatory response (26), this in turn represents a powerful predictor of mortality in the maintenance hemodialysis population (27–29) and this finding can be considered as an additional strength for both scores.
Some limitations of this study should be considered. First, this study is based on a relatively small sample size of prevalent hemodialysis patients from a single center, limiting the ability of our findings to be generalized. Second, this study used only an observational approach, without manipulation of exposure factors. A high drop-out rate (18 patients comprising 24% of the baseline population that did not complete the longitudinal measurements) may limit the statistical power of the study. Dietary intake assessed by 3-day food records is another limitation of the study, because results can be subjective and incomplete. Nevertheless, this study has the advantage of providing the first longitudinal validation of the accepted nutritional scores, MIS and GNRI, in prevalent hemodialysis patients.
In conclusion, our findings suggest that in the clinical setting, either MIS or GNRI are valid tools for longitudinal observation of nutritional status of prevalent hemodialysis patients and should be used for this purpose, taking into account both the type of score and its interrater and intrarater agreements. MIS is more comprehensive than GNRI because of its better concurrent and predictive validity. However, one potential problem with MIS is its subjective nature, which reduces its reproducibility; thus, small differences in MIS must be interpreted with great caution. In addition, repeated measurements of MIS for nutritional monitoring should be performed by the same observer. Furthermore, predictive validity of GNRI must be examined in maintenance hemodialysis patients in all major racial and ethnic groups.
Disclosures
None.
Acknowledgment
We wish to acknowledge our colleague and coauthor Joshua Weissgarten, an outstanding personality, clinical nephrologist, investigator, educator and medical director, who passed away on June 3, 2012, at the age of 63, shortly after submission of the manuscript.
Footnotes
aDeceased.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Carrero JJ, Chmielewski M, Axelsson J, Snaedal S, Heimbürger O, Bárány P, Suliman ME, Lindholm B, Stenvinkel P, Qureshi AR: Muscle atrophy, inflammation and clinical outcome in incident and prevalent dialysis patients. Clin Nutr 27: 557–564, 2008 [DOI] [PubMed] [Google Scholar]
- 2.de Mutsert R, Grootendorst DC, Axelsson J, Boeschoten EW, Krediet RT, Dekker FW, NECOSAD Study Group : Excess mortality due to interaction between protein-energy wasting, inflammation and cardiovascular disease in chronic dialysis patients. Nephrol Dial Transplant 23: 2957–2964, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Pablo AM, Izaga MA, Alday LA: Assessment of nutritional status on hospital admission: Nutritional scores. Eur J Clin Nutr 57: 824–831, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, Benazeth S, Cynober L, Aussel C: Geriatric Nutritional Risk Index: S new index for evaluating at-risk elderly medical patients. Am J Clin Nutr 82: 777–783, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Cooper BA, Bartlett LH, Aslani A, Allen BJ, Ibels LS, Pollock CA: Validity of subjective global assessment as a nutritional marker in end-stage renal disease. Am J Kidney Dis 40: 126–132, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Yamada K, Furuya R, Takita T, Maruyama Y, Yamaguchi Y, Ohkawa S, Kumagai H: Simplified nutritional screening tools for patients on maintenance hemodialysis. Am J Clin Nutr 87: 106–113, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH: A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis 38: 1251–1263, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Kalantar-Zadeh K, Kopple JD, Humphreys MH, Block G: Comparing outcome predictability of markers of malnutrition-inflammation complex syndrome in haemodialysis patients. Nephrol Dial Transplant 19: 1507–1519, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Rambod M, Bross R, Zitterkoph J, Benner D, Pithia J, Colman S, Kovesdy CP, Kopple JD, Kalantar-Zadeh K: Association of Malnutrition-Inflammation Score with quality of life and mortality in hemodialysis patients: A 5-year prospective cohort study. Am J Kidney Dis 53: 298–309, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalantar-Zadeh K, McAllister CJ, Lehn RS, Lee GH, Nissenson AR, Kopple JD: Effect of malnutrition-inflammation complex syndrome on EPO hyporesponsiveness in maintenance hemodialysis patients. Am J Kidney Dis 42: 761–773, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi I, Ishimura E, Kato Y, Okuno S, Yamamoto T, Yamakawa T, Mori K, Inaba M, Nishizawa Y: Geriatric Nutritional Risk Index, a simplified nutritional screening index, is a significant predictor of mortality in chronic dialysis patients. Nephrol Dial Transplant 25: 3361–3365, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Dessì M, Noce A, Agnoli A, De Angelis S, Fuiano L, Tozzo C, Taccone-Gallucci M, Fuiano G, Federici G: The usefulness of the prognostic inflammatory and nutritional index (PINI) in a haemodialysis population. Nutr Metab Cardiovasc Dis 19: 811–815, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Beberashvili I, Azar A, Sinuani I, Yasur H, Feldman L, Averbukh Z, Weissgarten J: Objective Score of Nutrition on Dialysis (OSND) as an alternative for the malnutrition-inflammation score in assessment of nutritional risk of haemodialysis patients. Nephrol Dial Transplant 25: 2662–2671, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Bross R, Noori N, Kovesdy CP, Murali SB, Benner D, Block G, Kopple JD, Kalantar-Zadeh K: Dietary assessment of individuals with chronic kidney disease. Semin Dial 23: 359–364, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilkens K, Brouns-Schiro K: Suggested Guidelines for Nutrition Care of Renal Patients, Chicago, IL, American Dietetic Association, 1992, p 40 [Google Scholar]
- 16.Depner TA, Daugirdas JT: Equations for normalized protein catabolic rate based on two-point modeling of hemodialysis urea kinetics. J Am Soc Nephrol 7: 780–785, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Noori N, Kopple JD, Kovesdy CP, Feroze U, Sim JJ, Murali SB, Luna A, Gomez M, Luna C, Bross R, Nissenson AR, Kalantar-Zadeh K: Mid-arm muscle circumference and quality of life and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol 5: 2258–2268, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bross R, Chandramohan G, Kovesdy CP, Oreopoulos A, Noori N, Golden S, Benner D, Kopple JD, Kalantar-Zadeh K: Comparing body composition assessment tests in long-term hemodialysis patients. Am J Kidney Dis 55: 885–896, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kyle UG, Genton L, Karsegard L, Slosman DO, Pichard C: Single prediction equation for bioelectrical impedance analysis in adults aged 20--94 years. Nutrition 17: 248–253, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Viera AJ, Garrett JM: Understanding interobserver agreement: The kappa statistic. Fam Med 37: 360–363, 2005 [PubMed] [Google Scholar]
- 21.Visser R, Dekker FW, Boeschoten EW, Stevens P, Krediet RT: Reliability of the 7-point subjective global assessment scale in assessing nutritional status of dialysis patients. Adv Perit Dial 15: 222–225, 1999 [PubMed] [Google Scholar]
- 22.Kalantar-Zadeh K, Kleiner M, Dunne E, Lee GH, Luft FC: A modified quantitative subjective global assessment of nutrition for dialysis patients. Nephrol Dial Transplant 14: 1732–1738, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Steiber A, Leon JB, Secker D, McCarthy M, McCann L, Serra M, Sehgal AR, Kalantar-Zadeh K: Multicenter study of the validity and reliability of subjective global assessment in the hemodialysis population. J Ren Nutr 17: 336–342, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Szeto CC, Kwan BC, Chow KM, Law MC, Li PK: Geriatric nutritional risk index as a screening tool for malnutrition in patients on chronic peritoneal dialysis. J Ren Nutr 20: 29–37, 2010 [DOI] [PubMed] [Google Scholar]
- 25.WHO Expert Consultation : Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363: 157–163, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Zoccali C, Tripepi G, Mallamaci F: Dissecting inflammation in ESRD: Do cytokines and C-reactive protein have a complementary prognostic value for mortality in dialysis patients? J Am Soc Nephrol 17[Suppl 3]: S169–S173, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Panichi V, Maggiore U, Taccola D, Migliori M, Rizza GM, Consani C, Bertini A, Sposini S, Perez-Garcia R, Rindi P, Palla R, Tetta C: Interleukin-6 is a stronger predictor of total and cardiovascular mortality than C-reactive protein in haemodialysis patients. Nephrol Dial Transplant 19: 1154–1160, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Barreto DV, Barreto FC, Liabeuf S, Temmar M, Lemke HD, Tribouilloy C, Choukroun G, Vanholder R, Massy ZA, European Uremic Toxin Work Group (EUTox) : Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int 77: 550–556, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Beberashvili I, Sinuani I, Azar A, Yasur H, Shapiro G, Feldman L, Averbukh Z, Weissgarten J: IL-6 levels, nutritional status, and mortality in prevalent hemodialysis patients. Clin J Am Soc Nephrol 6: 2253–2263, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]