Summary
Background and objectives
CKD is a common public health problem. Identifying biomarkers adds prognostic/diagnostic value by contributing to an understanding of CKD at the molecular level and possibly defining new drug targets. Metabolomics provides a snapshot of biochemical events at a particular time in the progression of CKD. This cross-sectional metabolomics study ascertained whether plasma metabolite profiles are significantly different in CKD stages 2, 3, and 4.
Design, setting, participants, & measurements
An analysis of plasma metabolites, using gas and liquid chromatography coupled to mass spectrometry, was conducted on 30 nondiabetic men ages 40–52 years, with 10 participants each in CKD stages 2, 3, and 4 based on their estimated GFR (calculated by the Modified Diet in Renal Disease formula). Participants were recruited in late 2008, and plasma samples were tested at Metabolon Inc and analyzed in 2012.
Results
Comparison of stage 3/stage 2 identified 62 metabolites that differed (P≤0.05), with 39 higher and 23 lower in stage 3 compared with stage 2; comparisons of stage 4/stage 2 identified 111 metabolites, with 66 higher and 45 lower; and comparisons of stage 4/stage 3 identified 11 metabolites, with 7 higher and 4 lower. Major differences in metabolite profiles with increasing stage of CKD were observed, including altered arginine metabolism, elevated coagulation/inflammation, impaired carboxylate anion transport, and decreased adrenal steroid hormone production.
Conclusions
Global metabolite profiling of plasma uncovered potential biomarkers of stages of CKD. Moreover, these biomarkers provide insight into possible pathophysiologic processes that may contribute to progression of CKD.
Introduction
CKD encompasses a spectrum of kidney diseases, ranging from kidney damage with normal kidney function to ESRD. The National Kidney Foundation has devised a five-stage classification system for CKD based on the level of GFR (1). Cardiovascular disease (CVD) is a major cause of morbidity and mortality in patients with all forms of CKD and contributes to the complexity of CKD (2–6). This complexity introduces new challenges in predicting and treating patients sufficiently early in the course of CKD to positively alter patient outcome. Until recently, most risk factor analysis of kidney diseases has focused on ESRD. Little is known about the effect of various risk factors at each stage of disease and how these contribute to the rate of progression to ESRD. In addition, there has been little study of the potential differences in risk factors for transition from one stage to the next and whether the risk factors for onset and transition from stage 1 to stage 2 may differ from those for transition to stage 3 and stage 4 CKD or for final development of ESRD. Currently, both the incidence and prevalence of CKD leading to ESRD continue to increase at an alarming rate in the United States. There are at least 19 million people in the United States with some degree of CKD (7), with enormous costs to society (8), prompting the Surgeon General to include CKD as a focus area for improving the nation’s health in Healthy People 2010. An understanding of the characteristics of early stage CKD, as well as the factors that differentially affect the progression of CKD from one stage to the next, is essential for determining appropriate therapy and predicting long-term outcomes.
Metabolomics, which is the most recent systems-biology approach to complement the genomic, transcriptomic, and proteomic efforts to characterize an entire biologic system, is increasingly being used to study kidney function (9–11). Because metabolites represent the end products of the genome and proteome, metabolomics holds the promise of providing an integrated physiologic phenotype of a system. Such metabolic profiling involves a comprehensive measurement of the types and concentrations of metabolites in a system at a specified time, such as each stage of CKD. Metabolomics also provides insight into metabolic pathways and networks downstream of gene expression. Complex metabolite profiles may provide the data required to enable the diagnosis, risk stratification, treatment, and evaluation of treatment response of patients. This may be through the identification of single biomarkers as in the more traditional methods or more likely by identification of patterns across many metabolites.
In this cross-sectional study, we determined plasma metabolite profiles of 30 participants; these were nondiabetic men aged 40–52 years, with 10 each in CKD stages 2, 3, and 4 based on their estimated GFR. Our goal was to determine whether there are significant differences in specific metabolites by stage of CKD and whether these may be useful stage-specific biomarkers. A related goal was to consider whether these differences might offer insight into potential pathophysiologic mechanisms that contribute to progression of CKD.
Materials and Methods
Study Participants
Plasma samples, which were obtained from the University of Pennsylvania from patients recruited in late 2008, were stored at −80°C for this study. Samples from 30 participants with CKD, 10 each in stages 2, 3 and 4, were selected for metabolite analysis at Metabolon Inc in 2012. Informed consent was obtained from all participants. Descriptions of the participants are summarized in Table 1.
Table 1.
Study participant baseline characteristics
| Kidney Disease Progression | |||
|---|---|---|---|
| CKD Stage 2 | CKD Stage 3 | CKD Stage 4 | |
| Number of patients | 10 | 10 | 10 |
| Sex | Male | Male | Male |
| Ethnicity | NHW | NHW | NHW |
| Age (yr) | 51.4±3.3 | 58.2±2.6 | 61.5±4.7 |
| Height (cm) | 168.1±6.9 | 174.5±5.3 | 175.5±6.1 |
| Weight (kg) | 92.9±9.6 | 97.3±7.9 | 100.5±10.0 |
| Body mass index | 32.8±2.6 | 31.9±2.2 | 32.5±1.8 |
| Estimated GFR (ml/min per 1.73 m2) | 63.6±13.2 | 37.9±9.9 | 27.4±4.4 |
Data are presented as mean ± SD. NHW, non-Hispanic white.
Sample Accessioning/Preparation
All mass spectrometry data were collected at Metabolon Inc. Each plasma sample was accessioned into the Metabolon LIMS system and was assigned by the LIMS unique identifier, which was associated with the original source identifier only. The nontargeted metabolic profiling platform utilized for this analysis combined three independent platforms: ultrahigh performance liquid chromatography–tandem mass spectrometry (UHPLC–MS/MS2) optimized for basic species, UHPLC–MS/MS2 optimized for acidic species, and gas chromatography/mass spectrometry (GC/MS). Samples were processed essentially as described previously (12,13). For each sample, 100 μl was used for analyses. Using an automated liquid handler (Hamilton LabStar, Salt Lake City, UT), protein was precipitated from the plasma with methanol that contained four standards to report on extraction efficiency. The resulting supernatant was split into equal aliquots for analysis on the three platforms. Aliquots, dried under nitrogen and vacuum-desiccated, were subsequently either reconstituted in 50 μl 0.1% formic acid in water (acidic conditions) or in 50 μl 6.5 mM ammonium bicarbonate in water, pH 8 (basic conditions) for the two UHPLC–MS/MS2 analyses, or were derivatized to a final volume of 50 μl for GC/MS analysis using equal parts bistrimethyl-silyl-trifluoroacetamide and solvent mixture acetonitrile:dichloromethane:cyclohexane (5:4:1) with 5% triethylamine at 60°C for 1 hour. In addition, three types of controls were analyzed in concert with the experimental samples: aliquots of a well characterized human plasma pool served as technical replicates throughout the data set, extracted water samples served as process blanks, and a cocktail of standards spiked into every analyzed sample allowed instrument performance monitoring. Experimental samples and controls were randomized across platform run days.
LC/MS, LC/MS2
For UHLC–MS/MS2 analysis, aliquots were separated using a Waters Acquity UPLC (Waters, Millford, MA) and were analyzed using an LTQ mass spectrometer (Thermo Fisher Scientific Inc, Waltham, MA) that consisted of an electrospray ionization source and linear ion-trap mass analyzer. The MS instrument scanned 99–1000 m/z and alternated between MS and MS2 scans using dynamic exclusion with approximately six scans per second.
GC/MS
Derivatized samples for GC/MS were separated on a 5% phenyldimethyl silicone column with helium as the carrier gas and a temperature ramp from 60°C to 340°C and then analyzed on a Thermo-Finnigan Trace DSQ MS (Thermo Fisher Scientific Inc.) operated at unit mass resolving power with electron impact ionization and a 50–750 atomic mass unit scan range.
Compound Identification
Compounds were identified by automated comparison of the ion features in the experimental samples with a reference library of chemical standard entries that included retention time, molecular weight (m/z), preferred adducts, and in-source fragments as well as associated MS spectra, and were curated by visual inspection for quality control using software developed at Metabolon (14). At present, >2500 commercially available purified standards are registered into LIMS for distribution to both the LC and GC platforms for determination of their analytical characteristics. Compound abundance was quantified by calculating the area under the curve for the quantification ion of the compound.
Statistical Analyses
To aid data visualization, the raw area counts for each biochemical were rescaled by dividing each sample value by the median value for that specific biochemical. For statistical analyses, any missing values were assumed to be below the limits of detection and these values were imputed with the compound minimum (minimum value imputation). Statistical analyses of log-transformed data were performed using “R” (http://cran.r-project.org/), which is a freely available, open-source software package. Welch’s t tests were performed to compare data between experimental groups. Multiple comparisons were accounted for by estimating the false discovery rate (FDR) using q values (15). CKD groups were classified using Random Forest analyses. Random Forests give an estimate of how well we can classify individuals in a new data set into each group, in contrast to a t test, which tests whether the unknown means for two populations are different or not. Random Forests create a set of classification trees based on continual sampling of the experimental units and compounds. Each observation is then classified based on the majority votes from all of the classification trees (16,17).
Results
Global Metabolite Determination
Initially, metabolites were measured in all plasma samples and were then evaluated by comparing values from CKD stage 3 with stage 2, CKD stage 4 with stage 2, and CKD stage 4 with stage 3. In total, 258 metabolites were identified. A subset of these metabolites was identified, with significant differences in one or more of these CKD stage comparisons (P≤0.05); an additional set of metabolites was identified that approached significance (0.05<P<0.10). Supplemental Table 1 lists the fold of the differences and statistical test results, including the q-value statistic, which is an estimate of the FDR in multiparametric datasets, for every metabolite detected in this study. Supplemental Table 2 shows the median re-scaled raw area counts with missing values imputed with the observed minimum detection value and represents the individual sample values that contributed to the statistical results in Supplemental Table 1. A total of 62 metabolites were identified that were significantly higher or lower in comparisons of CKD stage 3 with stage 2, with 39 higher and 23 lower; in comparisons of CKD stage 4 with stage 2, a total of 111 metabolites differed significantly, with 66 higher and 45 lower; and in comparisons of CKD stage 4 with stage 3, 11 metabolites were identified, with 7 higher and 4 lower. The number of different metabolites that differed significantly was 117. The raw data for all 258 metabolites are presented in the Supplemental Material.
Identification of Thematic Changes
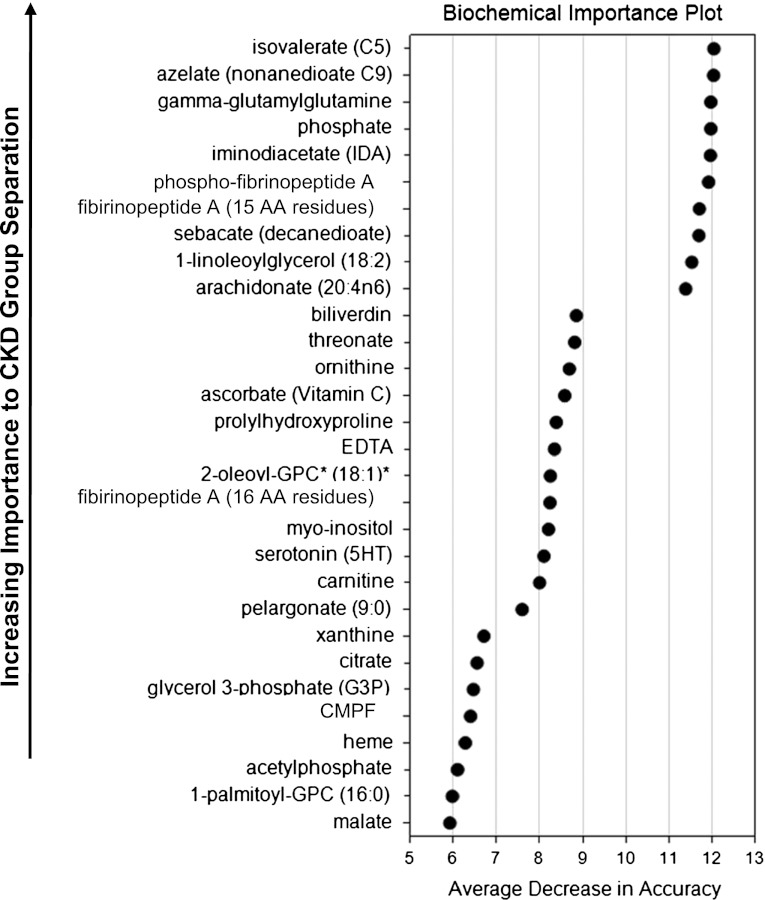
Differences in level of a specific metabolite among a large number may be significant by chance. Therefore, it was of interest to search for thematic differences in which multiple metabolites are significantly higher or lower, which would unlikely be due to chance. Random Forest classification of CKD stage 2 compared with stage 3 and of CKD stage 4 compared with stage 2 identified metabolites based upon their abilities to identify groups. The 30 top-ranking metabolites for the Random Forest classification that compared CKD stages 2 and 4 are listed in Figure 1, denoted as the biochemical importance plot. These metabolites were evaluated, along with related metabolites beyond the top 30, to identify thematic differences. These differences may reveal CKD stage-specific biomarkers. They may also reflect significant alterations of pathophysiology that promote progression from CKD stage 2 to higher stages. Several themes were identified.
Figure 1.
Top 30 metabolites important to CKD stage 2 versus stage 4 separation by Random Forest classification. Random Forest multivariate classification was conducted by randomly choosing CDK stage 2 and CDK stage 4 samples (“in-bag”), randomly choosing variables (metabolites) to build classification and regression trees (CART) based on the in-bag samples, and then using several CART trees (50,000) to classify the remaining “out-of-bag” samples between CDK stage 2 and CDK stage 4 (17). The final prediction is based on an aggregation of the predictions across all trees for which the observation was part of the out-of-bag samples. Average decrease in accuracy was obtained by computing the classification error, based on out-of-bag samples, for each CART tree, permuting each variable, and re-computing the error for each permuted tree. The average difference between the two errors was computed and scaled by dividing the SD of these differences yielding a relationship of the more important the variable to the classification, the higher its average decrease in accuracy.
Altered Arginine Metabolism
A large difference in relative metabolite concentration was observed for dimethylarginine, as shown in Table 2, as a combination of asymmetric and symmetric dimethylarginine. Dimethylarginine in CKD stage 3 is higher compared with stage 2 (8.1-fold) and in CKD stage 4 compared with stage 2 (4.8-fold). This represents one of the larger metabolite fold increases that were observed in this cross-sectional comparison of CKD stage 2 with higher stages. Other metabolites related to arginine metabolism that were also significantly different in CKD stages 3 and 4 compared with stage 2 include ornithine and citrulline. Ornithine was markedly lower in CKD stages 3 and 4 compared with CKD stage 2.
Table 2.
Ratios of significant changes of specific metabolites by stage of CKD (P≤0.05), mean values, and P values
| Fold of Change | Mean ± SD | P Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Stages 3/2 | Stages 4/2 | Stages 4/3 | CKD Stage 2 | CKD Stage 3 | CKD Stage 4 | Stages 3/2 | Stages 4/2 | Stages 4/3 | |
| Altered arginine metabolism | |||||||||
| Dimethylarginine (SDMA + ADMA) | 8.1 | 4.8 | 0.497±0.133 | 4.01±2.27 | 2.40±1.77 | 7.9E-05 | 0.01 | 0.17 | |
| Citrulline | 1.6 | 1.3a | 0.825±0.194 | 1.30±0.586 | 1.08±0.362 | 0.03 | 0.08 | ||
| Ornithine | 0.28 | 0.16 | 4.33±1.39 | 1.19±1.32 | 0.683±0.334 | 2.0E-4 | 1.4E-06 | ||
| Arginine | 1.5a | 1.5a | 0.675±0.238 | 1.02±0.480 | 1.03±0.559 | 0.06 | 0.10 | ||
| Elevated coagulation/inflammation | |||||||||
| Fibrinopeptide A | 689 | 827 | 0.002±0.001 | 1.03±0.765 | 1.24±0.795 | 3.0E-4 | 1.1E-14 | ||
| Phosphorylated fibrinopeptide A | 18 | 45 | 2.5 | 0.045±0.017 | 0.818±0.683 | 2.06±2.62 | 0.002 | 4.4E-08 | 0.04 |
| Proline-hydroxyproline | 2.5 | 4.5 | 0.466±0.251 | 1.16±1.26 | 2.08±1.68 | 0.03 | 8.3E-05 | ||
| Impaired carboxylate anion transport | |||||||||
| γ-Glutamylleucine | 1.3 | 0.821±0.283 | 1.07±0.161 | 0.03 | |||||
| γ-Glutamylisoleucine | 1.6 | 1.7 | 0.670±0.196 | 1.12±0.440 | 1.18±0.510 | 0.01 | 0.01 | ||
| γ-Glutamylglutamine | 3.8 | 4.8 | 0.275±0.166 | 1.05±0.417 | 1.31±0.349 | 5.5E-05 | 8.9E-06 | ||
| γ-Glutamylphenylalanine | 1.5 | 1.3 | 0.903±0.240 | 1.31±0.480 | 1.17±0.181 | 0.03 | 0.01 | ||
| CMPF | 18.3a | 23.6 | 0.307±0.279 | 5.61±11.9 | 7.23±7.20 | 0.06 | 5.0E-4 | ||
| Decreased adrenal steroid hormone production | |||||||||
| Dehydroisoandrosterone sulfate | 0.55 | 1.48±0.878 | 0.819±0.685 | 0.04 | |||||
| 4-androsten-3-β,17-β-diol disulfate | 0.26 | 3.91±3.76 | 1.02±0.958 | 0.01 | |||||
ADMA, asymmetric dimethylarginine; SDMA, symmetric dimethylarginine; CMPF, 3-Carboxy-4-methyl-5-propyl-2- furanpropanoate.
0.1>P>0.05.
Elevated Coagulation/Inflammation
The largest fold difference that was observed in comparisons of CKD stage 2 with stage 3, which remained elevated in stage 4, was the increase in coagulation/inflammation factor fibrinopeptide-A and phosphorylated fibrinopeptide-A (Table 2). The higher level of fibrinopeptide-A in CKD stage 3 compared with stage 2 (689-fold) is maintained in CKD stage 4 compared with stage 2 (827-fold). The higher level of phosphorylated fibrinopeptide-A (phosphorylated at serine-3) in CKD stage 3 compared with stage 2 (18-fold) remained elevated in stage 4 compared with stage 2 (45-fold) and was significantly higher in CKD stage 4 compared with stage 3 (2.5-fold). Proline-hydroxyproline dipeptide was significantly higher in CKD stage 3 compared with stage 2 (2.5-fold) and in stage 4 compared with stage 2 (4.5-fold), which may reflect matrix degradation in response to increased coagulation/inflammation.
Impaired Carboxylate Anion Transport
Numerous mono-and di-carboxylate anions are higher in CKD stages 3 and 4 compared with CKD stage 2 (Table 2). A number of these are γ-glutamyl amino acid dipeptides. γ-glutamylglutamine, for example, is higher in CKD stage 3 compared with stage 2 (3.8-fold) and in stage 4 compared with stage 2 (4.8-fold). The γ-glutamyl amino acid dipeptides in Table 2 are involved in the γ-glutamyl cycle, which is involved in glutathione homeostasis. These increases may reflect increased oxidative stress related to depletion of glutathione. Other carboxylate anions also are increased. 3-Carboxy-4-methyl-5-propyl-2-furanpropanoate (CMPF), a known uremic toxin that accumulates in ESRD, is higher in CKD stage 3 compared with stage 2 (18.3-fold) and in stage 4 compared with stage 2 (23.6-fold).
Decreased Adrenal Steroid Hormone Production
A number of adrenal steroid hormones, especially sulfated metabolites, were significantly lower in CKD stage 4 compared with stage 2 (Table 2). These are also anions. However, unlike the situation with carboxylate anions, in which higher levels were observed in higher stages of CKD, the sulfated metabolites were lower in the higher stages of CKD. Cortisol was also significantly lower in CKD stage 4 compared with stage 2 (0.62-fold). This suggests that a decrease in production of adrenal steroid hormones may explain these data.
Discussion
Major differences in metabolite profiles in the various stages of CKD were observed, consistent with altered arginine metabolism, elevated coagulation/inflammation, impaired carboxylate anion transport, and decreased adrenal steroid hormone production. These differences may reveal stage-specific biomarkers of CKD. Of particular interest are the major differences in metabolite profiles related to arginine metabolism and the significance of these changes with respect to impaired production of NO and the effect that this may have on endothelial function. Also of particular interest are the large fold increases in the levels of fibrinopeptide-A in comparisons of CKD stages 3 and 4 with CKD stage 2 and the significance of these increases with respect to development of a procoagulation/proinflammation state.
Arginine Metabolism
There are extensive data on the possible role of asymmetric dimethylarginine in kidney disease owing to its ability to inhibit nitric oxide synthase (NOS) and limit production of NO, thereby contributing to vascular complications associated with CKD (18,19). The presence of asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA) in human urine was first reported in 1970 (20). Inhibition of NOS by ADMA but not by SDMA was reported in 1992, with the observation that plasma levels of SDMA and ADMA were markedly elevated in CKD and ESRD and the suggestion that inhibition of NOS by ADMA may contribute to CVD, hypertension, and immune dysfunction associated with kidney disease (21). ADMA and SDMA are produced as post-translational modifications of selected arginine residues in specific proteins by methyl transfer from S-adenosylmethionine, which is catalyzed by protein arginine methyltransferases (PRMTs) (22,23). In turnover of proteins with methylated arginine residues, ADMA and SDMA, as well as mono-methylated arginine residues, are released. Most of the SDMA is released into plasma for clearance by the kidney. ADMA, however, is primarily converted into dimethylamine and citrulline, catalyzed by dimethylarginine dimethylaminohydrolases (DDAH-1 and DDAH-2) with distinct tissue distribution and regulation (24). Hydrolysis catalyzed by DDAH accounts for about 80% of the fate of ADMA. The remaining ADMA is excreted into the urine. It has been suggested that the plasma concentrations of ADMA are determined primarily by DDAH-1, which is highly expressed in kidney (25) and is colocalized with NOS (26). Thus, impaired kidney function may directly dictate the plasma concentrations of ADMA, which can inhibit endothelial NOS (eNOS) at low micromolar concentrations. In addition, methylarginines and dimethylarginines reduce uptake of arginine and other cationic amino acids by inhibition of amino acid transporters CAT-1 and CAT-2, which may also contribute to diminished production of NO (27).
Coagulation/Inflammation
Damage to the endothelium normally exposes collagen and other subendothelial proteins that are recognized by platelet receptors to initiate platelet activation. In addition, tissue factor is exposed on the damaged endothelium and can form a complex with factor VII to initiate the proteolysis cascades that produce thrombin. The activated platelets bind thrombin, which results in release of additional factors to recruit platelets to the site of clot formation. Platelet-bound thrombin also catalyzes the degradation of fibrinogen to form fibrin at the surface of the aggregated platelets, which is then cross-linked to form the clot. Patients with CKD can exhibit defects with any of these aspects of hemostasis, suggesting that CKD is a procoagulation state (28). Impaired platelet activation has been reported in CKD patients with mild-to-moderate CKD (29).
Fibrinopeptide-A is a 16 amino acid peptide derived from the thrombin-catalyzed proteolysis of the N-terminal end of the Aα-chain in fibrinogen. Accumulation of fibrinopeptide-A may reflect diminished capacity to clear this metabolite concomitant with a decrease in estimated GFR or may indicate development of a procoagulation state in the progression of CKD stage 2 to stages 3 and 4. Fibrinopeptide-A is a proinflammatory peptide (30), which suggests that this potential development of a procoagulation state is accompanied by the development of inflammation. Fibrinopeptide-A may be a useful stage-specific biomarker. However, the marked differences in levels of fibrinopeptide-A with stage of CKD may also reflect the development of a procoagulation/proinflammation state and may define the critical point of progression of CKD to states that eventually lead to ESRD. In support of this suggestion, recent studies of several animal models of CKD demonstrated the involvement of coagulation factor Xa and the ability of inhibitors of factor Xa to suppress development of some of the pathologic factors associated with CKD (31).
A critical component of endothelial control of hemostasis is the generation of NO at the appropriate time and levels. In experimental animal studies, eNOS production of NO regulates expression of tissue factor, suggesting that impairment of NO production will result in elevated tissue factor and promotion of coagulation (32). In addition, NO produced by the endothelium acts locally to inhibit platelet aggregation and therefore is essential for dampening the procoagulation response (33). The differences in levels of dimethylarginines (Table 2) observed in comparisons of CKD stage 2 with stage 3 suggests impaired NO production, which therefore may exacerbate the development of a procoagulation/proinflammation state in early stage CKD and enhance its progression (34).
Carboxylate Anions
CMPF is one of a number of uremic toxins that accumulate in ESRD, due to tight binding to albumin, and present problems in removal during hemodialysis (35). CMPF is toxic both to endothelial cells and to proximal tubular cells (36,37). CMPF was higher in CKD stage 3 compared with stage 2 and higher in stage 4 compared with stage 3.
In summary, in this cross-sectional metabolomics study, we determined metabolite patterns in different stages of CKD. The results demonstrated that, for the specific population that was studied, significant differences in metabolite patterns occur. Specifically, markedly higher levels of dimethylarginine and fibrinopeptide-A in comparisons of CKD stage 3 with CKD stage 2 suggest that these may be stage-specific biomarkers. These differences also point to endothelial dysfunction and suggest that a convergence of impaired NO production and enhanced production of coagulation/inflammation factor fibrinopeptide-A may collectively act to enhance progression of CKD beyond stage 2. These are testable hypotheses.
Study Limitations
This was an initial proof-of-concept study in which to address the question whether metabolomics might provide meaningful data for comparison of differences in CKD by stage. As such, it was a cross-sectional study with a limited study population. The results, although interesting and potentially meaningful for identification of stage-specific biomarkers and for insight into CKD progression, are preliminary to a longitudinal study of metabolite changes in CKD progression. The approved future longitudinal study will utilize the Chronic Renal Insufficiency Cohort population in order to compare CKD progression in diabetic and nondiabetic CKD populations that are further divided by age, sex, and ethnicity.
Disclosures
E.K. and K.L.P. are employees of Metabolon Inc and, as such, have affiliations with or financial involvement with Metabolon Inc.
Supplementary Material
Acknowledgments
This project was supported in part by an Innovative Pilot Grant from the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) (8UL1TR000041) and the University of New Mexico (UNM) Clinical and Translational Science Center. We also acknowledge support from grants from the National Center for Research Resources (5P20RR016480-12) and the National Institute of General Medical Sciences (8P20GM103451-12) of the NIH. The cost for clinical phenotyping was supported under a UNM Health Sciences-based Cardiovascular and Metabolic Diseases Signature Program. Portions of this work with R.R.T were supported under Grants DK-060984 and UL1RR024134 from NIH.
This work was presented in poster form at the American Society of Nephrology Kidney Week 2012, San Diego, CA, November 3, 2012.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05540512/-/DCSupplemental.
References
- 1.National Kidney Foundation : K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 2.Anakevar NS, Pfeffer MA: Cardiovascular risk in chronic kidney disease. Kidney Int Suppl 92: S11–S15, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW: The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol 35: 681–689, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Ruilope LM, Salvetti A, Jamerson K, Hansson L, Warnold I, Wedel H, Zanchetti A: Renal function and intensive lowering of blood pressure in hypertensive participants of the hypertension optimal treatment (HOT) study. J Am Soc Nephrol 12: 218–225, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Muntner P, He J, Hamm L, Loria C, Whelton PK: Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol 13: 745–753, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS: Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 41: 1–12, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Xue JL, Ma JZ, Louis TA, Collins AJ: Forecast of the number of patients with end-stage renal disease in the United States to the year 2010. J Am Soc Nephrol 12: 2753–2758, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Portilla D, Schnackenberg L, Beger RD: Metabolomics as an extension of proteomic analysis: Study of acute kidney injury. Semin Nephrol 27: 609–620, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toyohara T, Akiyama Y, Suzuki T, Takeuchi Y, Mishima E, Tanemoto M, Momose A, Toki N, Sato H, Nakayama M, Hozawa A, Tsuji I, Ito S, Soga T, Abe T: Metabolomic profiling of uremic solutes in CKD patients. Hypertens Res 33: 944–952, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Rhee EP, Thadhani R: New insights into uremia-induced alterations in metabolic pathways. Curr Opin Nephrol Hypertens 20: 593–598, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Ohta T, Masutomi N, Tsutsui N, Sakairi T, Mitchell M, Milburn MV, Ryals JA, Beebe KD, Guo L: Untargeted metabolomic profiling as an evaluative tool of fenofibrate-induced toxicology in Fischer 344 male rats. Toxicol Pathol 37: 521–535, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E: Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem 81: 6656–6667, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Dehaven CD, Evans AM, Dai H, Lawton KA: Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J Cheminform 2: 9, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Storey JD, Tibshirani R: Statistical significance for genomewide studies. Proc Natl Acad Sci U S A 100: 9440–9445, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breiman L: Random Forests. Mach Learn 45: 5–32, 2001 [Google Scholar]
- 17.Goldstein BA, Hubbard AE, Cutler A, Barcellos LF: An application of Random Forests to a genome-wide association dataset: methodological considerations & new findings. BMC Genet 11: 49, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwedhelm E, Böger RH: The role of asymmetric and symmetric dimethylarginines in renal disease. Nat Rev Nephrol 7: 275–285, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Wilcox CS: Asymmetric dimethylarginine and reactive oxygen species. Hypertension 59: 375–381, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kakimoto Y, Akazawa S: Isolation and identification of N-G,N-G- and N-G,N’-G-dimethyl-arginine, N-ε-mono-, di-, and trimethyllysine, and glucosylgalactosyl- and galactosyl-delta-hydroxylysine from human urine. J Biol Chem 245: 5751–5758, 1970 [PubMed] [Google Scholar]
- 21.Vallance P, Leone A, Calver A, Collier J, Moncada S: Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 339: 572–575, 1992 [DOI] [PubMed] [Google Scholar]
- 22.Durban E, Lee HW, Kim S, Paik WK: Purification and characterization of protein methylase I (S-adenosylmethionine: Protein-arginine methyltransferase; EC 2.1.1.23) from calf brain. Methods Cell Biol 19: 59–67, 1978 [DOI] [PubMed] [Google Scholar]
- 23.Rawal N, Rajpurohit R, Lischwe MA, Williams KR, Paik WK, Kim S: Structural specificity of substrate for S-adenosylmethionine:protein arginine N-methyltransferases. Biochim Biophys Acta 1248: 11–18, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Leiper JM, Santa Maria J, Chubb A, MacAllister RJ, Charles IG, Whitley GS, Vallance P: Identification of two human dimethylarginine dimethylaminohydrolases with distinct tissue distributions and homology with microbial arginine deiminases. Biochem J 343: 209–214, 1999 [PMC free article] [PubMed] [Google Scholar]
- 25.Kimoto M, Whitley GS, Tsuji H, Ogawa T: Detection of NG,NG-dimethylarginine dimethylaminohydrolase in human tissues using a monoclonal antibody. J Biochem 117: 237–238, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Tojo A, Welch WJ, Bremer V, Kimoto M, Kimura K, Omata M, Ogawa T, Vallance P, Wilcox CS: Colocalization of demethylating enzymes and NOS and functional effects of methylarginines in rat kidney. Kidney Int 52: 1593–1601, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Closs EI, Simon A, Vékony N, Rotmann A: Plasma membrane transporters for arginine. J Nutr 134[Suppl]: 2752S–2759S, discussion 2765S–2767S, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Jalal DI, Chonchol M, Targher G: Disorders of hemostasis associated with chronic kidney disease. Semin Thromb Hemost 36: 34–40, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Thijs A, Nanayakkara PW, Ter Wee PM, Huijgens PC, van Guldener C, Stehouwer CD: Mild-to-moderate renal impairment is associated with platelet activation: A cross-sectional study. Clin Nephrol 70: 325–331, 2008 [PubMed] [Google Scholar]
- 30.Jennewein C, Tran N, Paulus P, Ellinghaus P, Eble JA, Zacharowski K: Novel aspects of fibrin(ogen) fragments during inflammation. Mol Med 17: 568–573, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ono T: [Roles of coagulation pathway and factor Xa in chronic kidney disease (CKD)]. Yakugaku Zasshi 132: 449–453, 2012 [DOI] [PubMed] [Google Scholar]
- 32.van Hinsbergh VWM: Endothelium—role in regulation of coagulation and inflammation. Semin Immunopathol 34: 93–106, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solovey A, Kollander R, Milbauer LC, Abdulla F, Chen YIE, Kelm RJ, Jr, Hebbel RP: Endothelial nitric oxide synthase and nitric oxide regulate endothelial tissue factor expression in vivo in the sickle transgenic mouse. Am J Hematol 85: 41–45, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Moncada S, Higgs A: The L-arginine-nitric oxide pathway. N Engl J Med 329: 2002–2012, 1993 [DOI] [PubMed] [Google Scholar]
- 35.Jourde-Chiche N, Dou L, Cerini C, Dignat-George F, Vanholder R, Brunet P: Protein-bound toxins—update 2009. Semin Dial 22: 334–339, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Itoh Y, Ezawa A, Kikuchi K, Tsuruta Y, Niwa T: Protein-bound uremic toxins in hemodialysis patients measured by liquid chromatography/tandem mass spectrometry and their effects on endothelial ROS production. Anal Bioanal Chem 403: 1841–1850, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Miyamoto Y, Iwao Y, Mera K, Watanabe H, Kadowaki D, Ishima Y, Chuang VT, Sato K, Otagiri M, Maruyama T: A uremic toxin, 3-carboxy-4-methyl-5-propyl-2-furanpropionate induces cell damage to proximal tubular cells via the generation of a radical intermediate. Biochem Pharmacol 84: 1207–1214, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.