Summary
Background and objectives
Abnormal left ventricular structure and function are associated with increased risk of adverse outcomes among patients with CKD and ESRD. A better understanding of changes in left ventricular mass and ejection fraction during the transition from CKD to ESRD may provide important insights to opportunities to improve cardiac outcomes.
Design, setting, participants, & measurements
This was a longitudinal study of a subset of participants of the Chronic Renal Insufficiency Cohort who were enrolled from 2003 to 2007 and followed through January of 2011. Participants were included if they had serial echocardiograms performed at advanced CKD (defined as estimated GFR<20 ml/min per 1.73 m2) and again after ESRD (defined as need for hemodialysis or peritoneal dialysis).
Results
A total of 190 participants (44% female, 66% black) had echocardiograms during advanced CKD and after ESRD. Mean (SD) estimated GFR at advanced CKD was 16.9 (3.5) ml/min per 1.73 m2. Mean (SD) time between the advanced CKD echocardiogram and ESRD echocardiogram was 2.0 (1.0) years. There was no significant change in left ventricular mass index (62.3–59.5 g/m2.7, P=0.10) between advanced CKD and ESRD; however, ejection fraction significantly decreased (53%–50%, P=0.002). Interactions for age, race, dialysis modality, and diabetes status were not significant (P>0.05).
Conclusions
Mean left ventricular mass index did not change significantly from advanced CKD to ESRD; however, ejection fraction declined during this transition period. Although left ventricular mass index is fixed by advanced stages of CKD, ejection fraction decline during more advanced stages of CKD may be an important contributor to cardiovascular disease and mortality after dialysis.
Introduction
Patients with CKD have a tremendous burden of cardiovascular disease, and patients with ESRD are at the greatest risk for cardiovascular events and death (1–3). In patients with CKD and ESRD, abnormalities in left ventricular (LV) structure and function are important subclinical measures that have been associated with adverse clinical outcomes.
Approximately three quarters of incident dialysis patients have increased LV mass (LVM) (4,5), an independent predictor of cardiovascular events and death after onset of ESRD (6,7). Similarly, low ejection fraction (EF), even in the absence of clinical heart failure, has also been shown to be a risk factor for cardiovascular and all-cause mortality among patients with both CKD and ESRD (8,9).
Prior studies of LV structure and function have been limited by cross-sectional study design or use of clinical trial participants (10), which is generally less representative compared with enrollees in observational studies (10–15). For example, in a posthoc analysis of the Cardiovascular Risk Reduction by Early Anemia Treatment with Epoetin β trials, GN was the most common cause of CKD (10). Additionally, an observational study of CKD patients excluded patients with diabetes (12). No previous studies to our knowledge have followed patients over time from CKD to ESRD. The period of advanced CKD before the initiation of dialysis is a vulnerable period when many key medical interventions are implemented, such as changes in BP regimens (e.g., management of angiotensin converting enzyme [ACE] inhibitors), initiation of erythropoitin stimulating agents, and decisions on dialysis modality; all of these interventions may impact outcomes after initiation of dialysis. A better understanding of the change in LV structure and function from advanced CKD to after onset of ESRD may identify modifiable risk factors to improve cardiovascular outcomes after ESRD. To address this knowledge gap, we performed serial echocardiograms in a subset of study participants enrolled in a prospective cohort study, the Chronic Renal Insufficiency Cohort (CRIC) Study, at the time of advanced CKD and again after reaching ESRD. Our original hypothesis was that abnormalities in LV structure and function at the onset of ESRD were already present during advanced CKD.
Materials and Methods
Source Population
This study analyzed data from CRIC, a National Institutes of Health-sponsored multicenter prospective cohort study of persons with CKD recruited from 7 clinical centers (with 13 enrolling sites); inclusion and exclusion criteria and baseline characteristics have been previously published (16,17). The CRIC study included participants with estimated GFR (eGFR)=20–70 ml/min per 1.73 m2 by the Modification of Diet in Renal Disease (MDRD) equation at cohort entry and excluded participants with New York Heart Association class III or IV heart failure. Institutional review board approval was obtained from all participating institutions, and informed consent to participate in CRIC was obtained from all subjects.
CRIC participants have annual in-person visits and 6-month phone contacts. At the annual in-person visits, participants complete questionnaires updating interim medical events and medication use and undergo laboratory testing. Estimated GFR is calculated from calibrated serum creatinine measurements at each visit using the four-variable MDRD equation.
Per the CRIC study protocol, research echocardiograms are performed at year 1 study visit, year 4 study visit, advanced CKD study visit, and ESRD study visit (these visits may overlap).
Study Population
Our study population included only CRIC participants who progressed to ESRD and had an echocardiogram performed at ESRD through January 5, 2011. ESRD was defined as the first CRIC study visit after a patient initiated chronic maintenance dialysis or received a kidney transplant. Participants on hemodialysis were targeted to have their echocardiogram performed 1 day after dialysis (which was achieved in 80% of participants in our study).
In our primary analysis, we included participants who had an echocardiograms performed at both advanced CKD (within 3 months) and ESRD. Advanced CKD was defined as either the visit at which a participant was observed to have eGFR<20 ml/min per 1.73 m2 or a visit at which there was a high predicted likelihood of a participant reaching eGFR<20 ml/min per 1.73 m2. This predicted likelihood was determined using an internal CRIC multivariable regression model that took into account variables, such as prior levels of eGFR, proteinuria, and BP.
In extended analyses, we studied a subset of participants who had serial echocardiograms performed at moderate stages of CKD, advanced CKD, and ESRD. Moderate CKD was defined as the visit preceding the advanced CKD visit. The echocardiogram corresponding to the moderate CKD visit was the year 1 echocardiogram in the majority of cases (1 year after entry into CRIC).
Description of Echocardiogram Parameters
Assessments of cardiac structure and function were performed by echocardiography according to American Society of Echocardiography guidelines (18). The echocardiograms are standard transthoracic echocardiograms done at the individual sites in accordance with a standard imaging protocol. Sonographers were initially trained in telephone conference calls and provided with a detailed scanning manual complete with a checklist. The core laboratory monitored quality control and adherence to the scanning protocol, and it provided critiques of the first several hundred echoes to the sites by fax. Supplemental training was provided as needed. Quality of echocardiograms was relatively uniform across sites. All echocardiograms were then quantified at the CRIC Central Echocardiography Laboratory at the University of Pennsylvania solely by one highly trained Registered Diagnostic Cardiac Sonographer with greater than 30 years of experience. This sonographer was not provided reference to measurements or images from the same patient done on a different date.
The following echocardiographic parameters were ascertained: LVM index (LVMI), relative wall thickness (RWT), LV EF, LV geometry, and diastolic dysfunction. LVM was calculated from two-dimensional images of the LV short axis muscle area and apical LV length (LVM = 5/6 area×length). Two-dimensional echocardiography had been selected by the CRIC Steering Committee to be used in all primary analyses of CRIC echocardiographic data. Left ventricular hypertrophy (LVH) was defined a priori using the Cornell Criteria; normal was considered <50 g/m2.7 for men and <47 g/m2.7 for women (18). RWT<0.45 was considered normal. EF was calculated using diastolic and systolic LV volumes measured by the single-plane Simpson’s rule method: EF=((Dvol−Svol)/Dvol)×100. EF was examined as a both a continuous and dichotomous variable, with a cutoff of ≤50% based on prior literature of preserved systolic function (19–21). LV geometry was based on LVMI and RWT and divided into four categories: (1) normal (normal LVMI and normal RWT), (2) concentric remodeling (normal LVMI and high RWT), (3) eccentric hypertrophy (elevated LVMI and normal RWT), and (4) concentric hypertrophy (elevated LVMI and elevated RWT). Multiple reproducibility, intrareader reliability, and reader drift analyses were performed throughout the course of this large-scale prospective cohort study yearly on a 2% random sample. The intrareader reliability correlation coefficients for the echocardiographic measures are 0.92 for LVM, 0.69 for RWT, and 0.85 for LV EF; 6% of patients were missing all echocardiographic measurements of interest and could not be included in the analyses.
Covariates
Information on covariates was obtained from the moderate CKD, advanced CKD, and ESRD visits (as appropriate). Covariates included demographic characteristics, physical examination components, self-reported comorbid conditions, tobacco and alcohol use, and medication use within 30 days of study visit. Self-reported history of cardiovascular disease included coronary heart disease, myocardial infarction or revascularization, heart failure, and stroke or peripheral vascular disease, and these factors were tallied cumulatively from earlier to later visits. BP measurement was performed in a quiet, standardized setting. Three seated resting BP readings were obtained at each visit, and the average of the three seated resting BP readings was used for this study. Urine proteinuria was determined from timed 24-hour urine collections. Dialysis treatment-related information was abstracted from the dialysis unit charts for each participant approximately 6 months after the initiation of renal replacement therapy.
Statistical Considerations
We compared differences in participant characteristics between visits at advanced CKD and ESRD using Generalized Estimating Equation models, because these analyses were paired (SAS 9.2; SAS, Cary, NC). To test the generalizability of our study population with serial echocardiograms to the larger CRIC cohort, we compared baseline characteristics of these two groups using chi-squared tests for categorical variables and t test for continuous variables.
We then compared echocardiogram measures at advanced CKD with echocardiogram with echocardiogram measures at ESRD using Generalized Estimating Equation models. We initially compared echocardiogram measures at these two time points in the whole study population (n=190). We tested for interactions by dialysis modality, age, race, sex, and diabetes status.
In extended analyses, we investigated the change in LV structure and function from moderate CKD to advanced CKD to ESRD using trend tests.
Results
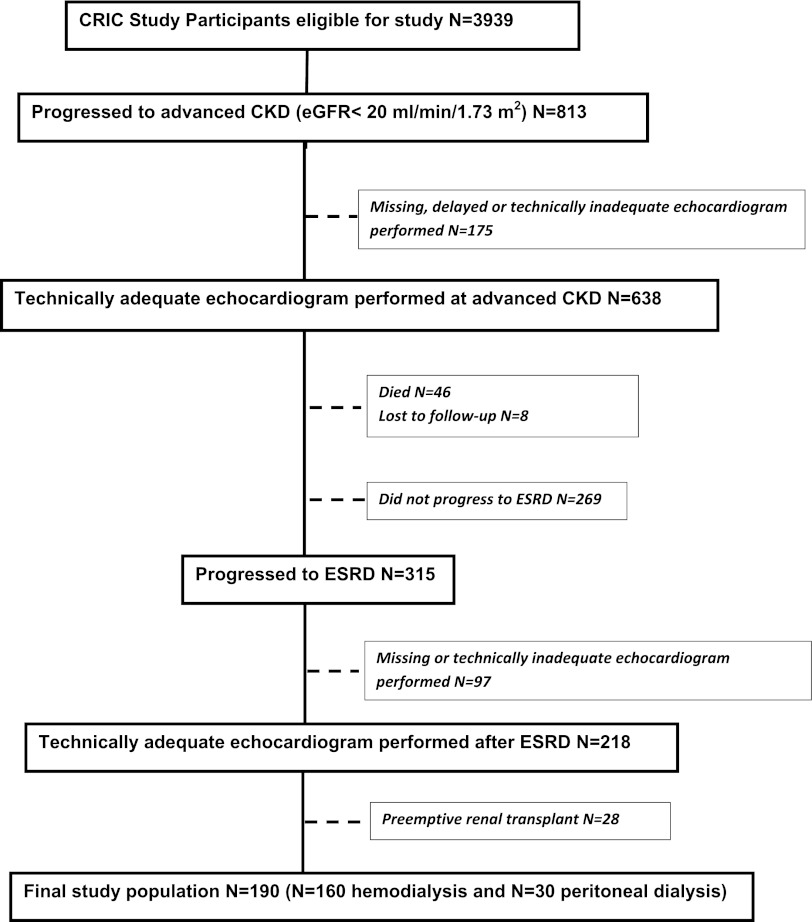
Of the total CRIC study population, 813 participants progressed to advanced CKD, of which 638 participants had an echocardiogram performed (Figure 1). Of 638 participants, 315 participants progressed to ESRD, of which 218 participants had a repeat echocardiogram after ESRD. Of 218 participants, we excluded 28 participants who underwent preemptive renal transplantation. Thus, our final study population consisted of 190 participants (160 hemodialysis and 30 peritoneal dialysis patients) (Figure 1). Of 190 participants, there were 89 participants available for our extended analysis to compare changes in three serial echocardiograms (at moderate CKD, advanced CKD, and ESRD).
Figure 1.
Derivation of study population.
To assess the generalizability of our study population with the CRIC population, we compared characteristics of participants who did versus did not have an echocardiogram performed. At advanced CKD, the participants who did not have an echocardiogram (Figure 1) were less likely to be black, had higher levels of proteinuria, and had lower hemoglobin compared with those participants who did have an echocardiogram (Supplemental Table 1). At ESRD, the participants who did not have an echocardiogram were older in age, were less likely to be black, and had lower body mass index compared with those participants who did have an echocardiogram performed at ESRD (Supplemental Table 2).
Characteristics of the Study Population at Advanced CKD and ESRD
The study population of 190 participants was diverse, with 44% women and 66% black participants. At advanced CKD, the mean (SD) age was 57.5 (11.2) years, median proteinuria was 2.75 g/24 hours (interquartile range=1.21–5.55 g/24 hours), mean eGFR was 16.9 (3.5) ml/min per 1.73 m2, and mean observed eGFR slope from the baseline study visit to advanced CKD was −7.7 (6.3) ml/min per 1.73 m2 per year.
Among the 160 participants who initiated hemodialysis, the mean intradialytic weight gain was 2.5 kg, mean predialysis BP was 154/80, and postdialysis BP was 144/76. Mean urea reduction ratio was 70%, and mean Kt/V was 1.53. Mean hemoglobin was 11.7 g/dl, and mean albumin was 3.8 mg/dl. Among the 30 participants who initiated peritoneal dialysis, mean weekly Kt/V was 1.95, mean hemoglobin was 11.8 g/dl, and mean albumin was 3.4 mg/dl.
From advanced CKD to ESRD, body mass index decreased, hemoglobin increased, BP decreased, and prevalence of self-reported cardiovascular disease increased among the study participants (Table 1).
Table 1.
| Characteristic | Time of First Echocardiogram at Advanced CKD | Time of Second Echocardiogram at ESRD | P Value |
|---|---|---|---|
| Body mass index (kg/m2; median [IQR]) | 31.9 (27.3, 36.9) | 30.4 (25.7, 35.1) | 0.01 |
| Hemoglobin (g/dl; mean [SD]) | 11.2 (1.5) | 12.3 (1.5) | <0.001 |
| Systolic BP (mmHg; mean [SD]) | 143.2 (26.8) | 131.7 (26.0) | <0.001 |
| Diastolic BP (mmHg; mean [SD]) | 72.1 (13.0) | 66.4 (13.0) | <0.001 |
| ACE inhibitor or ARB use (%) | 62 | 55 | 0.10 |
| Hypertensionc (%) | 100 | 100 | 1.00 |
| Diabetesc (%) | 69 | 70 | 0.30 |
| Cardiovascular diseasec (%) | 51 | 64 | <0.001 |
| Peripheral vascular diseasec (%) | 9 | 11 | 0.08 |
| Congestive heart failurec (%) | 15 | 26 | <0.001 |
| Strokec (%) | 14 | 18 | 0.008 |
| Myocardial infarction or revascularizationc (%) | 26 | 38 | <0.001 |
| Hypercholesterolemiac (%) | 96 | 97 | 0.20 |
| Current tobacco usec (%) | 11 | 11 | 0.70 |
| Current alcohol usec (%) | 47 | 42 | 0.08 |
IQR, interquartile range; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker.
Defined as estimated GFR<20 ml/min per 1.73 m2.
Defined as receipt of either peritoneal dialysis or hemodialysis.
By self-report.
Change in LV Structure and Function from Advanced CKD to ESRD
The mean (SD) time between the advanced CKD echocardiogram and ESRD echocardiogram was 2.0 (1.0) years. The mean time between the advanced CKD echocardiogram and start of dialysis was 1.1 (0.9) years.
The majority of participants (79%) had LVH at ESRD, similar to the proportion of LVH at advanced CKD (85%, P=0.10) (Table 2). There was no significant change in LVMI between the advanced CKD (62.3 [15.0] g/m2.7) and ESRD (59.5 [14.1] g/m2.7) echocardiograms (P=0.10).
Table 2.
| Variable | Advanced CKD | ESRD | P Value |
|---|---|---|---|
| Left ventricular hypertrophy (%) | 85 | 79 | 0.10 |
| Mean (SD) left ventricular mass index (g/m2.7) | 62.3 (15.0) | 59.5 (14.1) | 0.10 |
| Mean (SD) left ventricular ejection fraction (%) | 52.5 (7.8) | 49.7 (8.4) | 0.002 |
| Ejection fraction≤50% (%) | 29 | 48 | <0.001 |
| Left ventricular geometry (%) | 0.30 | ||
| Normal | 6 | 9 | |
| Concentric remodeling | 10 | 13 | |
| Eccentric hypertrophy | 25 | 21 | |
| Concentric hypertrophy | 60 | 58 |
Defined as estimated GFR<20 ml/min per 1.73 m2.
Defined as receipt of either peritoneal dialysis or hemodialysis.
In contrast, over this 2-year period as participants progressed from advanced CKD to ESRD, there was a decrease in mean EF from 53% to 50% (P=0.002). The proportion of participants with EF≤50% increased from 29% to 48% (P<0.001) (Table 2). Interactions for age, race, dialysis modality, and diabetes status were not significant (P>0.05). Interaction for sex was borderline statistically significant (P=0.05); however, both men and women had decline in EF from advanced CKD to ESRD.
Change in LV Structure and Function from Moderate CKD to Advanced CKD to ESRD
In extended analysis among the subgroup of 89 participants with three serial echocardiograms (the first echocardiogram at moderate CKD, the second echocardiogram at advanced CKD, and the third echocardiogram after ESRD), the mean eGFR at the time of the earliest echocardiogram was 28.6 (8.0) ml/min per 1.73 m2, and the mean eGFR at the time of the second echocardiogram was 17.4 (4.0) ml/min per 1.73 m2. The third echocardiogram was performed after ESRD. The mean time between the first CKD echocardiogram and the ESRD echocardiogram was 3.6 (1.0) years. The demographic characteristics of this subgroup were similar to the larger cohort of 190 participants (data not shown). In this extended analysis, we also found that there was little change in LVMI (58.2, 63.6, and 60.2 g/m2.7, respectively, P=0.20). A decline in mean EF was again observed as kidney disease progressed (from 54% to 51% to 50%, P=0.02) (Table 3). The proportion of participants who had EF≤50% rose progressively from 21% to 33% to 46% (P=0.005).
Table 3.
| Variable | Moderate CKD | Advanced CKD | ESRD | P Value |
|---|---|---|---|---|
| Left ventricular hypertrophy (%) | 70 | 83 | 85 | 0.20 |
| Mean (SD) left ventricular mass index (g/m2.7) | 58.2 (15.5) | 63.6 (15.7) | 60.2 (13) | 0.20 |
| Mean (SD) left ventricular ejection fraction (%) | 53.5 (7.0) | 51.4 (6.5) | 50.4 (8.5) | 0.02 |
| Ejection fraction≤50% (%) | 21 | 33 | 46 | 0.005 |
| Left ventricular geometry (%) | 0.60 | |||
| Normal | 10 | 7 | 7 | |
| Concentric remodeling | 21 | 12 | 7 | |
| Eccentric hypertrophy | 17 | 21 | 24 | |
| Concentric hypertrophy | 52 | 60 | 62 |
Defined as estimated GFR=20–60 ml/min per 1.73 m2.
Defined as estimated GFR<20 ml/min per 1.73 m2.
Defined as receipt of either peritoneal dialysis or hemodialysis.
Predictors of Decline in EF from Advanced CKD to ESRD
Participants who had deteriorations in EF from advanced CKD to ESRD had a higher prevalence of peripheral vascular disease at advanced CKD, but they were otherwise similar to those participants who had stable or increased EF from advanced CKD to ESRD (data not shown). In multivariable analyses, we found that lower systolic BP at advanced CKD was an independent predictor of decline in EF (0.8% [0.1%–1.5%]; additional decrement of EF for every 10 mmHg lower systolic BP, P=0.03). Age, sex, race, hemoglobin level, diastolic BP, eGFR level, proteinuria, and use of ACE inhibitors/angiotensin receptor blockers at advanced CKD were not significantly associated with subsequent EF decline from advanced CKD to ESRD.
Discussion
To our knowledge, this study is the first that has longitudinally examined change in LV structure and function during the transition from CKD to ESRD. In this racially diverse population, we found that, although LVM was abnormally high, it did not significantly change from CKD to ESRD, even over a time span extending 2 years from CKD to ESRD (and in a subgroup, over 3.6 years). However, we noted a decline in EF from CKD to ESRD over this time period. Our results suggest that abnormally elevated LVM is relatively fixed by moderate to advanced stages of CKD, whereas EF deteriorates during this important physiologic transition.
The lack of increase in LVMI during the transition from CKD to ESRD contrasts with prior cross-sectional studies showing that there is a higher prevalence of LVH with stepwise lower eGFR (12,22). However, longitudinal studies are superior to cross-sectional studies in providing information about disease evolution over time. In addition, prior cross-sectional studies have generally examined higher ranges of eGFR (e.g., eGFR≥60 versus <60 ml/min per 1.73 m2) and have not focused on finer gradations of lower eGFR level in advanced CKD and incident ESRD. By prospectively following patients from advanced stages of CKD to ESRD, our data provide novel insight into the natural history of cardiovascular disease evolution in patients who survive earlier stages of CKD and reach ESRD.
Our study found that, although LVMI was elevated in our study population, there was little change with progression from CKD to ESRD, whereas EF declined during this time period. These results are consistent with heart failure literature, which describes LVH as a precursor state to eventual systolic dysfunction. Pathologic processes to increase afterload (e.g., hypertension) are likely initiated early in CKD, thus leading to LVH, an adaptive response that initially normalizes wall stress and maintains a normal EF. Over time, LVH is associated with subendocardial ischemia and fibrosis, which can lead to eventual systolic dysfunction (23). The transition from compensated LVH to early heart failure is heralded by LV dilation, impairment of systolic function, and progression of the abnormalities in LV filling (24,25). Thus, the early impairment of EF that we found in our study population may be a sign of early heart failure. Our results suggest that there may be different opportunities for cardiovascular disease modification along the spectrum of CKD progression. For example, we found that, by advanced CKD, it may be too late to intervene in LVH; therefore, interventions to target LV structural disease must start early in the course of CKD, even years before ESRD. In contrast, therapies targeted to the preservation of LV function may be especially important at advanced CKD.
Few previous studies have assessed longitudinal change in EF in patients with kidney disease, although several papers have highlighted the significance of even modest decreases in EF. A prior analysis of patients with CKD showed that an EF threshold of 50% was an important independent predictor of mortality (9). Another investigation of 230 ESRD patients reported that a modest decrease in EF to ≤48% was the most significant predictor of sudden cardiac death (26). One longitudinal study of prevalent ESRD patients observed a decrease in LV systolic function over a mean time of 17 months apart, which was associated with a 51% increase in cardiovascular events over the subsequent 3 years (27). In the heart failure population, any or even small declines in EF have been shown to be clinically significant predictors of increased mortality (28,29). Our study is the first to look at change in LV systolic function specifically during the transition from CKD to ESRD, an important period of time associated with heightened cardiovascular risk.
In our study, lower systolic BP at advanced CKD was independently associated with subsequent EF decline from advanced CKD to ESRD (30–32). Interestingly, lower BP has been shown to be paradoxically associated with worse survival among patients with ESRD on hemodialysis (33–36) because of reasons that remain unclear. It is possible that this paradoxical association between BP and outcomes originates in the months to years before starting ESRD, a period that has been understudied in previous epidemiologic studies. We also found that demographic characteristics, measures of kidney function, and use of ACE inhibitors or angiotensin receptor blockers were not associated with EF decline in our study population. It is possible that novel uremia-related toxins may accumulate at advanced stages of CKD and play an important role in EF decline from CKD to ESRD. Additional investigation of novel cardiovascular risk factors that may contribute to decline in EF is warranted.
Prior studies have focused on patients with eGFR<60 ml/min per 1.73 m2 as a whole; however, greater granularity in studying evolution of cardiovascular disease may be necessary for more targeted and timely interventions. Our results suggest that cardiac function may be dynamic during advanced CKD through ESRD, and there may be different opportunities for cardiovascular disease modification along the spectrum of CKD progression.
Our study had several strengths. We used a unique prospective longitudinal cohort of patients with CKD. Serial research-grade echocardiograms were performed at key transition points (e.g., advanced CKD and after ESRD) and quantified by a central laboratory by a single reader. Our study had some limitations as well. Although this study is the largest study to date, the overall sample size was limited. We may, thus, be underpowered to detect modifiable risk factors for this decline in EF. Race, comorbid diseases, and cardiovascular events were ascertained by self-report. The ESRD echocardiogram was not performed immediately at incident ESRD but at a mean time of 1 year later. However, this result is entirely consistent with other studies, where echocardiograms performed 12–18 months after dialysis initiation were taken to represent incident ESRD pathophysiology (5,13,14,37,38). We did not have enough participants undergoing peritoneal dialysis to compare outcomes between these patients and patients on hemodialysis, although the interaction for dialysis modality was not significant. In this study population, adjudication of cardiovascular events was not complete, and the number of deaths was small; therefore, we were not able to examine associations between EF decline and important clinical end points. This study was of research volunteers, and therefore, results may not be generalizable to all CKD patients, although our estimates of LVH among new dialysis patients were very consistent with prior studies (39).
In conclusion, our findings suggest that there is significant decline in EF from advanced CKD to ESRD, whereas LVM is relatively stable. Additional studies are needed to elucidate the pathologic mechanism for this decline in EF, define its correlation with clinical outcomes, and explore possible interventions to preserve systolic function from CKD to ESRD.
Disclosures
None.
Supplementary Material
Acknowledgments
Funding for the Chronic Renal Insufficiency Cohort Study was obtained under a cooperative agreement from the National Institute of Diabetes and Digestive and Kidney Diseases (Grants U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by University of Pennsylvania Grant Clinical and Translational Research Center (CTRC) Clinical and Translational Science Awards (CTSA) UL1 RR-024134, Johns Hopkins University Grant UL1 RR-025005, University of Maryland Grant General Clinical Research Center (GCRC) M01 RR-16500, the Clinical and Translational Science Collaborative of Cleveland, Grant UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and National Institutes of Health roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) Grant UL1RR024986, University of Illinois at Chicago Grant CTSA UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology Grant P30GM103337, Kaiser National Institutes of Health/National Center for Research Resources (NCRR) Grant UCSF Clinical and Translational Science Institute (UCSF-CTSI) UL1 RR-024131, and American Kidney Fund Grants K23DK088865, K24DK092291, and R01DK070939.
Chronic Renal Insufficiency Cohort Study Investigators: Lawrence J. Appel; Harold I. Feldman; Alan S. Go; Jiang He; John W. Kusek; James P. Lash; Akinlolu Ojo; Mahboob Rahman; and Raymond R. Townsend.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06020612/-/DCSupplemental.
See related editorial, “Echocardiography: Providing Additional Insights into Cardiovascular Structural and Functional Abnormalities in Advanced CKD,” on pages 339–341.
References
- 1.Herzog CA, Ma JZ, Collins AJ: Poor long-term survival after acute myocardial infarction among patients on long-term dialysis. N Engl J Med 339: 799–805, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Chan KE, Maddux FW, Tolkoff-Rubin N, Karumanchi SA, Thadhani R, Hakim RM: Early outcomes among those initiating chronic dialysis in the United States. Clin J Am Soc Nephrol 6: 2642–2649, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE: Impact of hypertension on cardiomyopathy, morbidity and mortality in end-stage renal disease. Kidney Int 49: 1379–1385, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray DC, Barre PE: Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant 11: 1277–1285, 1996 [PubMed] [Google Scholar]
- 6.Foley RNPP, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barré PE: The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J Am Soc Nephrol 5: 2024–2031, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Silberberg JS, Barre PE, Prichard SS, Sniderman AD: Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int 36: 286–290, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Yamada S, Ishii H, Takahashi H, Aoyama T, Morita Y, Kasuga H, Kimura K, Ito Y, Takahashi R, Toriyama T, Yasuda Y, Hayashi M, Kamiya H, Yuzawa Y, Maruyama S, Matsuo S, Matsubara T, Murohara T: Prognostic value of reduced left ventricular ejection fraction at start of hemodialysis therapy on cardiovascular and all-cause mortality in end-stage renal disease patients. Clin J Am Soc Nephrol 5: 1793–1798, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu IW, Hung MJ, Chen YC, Hsu HJ, Cherng WJ, Chang CJ, Wu MS: Ventricular function and all-cause mortality in chronic kidney disease patients with angiographic coronary artery disease. J Nephrol 23: 181–188, 2010 [PubMed] [Google Scholar]
- 10.Eckardt KU, Scherhag A, Macdougall IC, Tsakiris D, Clyne N, Locatelli F, Zaug MF, Burger HU, Drueke TB: Left ventricular geometry predicts cardiovascular outcomes associated with anemia correction in CKD. J Am Soc Nephrol 20: 2651–2660, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levin A, Thompson CR, Ethier J, Carlisle EJ, Tobe S, Mendelssohn D, Burgess E, Jindal K, Barrett B, Singer J, Djurdjev O: Left ventricular mass index increase in early renal disease: Impact of decline in hemoglobin. Am J Kidney Dis 34: 125–134, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Paoletti E, Bellino D, Cassottana P, Rolla D, Cannella G: Left ventricular hypertrophy in nondiabetic predialysis CKD. Am J Kidney Dis 46: 320–327, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Foley RN, Curtis BM, Randell EW, Parfrey PS: Left ventricular hypertrophy in new hemodialysis patients without symptomatic cardiac disease. Clin J Am Soc Nephrol 5: 805–813, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foley RN, Parfrey PS, Kent GM, Harnett JD, Murray DC, Barre PE: Serial change in echocardiographic parameters and cardiac failure in end-stage renal disease. J Am Soc Nephrol 11: 912–916, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Levin A, Singer J, Thompson CR, Ross H, Lewis M: Prevalent left ventricular hypertrophy in the predialysis population: Identifying opportunities for intervention. Am J Kidney Dis 27: 347–354, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER, 3rd, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT, Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol 14[Suppl 2]: S148–S153, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Chronic Renal Insufficiency Cohort (CRIC) Study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 4: 1302–1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ, Chamber Quantification Writing Group. American Society of Echocardiography’s Guidelines and Standards Committee. European Association of Echocardiography : Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18: 1440–1463, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP: Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 355: 260–269, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Hsich EM, Grau-Sepulveda MV, Hernandez AF, Peterson ED, Schwamm LH, Bhatt DL, Fonarow GC: Sex differences in in-hospital mortality in acute decompensated heart failure with reduced and preserved ejection fraction. Am Heart J 163: 430–437, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Masoudi FA, Havranek EP, Smith G, Fish RH, Steiner JF, Ordin DL, Krumholz HM: Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol 41: 217–223, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Rahman M, Brown CD, Coresh J, Davis BR, Eckfeldt JH, Kopyt N, Levey AS, Nwachuku C, Pressel S, Reisin E, Walworth C, Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group : The prevalence of reduced glomerular filtration rate in older hypertensive patients and its association with cardiovascular disease: A report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Arch Intern Med 164: 969–976, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Condorelli G, Morisco C, Stassi G, Notte A, Farina F, Sgaramella G, de Rienzo A, Roncarati R, Trimarco B, Lembo G: Increased cardiomyocyte apoptosis and changes in proapoptotic and antiapoptotic genes bax and bcl-2 during left ventricular adaptations to chronic pressure overload in the rat. Circulation 99: 3071–3078, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Litwin SE, Katz SE, Weinberg EO, Lorell BH, Aurigemma GP, Douglas PS: Serial echocardiographic-Doppler assessment of left ventricular geometry and function in rats with pressure-overload hypertrophy. Chronic angiotensin-converting enzyme inhibition attenuates the transition to heart failure. Circulation 91: 2642–2654, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Katz AM: Cardiomyopathy of overload. A major determinant of prognosis in congestive heart failure. N Engl J Med 322: 100–110, 1990 [DOI] [PubMed] [Google Scholar]
- 26.Wang AY, Lam CW, Chan IH, Wang M, Lui SF, Sanderson JE: Sudden cardiac death in end-stage renal disease patients: A 5-year prospective analysis. Hypertension 56: 210–216, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Zoccali C, Benedetto FA, Tripepi G, Mallamaci F, Rapisarda F, Seminara G, Bonanno G, Malatino LS: Left ventricular systolic function monitoring in asymptomatic dialysis patients: A prospective cohort study. J Am Soc Nephrol 17: 1460–1465, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Sato Y, Yamada T, Taniguchi R, Nagai K, Makiyama T, Okada H, Kataoka K, Ito H, Matsumori A, Sasayama S, Takatsu Y: Persistently increased serum concentrations of cardiac troponin t in patients with idiopathic dilated cardiomyopathy are predictive of adverse outcomes. Circulation 103: 369–374, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Horwich TB, Patel J, MacLellan WR, Fonarow GC: Cardiac troponin I is associated with impaired hemodynamics, progressive left ventricular dysfunction, and increased mortality rates in advanced heart failure. Circulation 108: 833–838, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Davis BR, Kostis JB, Simpson LM, Black HR, Cushman WC, Einhorn PT, Farber MA, Ford CE, Levy D, Massie BM, Nawaz S, ALLHAT Collaborative Research Group : Heart failure with preserved and reduced left ventricular ejection fraction in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Circulation 118: 2259–2267, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ekundayo OJ, Allman RM, Sanders PW, Aban I, Love TE, Arnett D, Ahmed A: Isolated systolic hypertension and incident heart failure in older adults: A propensity-matched study. Hypertension 53: 458–465, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haider AW, Larson MG, Franklin SS, Levy D, Framingham Heart Study : Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med 138: 10–16, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Li Z, Lacson E, Jr, Lowrie EG, Ofsthun NJ, Kuhlmann MK, Lazarus JM, Levin NW: The epidemiology of systolic blood pressure and death risk in hemodialysis patients. Am J Kidney Dis 48: 606–615, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Zager PG, Nikolic J, Brown RH, Campbell MA, Hunt WC, Peterson D, Van Stone J, Levey A, Meyer KB, Klag MJ, Johnson HK, Clark E, Sadler JH, Teredesai P: “U” curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc. Kidney Int 54: 561–569, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Port FK, Hulbert-Shearon TE, Wolfe RA, Bloembergen WE, Golper TA, Agodoa LY, Young EW: Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis 33: 507–517, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Duranti E, Imperiali P, Sasdelli M: Is hypertension a mortality risk factor in dialysis? Kidney Int Suppl 55: S173–S174, 1996 [PubMed] [Google Scholar]
- 37.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE: Hypoalbuminemia, cardiac morbidity, and mortality in end-stage renal disease. J Am Soc Nephrol 7: 728–736, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, Barre PE: Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int 47: 186–192, 1995 [DOI] [PubMed] [Google Scholar]
- 39.Foley RN, Parfrey PS, Kent GM, Harnett JD, Murray DC, Barre PE: Long-term evolution of cardiomyopathy in dialysis patients. Kidney Int 54: 1720–1725, 1998 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.