Summary
Background and objective
The association of large arterial rigidity and kidney function decline in longitudinal analyses is not well established. This study evaluated the association of aortic pulse wave velocity (aPWV) and pulse pressure (PP) with rapid kidney function decline and incident CKD in the Health, Aging and Body Composition study.
Design, setting, participants, & measurements
Participants were 2129 older adults with a baseline measurement of aPWV, PP, and cystatin C and at least one additional measurement of cystatin C, either at year 3 or year 10. Outcomes were rapid kidney function decline (estimated GFRcysC loss of >3 ml/min per 1.73 m2 per year) and incident CKD (eGFRcysC < 60 ml/min per 1.73 m2 in participants with baseline estimated GFR > 60 ml/min per 1.73 m2). Multivariate regression models were used to evaluate association of aPWV and PP with each outcome.
Results
Mean (SD) baseline estimated GFRcysC was 79±29 ml/min per 1.73 m2. Median follow-up duration was 8.9 years. In multivariable analyses, aPWV was not associated with rapid decline (odds ratio [OR], 95% confidence interval [CI] 1.16, 0.89–1.52) but was associated with incident CKD (incident rate ratio [IRR], 95% CI, 1.39, 1.09–1.77) and PP was associated with both rapid decline (OR, 95% CI 1.10, 1.04–1.16) and incident CKD (IRR, 95% CI, 1.06, 1.01–1.11).
Conclusions
Large arterial stiffness assessed by aPWV and pulsatility assessed by PP were associated with incident CKD among older adults. Pulsatility assessed by PP was associated with rapid kidney function decline and incident CKD. Future research should determine whether interventions targeting arterial rigidity will prevent CKD development and progression.
Introduction
Increased arterial stiffness is associated with adverse alterations in cardiac structure and function that predispose to an increased risk of death from cardiovascular disease (CVD) (1–3). Because large-vessel arterial stiffness is common in CKD, it is believed to be one of the mechanisms accounting for the increased risk of CVD in CKD. There are fewer data on the relationship of vascular stiffness to longitudinal kidney function decline. An analysis of well functioning older adults from the Health, Aging and Body Composition (Health ABC) study, we noted that higher levels of cystatin C, a marker of impaired kidney function, but not creatinine, were associated with higher pulse wave velocity (aPWV) (4). This study was cross-sectional, so the direction of association is unclear. Although several mechanisms can explain how CKD may cause arterial stiffness, there are also potential mechanisms through which vascular stiffness may promote progression of kidney disease (5–10).
A few recent studies have evaluated the longitudinal relationship between arterial stiffness and kidney function decline (11–16), but most of them have been limited by small size, short-term follow-up, no measurement of cystatin C or gold standard assessment of arterial stiffness, or lack of focus on the elderly (the population with the highest prevalence of CKD) (17). Therefore, this study sought to evaluate the association between arterial rigidity, measured by both aPWV and pulse pressure (PP), with kidney function decline and incident CKD and to determine whether this association is independent of BP and other potential confounding factors. The Health ABC study provides an ideal cohort in which to evaluate this association given the large sample; detailed ascertainment of potential covariates; baseline measurement of aPWV and PP as markers of aortic stiffness and pulsatility, respectively; repeated measures of kidney function; and moderate length of follow-up.
Materials and Methods
Participants
Health ABC is a population-based, prospective study designed to evaluate the effect of weight and body composition on age-related physiologic and functional changes. Details of the study design have been described elsewhere (18). Briefly, individuals aged 70–79 years were recruited from March 1997 through July 1998 at two field centers located in Pittsburgh, Pennsylvania, and Memphis, Tennessee. White participants were drawn from a random sample of Medicare beneficiaries residing in ZIP codes from the metropolitan areas surrounding Pittsburgh and Memphis, and black participants were recruited from all age-eligible persons in the selected ZIP codes. The participants made a day-long visit to the clinic, during which baseline data were recorded, including medical history, physical examination, and aPWV measurement. The cohort consisted of 3075 men (48.4%) and women (51.6%), of whom 41.6% were black. Eligibility for the study included the requirement that participants report no difficulty in walking a quarter mile, climbing 10 steps, or performing the basic activities of daily living. Aortic PWV measurements were not available for 354 participants (12%) because of equipment problems. Another 233 participants (8%) had waveforms that were unusable for analysis for various reasons. This analysis included 2129 older adults with a baseline measurement of aPWV and cystatin C and an additional measurement of cystatin C obtained at year 3 (n=2068) or year 10 (n=1221) of follow-up.
Measurements
Cystatin C was measured in plasma specimens that had been stored at −70°C at the University of Vermont (which is not the Health ABC Core Laboratory), by means of a BNII Nephelometer (Dade Behring, Deerfield, IL), incorporating a particle-enhanced immunonephelometric assay (N Latex Cystatin C) (19). The intraindividual coefficient of variation was 7.7%. The assay range is 0.195–7.330 mg/L, with the reference range for young, healthy individuals reported as 0.530–0.950 mg/L. Because of drift, the calibration factor for cystatin C included a 20% increase for levels assayed after 2006 (20). GFR was estimated (eGFRcysC) using the following formula:
This formula was developed from the pooling of several cohorts with GFR measured from iothalamate (21).
Creatinine was assayed by a colorimetric technique on a Johnson & Johnson Vitros 950 analyzer (New Brunswick, NJ); the intraindividual coefficient of variation was approximately 1.5% in Health ABC reproducibility studies.
aPWV.
The methods for measuring aPWV in Health ABC have been described in detail (22). In brief, aPWV was ascertained at the baseline study visit from simultaneous Doppler flow signals obtained from the right carotid and right femoral arteries, using nondirectional transcutaneous Doppler flow probes (model 810A, 9.0–10-MHz probes; Parks Medical Electronics, Aloha, OR). The distance between the carotid and femoral sampling sites was measured above the surface of the body with a metal tape measure. Digitized data were recorded by custom programming for subsequent analysis. Less compliant vessels are associated with a faster aPWV. Results from all acceptable runs were averaged for the final aPWV measure used in the analyses. Replicate measures of aPWV in 14 individuals revealed intraclass correlations of 0.88 between sonographers and 0.84 between readers.
PP.
Brachial systolic BP (SBP) and diastolic BP (DBP) measurements were obtained as the average of two seated measurements. PP was defined as SBP minus DBP from the average of two seated measurements.
Outcomes
Change in kidney function was defined as eGFRcysC change in ml/min per 1.73 m2 per year. Rapid kidney function decline was defined as eGFRcysC loss of > 3 ml/min per 1.73 m2 per year because prior studies have demonstrated that this rate is associated with increased CVD morbidity and mortality, independent of baseline eGFR (23,24).
Incident CKD was defined as a follow-up eGFRcysC < 60 ml/min per 1.73 m2 in individuals with baseline GFR > 60 ml/min per 1.73m2. To avoid participants with minor fluctuations in eGFRcysC that may be due to “noise,” the definition also included a 1–ml/min per 1.73 m2 per year decrease in eGFR.
Our primary analyses were based on cystatin C for several reasons. Compared with creatinine, cystatin C is less influenced by sex, race, and muscle mass and may therefore more accurately reflect kidney function in an elderly population (19). Second, cystatin C appears to be more sensitive to changes in kidney function (23) and is more strongly associated with adverse outcomes in the elderly (25,26). Finally, we were unable to adjust for drift or calibration of creatinine given that year 3 and 10 creatinine values were standardized to IDMS while baseline was not.
Covariates
These included sociodemographic factors (age, sex, race, clinical site, education level); lifestyle factors (current smoking [defined by current versus former or never], alcohol use [defined by ≤1 drink per day versus >1 drink per day], and body mass index); comorbid conditions (impaired fasting glucose, defined as fasting glucose from 100 to 125 mg/dl; impaired glucose tolerance, defined as a 2-hour glucose tolerance of 140–200 mg/dl; diabetes, defined by use of hypoglycemic agents, self-report, fasting plasma glucose level >126 mg/dl or an oral glucose tolerance test result >200 mg/dl; hypertension, defined by either self-report plus use of antihypertensive medications or measured SBP >140 mmHg or DBP >90 mmHg; and MAP, heart failure, and coronary heart disease, defined as coronary heart disease, myocardial infarction, angina, and coronary artery bypass). Additional candidate covariates included HDL and LDL cholesterol.
Statistical Analyses
We categorized participants by quartiles of aPWV and compared the distribution of demographic characteristics and covariates across quartiles using a chi-squared test for categorical variables and ANOVA or Kruskal–Wallis tests for continuous variables, as appropriate. Spearman correlation was used to evaluate the correlation between PP and aPWV. Change in kidney function was defined by calculating the rates of change in eGFRcysC using two or three measurements of cystatin C (baseline to year 3 or year 10 of follow-up).
Multivariate linear regression models were used to evaluate the association between aPWV and PP with change in kidney function. The value of aPWV was right-skewed, and therefore the data were log-transformed to approximate a Gaussian distribution. Multivariable logistic regression models were used to evaluate the association between aPWV and PP with rapid decline as well as incident CKD. Analyses were conducted as continuous models for aPWV (β values represent a decline in ml/min per year per doubling of aPWV) and PP (per 10-mmHg increase) and then categorized into quartiles. Models were adjusted as follows: Model 1 adjusted for demographic variables (age, sex, race, and site). Model 2 adjusted for demographic variables, SBP, and hypertensive medications. Model 3 adjusted for model 2 variables plus smoking, LDL cholesterol, HDL cholesterol, prevalent coronary heart disease, and prevalent heart failure. Model 4 adjusted for all variables in model 3, excluded SBP, but added MAP. PP is based on SBP and DBP, and therefore PP models adjusted for antihypertensive medications and MAP but not for SBP or DBP. Unadjusted splines incorporating the 2.5th to the 97.5th percentiles were created to evaluate the association between log-transformed PWV and PP with incident CKD. All analyses were performed using S-Plus, release 8.0 (Insightful Inc., Seattle, WA), and SPSS software, release 16.0.1 (SPSS Inc., Chicago, IL).
Sensitivity Analyses
We performed two sensitivity analyses: the first repeating all the analyses using the CKD-Epidemiology Collaboration (CKD-EPI) equation (27) in place of eGFRcysC and the second repeating the analyses exclusively in individuals with three measures of kidney function.
Results
Among the 2129 study participants, the mean age ± SD was 74±3 years, 53% were female, 38% were black, 14% had diabetes, 26% had prevalent CVD, and 2% had prevalent heart failure. Mean ± SD baseline cystatin C and eGFRcysC were 1.03±0.25 mg/L and 79±29 ml/min per 1.73 m2, respectively. The correlation between aPWV and PP was 0.20. Participants in the highest aPWV quartile had a higher percentage of blacks and men and had worse cardiovascular risk profiles (Table 1). There were 211 participants without usable aPWV waveforms. This subgroup had a higher percentage of men and a higher prevalence of coronary heart disease but lower serum albumin, total cholesterol, and BP levels. Median length of follow-up was 8.85 years. The mean eGFRcysC was 76±20 ml/min per 1.73 m2 at year 3 and 71±21 ml/min per 1.73 m2 at year 10. A total of 568 (27%) and 344 (19%) of the cohort had rapid decline and incident CKD, respectively. The mean yearly change for eGFRcysC in the cohort was –1.59± 4.84 ml/min per year.
Table 1.
Baseline characteristics by quartiles of aortic pulse wave velocity
| Characteristic | All Patients (n = 2129) | aPWV Quartile | P Value | |||
|---|---|---|---|---|---|---|
| 1 (312–641 cm/s) (n = 546) | 1 (642–808 cm/s) (n =546) | 3 (809–1052 cm/s) (n = 518) | 4 (1053–2998 cm/s) (n = 519) | |||
| Age (yr) | 74±3 | 73±3 | 74±3 | 74±3 | 74±3 | <0.001 |
| Female (%) | 53 | 58 | 56 | 50 | 46 | <0.001 |
| Black (%) | 38 | 34 | 39 | 38 | 43 | 0.004 |
| Hypertension (%) | 46 | 36 | 45 | 50 | 57 | <0.001 |
| SBP (mmHg) | 136±21 | 130±19 | 134±19 | 138±21 | 141±22 | <0.001 |
| DBP (mmHg) | 72±11 | 70±11 | 72±11 | 72±11 | 73±12 | <0.001 |
| Diabetes (%) | 14 | 8 | 11 | 17 | 21 | <0.001 |
| Smoking (%) | 0.003 | |||||
| Never | 45 | 51 | 45 | 41 | 43 | |
| Former | 46 | 40 | 46 | 52 | 47 | |
| Current | 9 | 9 | 9 | 8 | 10 | |
| BMI (kg/m2) | 27±5 | 26.2±4.4 | 27.7±(4.6) | 28.0±4.9 | 27.6±4.9 | <0.001 |
| Total cholesterol (mg/dl) | 204±38 | 203±35 | 205±(37) | 203±41 | 203±40 | 0.71 |
| HDL cholesterol (mg/dl) | 54±17 | 57±17 | 55±16 | 53±17 | 53±18 | <0.001 |
| LDL cholesterol (mg/dl) | 122±35 | 121±32 | 123±34 | 122±36 | 122±36 | 0.77 |
| Triglycerides (mg/dl)a | 119 (89–166] | 114 (83–151) | 119 (91–172) | 123 (92–172) | 121 (90–174) | 0.001 |
| Albumin (g/dl) | 4.00±0.31 | 3.99±0.33 | 4.03±0.30 | 4.00±0.31 | 4.01±0.30 | 0.58 |
| eGFRcysC (ml/min per 1.73 m2) | 79±19 | 82±19 | 80±18 | 77±19 | 76±20 | <0.001 |
| AAI < 0.9 (%) | 12 | 8 | 12 | 13 | 17 | <0.001 |
| MAP (mmHg) | 93±13 | 90±12 | 92±12 | 94±13 | 96±13 | <0.001 |
| Pulse pressure (mmHg) | 64±18 | 60±16 | 63±16 | 66±18 | 69±19 | <0.001 |
| Prevalent disease (%) | ||||||
| Heart failure | 2 | 2 | 2 | 3 | 3 | 0.61 |
| Coronary heart disease | 23 | 20 | 19 | 27 | 24 | 0.01 |
| Peripheral artery disease | 5 | 5 | 4 | 6 | 5 | 0.46 |
Values expressed with a plus/minus sign are the mean ± SD. aPWV, aortic pulse wave velocity; SBP, systolic BP; DBP, diastolic BP; BMI, body mass index; eGFRcysC, GFR estimated using cystatin C; AAI, ankle arm index; MAP, mean arterial pressure.
Median (interquartile range).
Change in Kidney Function
Higher log-transformed aPWV was associated with faster rates of decline in unadjusted and demographic variable–adjusted models. Adjustment for SBP, CVD risk factors, and prevalent CVD attenuated this association substantially and rendered the results nonsignificant (Table 2). In analyses that considered quartiles of aPWV, the highest quartiles were associated with kidney function decline in unadjusted and demographic variable–adjusted models, which was attenuated after adjustment for SBP and in fully adjusted models. In continuous analysis, higher PP was associated with kidney function decline in unadjusted and fully adjusted models. The highest quartile of PP was also associated with kidney function decline in fully adjusted models (Table 2).
Table 2.
Association of aortic pulse wave velocity and pulse pressure with change in kidney function by GFR estimated using cystatin C
| Variable | Patients (n) | β Value (95% Confidence Interval)a | ||||
|---|---|---|---|---|---|---|
| Unadjusted | Demographic Variable–Adjustedb | BP-Adjustedc | Fully Adjustedd | Mean Arterial Pressure–Adjustede | ||
| Pulse wave velocity | ||||||
| Continuous | ||||||
| Log transformeda | 2129 | −0.26 (−0.51 to -0.02)f | −0.28 (−0.52 to -0.03)f | −0.15 (−0.40 to 0.11) | −0.11 (−0.37 to 0.14) | −0.15 (−0.40 to 0.10) |
| Quartiles | ||||||
| <642 cm/s | 546 | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) |
| 642–808 cm/s | 546 | −0.07 (−0.34 to 0.19) | −0.08 (−0.34 to 0.19) | −0.04 (−0.30 to 0.23) | −0.02 (−0.29 to 0.25) | −0.03 (−0.30 to 0.24) |
| 809–1052 cm/s | 518 | −0.33 (−0.60 to −0.05)f | −0.33 (−0.61 to −0.05)f | −0.24 (−0.53 to 0.04) | −0.26 (−0.54 to 0.03) | −0.28 (−0.57 to 0.01) |
| >1052 cm/s | 519 | −0.27 (−0.54 to −0.01)f | −0.29 (−0.56 to −0.02)f | −0.17 (−0.44 to 0.11) | −0.13 (−0.40 to 0.15) | −0.16 (−0.43 to 0.12) |
| Pulse pressureg | ||||||
| Continuous (per 10 mmHg) | 2129 | −0.12 (−0.17 to −0.06)f | −0.11 (−0.17 to −0.06) f | −0.10 (−0.16 to −0.04) f | −0.10 (−0.16 to −0.04) f | −0.09 (−0.15 to −0.02) f |
| Quartiles | ||||||
| <53 mmHg | 550 | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) |
| 53–62 mmHg | 520 | −0.21 (−0.48 to 0.05) | −0.19 (−0.46 to 0.07) | −0.18 (−0.44 to 0.09) | −0.14 (−0.41 to 0.13) | −0.12 (−0.39 to 0.16) |
| 63–75 mmHg | 575 | −0.24 (−0.50 to 0.01) | −0.22 (−0.48 to 0.04) | −0.20 (−0.45 to 0.06) | −0.19 (−0.45 to 0.07) | −0.15 (−0.42 to 0.12) |
| >75 mmHg | 484 | −0.57 (−0.86 to −0.28) f | −0.54 (−0.83 to −0.25) f | −0.49 (−0.78 to -0.19) f | −0.44 (−0.74 to −0.14) f | −0.38 (−0.71 to −0.05) f |
β values represent a decline in ml/min per 1.73 m2 per year per doubling of aortic pulse wave velocity.
Adjusted for age, sex, race, and site.
Adjusted for age, sex, race, site, systolic BP, and antihypertensive medications.
Adjusted for age, sex, race, site, systolic BP, antihypertensive medications, diabetes mellitus, smoking, LDL cholesterol, HDL cholesterol, prevalent coronary heart disease, and prevalent heart failure.
Adjusted for age, sex, race, site, antihypertensive medications, diabetes mellitus, smoking, LDL cholesterol, HDL cholesterol, prevalent coronary heart disease, prevalent heart failure, and mean arterial pressure.
P<0.05.
Neither systolic nor diastolic BP is included in the models for pulse pressure. BP adjustment is only for antihypertensive medications and mean arterial pressure.
Rapid Kidney Function Decline
Higher aPWV was associated with “rapid decline” in demographic variable–adjusted models, but the relationship was attenuated after adjustment for SBP. Higher quartiles of aPWV were associated with rapid kidney function decline in univariate and demographic variable–adjusted models, but the relationships were attenuated after SBP adjustment (Table 3).
Table 3.
Association of aortic pulse wave velocity and pulse pressure with rapid kidney function decline by GFR estimated using cystatin C
| Variable | Patients (n) | Rapid Decline, n (%) | Odds Ratio (95% Confidence Interval) | ||||
|---|---|---|---|---|---|---|---|
| Unadjusted | Demographic Variable–Adjusteda | BP-Adjustedb | Fully Adjustedc | Mean Arterial Pressure–Adjustedd | |||
| Pulse wave velocity | |||||||
| Continuous | 2129 | 568 (27) | 1.45 (1.13–1.86) | 1.33 (1.03–1.72) | 1.21 (0.93–1.58) | 1.16 (0.89–1.52) | 1.20 (0.92–1.57) |
| Log transformede | |||||||
| Quartiles | |||||||
| <642 cm/s | 546 | 120 (22) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 642–808 cm/s | 546 | 136 (25) | 1.13 (0.85–1.51) | 1.07 (0.80–1.43) | 1.04 (0.78–1.39) | 1.01 (0.76–1.36) | 1.02 (0.77–1.37) |
| 809–1052 cm/s | 518 | 159 (31) | 1.54 (1.16–2.03) | 1.41 (1.06–1.88) | 1.32 (0.99–1.77) | 1.26 (0.94–1.69) | 1.28 (0.95–1.72) |
| >1052 cm/s | 519 | 153 (30) | 1.46 (1.10–1.94) | 1.33 (1.00–1.77) | 1.21 (0.90–1.63) | 1.16 (0.86–1.57) | 1.20 (0.89–1.62) |
| Pulse pressuref | |||||||
| Continuous (per 10 mmHg) | 2129 | 568 (27) | 1.12 (1.06–1.19) | 1.10 (1.05–1.17) | 1.10 (1.04–1.17) | 1.10 (1.04–1.16) | 1.09 (1.02–1.16) |
| Quartiles | |||||||
| <53 mmHg | 550 | 119 (22) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 53–62 mmHg | 520 | 134 (26) | 1.22 (0.92–1.63) | 1.21 (0.91–1.62) | 1.20 (0.90–1.61) | 1.18 (0.88–1.58) | 1.16 (0.86–1.55) |
| 63–75 mmHg | 575 | 150 (26) | 1.27 (0.96–1.68) | 1.22 (0.92–1.62) | 1.21 (0.91–1.60) | 1.18 (0.89–1.57) | 1.14 (0.85–1.54) |
| >75 mmHg | 484 | 165 (34) | 1.77 (1.24–2.35) | 1.70 (1.28–2.26) | 1.66 (1.24–2.22) | 1.60 (1.20–2.14) | 1.52 (1.10–2.09) |
Adjusted for age, sex, race, and site.
Adjusted for age, sex, race, site, systolic BP, and hypertension medications.
Adjusted for age, sex, race, site, systolic BP, antihypertensive medications, diabetes mellitus, smoking, LDL cholesterol, HDL cholesterol, prevalent coronary heart disease, and prevalent heart failure.
Adjusted for age, sex, race, site, antihypertensive medications, diabetes mellitus, smoking, LDL cholesterol, HDL cholesterol, prevalent coronary heart disease, prevalent heart failure, and mean arterial pressure.
Odds ratios are per doubling of aortic pulse wave velocity.
Neither systolic nor diastolic BP is included in the models for pulse pressure. BP adjustment is only for hypertension medications and mean arterial pressure.
In continuous analyses, higher PP was associated with rapid kidney function decline in all models. The highest quartile of PP was also associated with rapid kidney function decline in multivariate models (Table 3).
Incident CKD
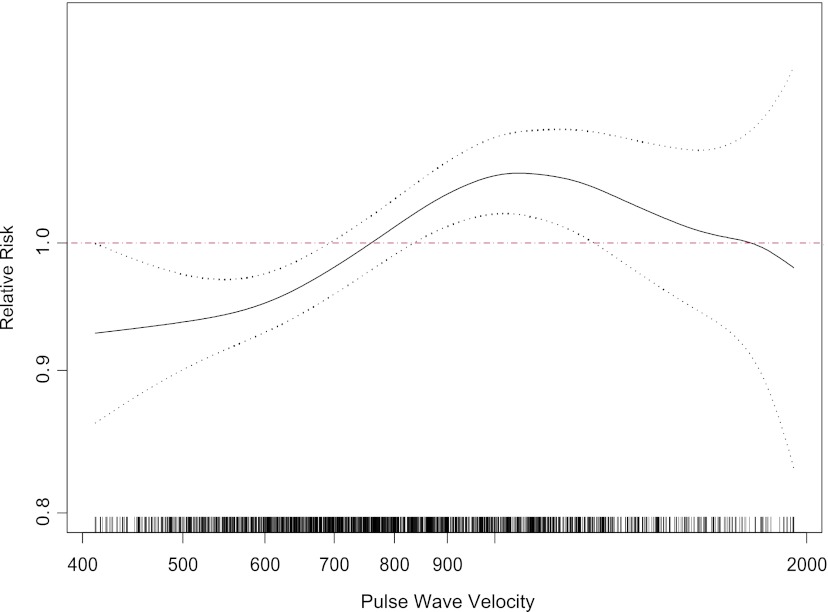
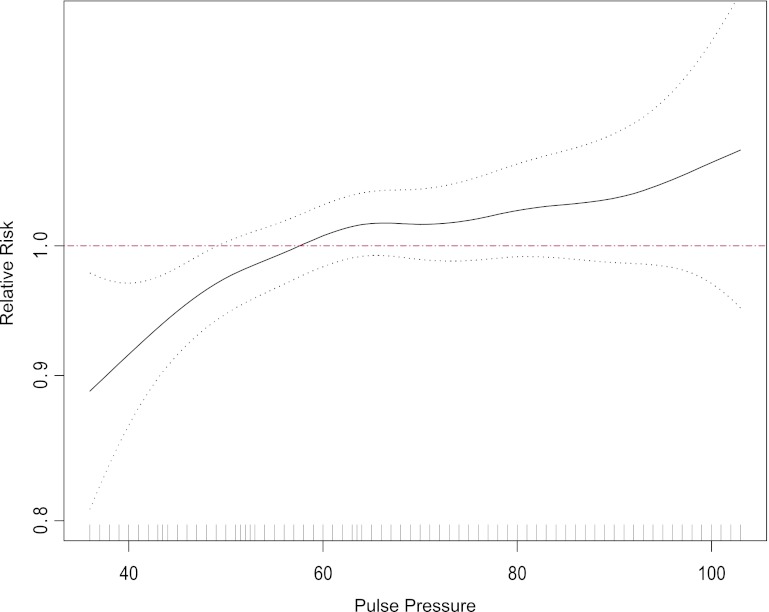
Higher aPWV and PP were associated with incident CKD in the spline analysis (Figures 1 and 2). Higher aPWV was associated with incident CKD in all models (Table 4), and results were consistent in the quartile analyses. Although the point estimate for the third quartile is higher than that for the fourth quartile, the confidence intervals overlap, suggesting perhaps a threshold effect. The P value for the deviation from linearity, however, was 0.235, suggesting no overt deviation from linearity. Higher PP was associated with incident CKD in continuous models, and the highest quartile of PP was also associated with incident CKD compared with the reference quartile (Table 4).
Figure 1.
Spline of pulse wave velocity and incident CKD. Dotted black lines are 95% confidence intervals of the spline; red line is the reference. Distribution of the population is shown along the x axis.
Figure 2.
Spline of pulse pressure and incident CKD. Dotted black lines are 95% confidence intervals of the spline; red line is the reference. Distribution of the population is shown along the x axis.
Table 4.
Association of aortic pulse wave velocity and pulse pressure with incident CKD by GFR estimated using cystatin C
| Variable | Patients (n) | Incident CKD, n (%) | Incident Rate Ratio (95% Confidence Interval) | ||||
|---|---|---|---|---|---|---|---|
| Unadjusted | Demographic Variable–Adjusteda | BP-Adjustedb | Fully Adjustedc | Mean Arterial Pressure–Adjustedd | |||
| Pulse wave velocity | |||||||
| Continuous | |||||||
| Log transformede | 1788 | 344 (19) | 1.77 (1.42–2.21) | 1.75 (1.40–2.19) | 1.58 (1.26–1.99) | 1.39 (1.09–1.77) | 1.42 (1.12–1.81) |
| Quartiles | |||||||
| <642 cm/s | 477 | 56 (12) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 642–808 cm/s | 476 | 92 (19) | 1.74 (1.28–2.36) | 1.77 (1.30–2.40) | 1.73 (1.28–2.35) | 1.60 (1.18–2.16) | 1.60 (1.19–2.17) |
| 809–1052 cm/s | 420 | 106 (25) | 2.33 (1.73–3.13) | 2.30 (1.71–3.10) | 2.15 (1.59–2.90) | 1.79 (1.32–2.43) | 1.83 (1.35–2.47) |
| >1052 cm/s | 415 | 90 (22) | 2.09 (1.53–2.84) | 2.07 (1.52–2.81) | 1.90 (1.39–2.58) | 1.59 (1.17–2.17) | 1.63 (1.20–2.22) |
| Pulse pressuref | |||||||
| Continuous (per 10 mmHg) | 1788 | 344 (19) | 1.10 (1.05–1.15) | 1.09 (1.03–1.14) | 1.06 (1.01–1.11) | 1.06 (1.01–1.11) | 1.06 (1.01–1.12) |
| Quartiles | |||||||
| <53 mmHg | 463 | 73 (16) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 53–62 mmHg | 448 | 80 (18) | 1.23 (0.92–1.64) | 1.23 (0.92–1.64) | 1.20 (0.90–1.59) | 1.24 (0.92–1.64) | 1.23 (0.93–1.64) |
| 63–75 mmHg | 485 | 100 (21) | 1.34 (1.02–1.76) | 1.30 (0.99–1.71) | 1.24 (0.94–1.62) | 1.24 (0.94–1.64) | 1.23 (0.93–1.63) |
| >75 mmHg | 392 | 91 (23) | 1.64 (1.24–2.17) | 1.56 (1.18–2.07) | 1.39 (1.05–1.84) | 1.41 (1.07–1.87) | 1.39 (1.02–1.89) |
Adjusted for age, sex, race, and site.
Adjusted for age, sex, race, site, systolic BP, and antihypertensive medications.
Adjusted for age, sex, race, site, systolic BP, antihypertensive medications, diabetes mellitus, smoking, LDL cholesterol, HDL cholesterol, prevalent heart failure, and baseline GFR estimated using cystatin C.
Adjusted for age, sex, race, site, antihypertensive medications, diabetes mellitus, smoking, LDL cholesterol, HDL cholesterol, prevalent coronary heart disease, prevalent heart failure, baseline GFR estimated using cystatin C, and mean arterial pressure.
Incident rate ratios are per doubling of aortic pulse wave velocity.
Neither systolic nor diastolic BP is included in the models for pulse pressure. BP adjustment is only for antihypertensive medications and mean arterial pressure.
Analyses Adjusted by CKD-EPI.
When the analyses were repeated using creatinine-based eGFR (CKD-EPI), the following results were observed:
Change in kidney function: The results for eGFR (CKD-EPI) and change in kidney function differed slightly from our original data. Higher aPWV was associated with kidney function decline in univariate but also in the fully adjusted models for both continuous and categorical analyses. Results for PP and change in kidney function were similar to those seen with the eGFRcysC models for both continuous and categorical analyses (Supplemental Table 1).
Rapid kidney function decline: The results for aPWV and rapid kidney function decline using CKD EPI were essentially unchanged. Results for PP and kidney function decline were similar to the original results with the exception of the highest quartile of PP. This variable was associated with rapid kidney function decline in univariate models, but the results were attenuated after adjustment for BP (Supplemental Table 2).
Incident CKD: The results for eGFR (CKD-EPI) with incident CKD differed from the original data because there was no significant association between aPWV and incident CKD in unadjusted or adjusted models for both continuous and categorical models. Likewise, in both continuous and categorical models, PP was associated with incident CKD in unadjusted models, but this effect was attenuated after adjustment for BP (Supplemental Table 3).
Analyses Incorporating Only Individuals with Three Measures of Kidney Function.
As above, the mean GFR decline was −1.59±4.84 ml/min per 1.73 m2 per year in those with two or three (n=2129) measures of kidney function versus −1.13±1.77 ml/min per 1.73 m2 per year in those with three (n=1160) measures of kidney function.
When the analyses were repeated using patients with three measures of kidney function, results for aPWV and PP with change in kidney function in both continuous and categorical models were similar to those of the analyses using patients with two or three measures of kidney function. The relationship between aPWV with rapid kidney function decline was not significant, however, in univariate models for both continuous and categorical analyses, although the effect size was similar to the original results. Results for PP and rapid kidney function decline were consistent with the original data for both continuous and categorical models. Likewise, the results for aPWV and incident CKD were consistent with our original data using two or three measures of kidney function. There was a similar effect size in the association between higher PP and incident CKD for both continuous and categorical data, but in contrast to the original data these results did not reach statistical significance (data not shown).
Discussion
In Health ABC, a cohort of initially well functioning older persons, higher aPWV and PP were associated with various measures of change in kidney function, including rapid kidney function decline and incident CKD. Arterial PWV was associated with rapid decline, which was attenuated after adjustment for BP and incident CKD in multivariable analysis. PP was associated with both rapid decline and incident CKD.
These results are consistent with most studies that have evaluated the relationship of central aortic rigidity with kidney function decline (10–16). In patients with CKD stages 3–4, aPWV and PP were associated with GFR decline (10–13), but some studies did not adjust for BP, an important potential confounder in this association (10,12,13). For example, in the study by Briet et al., carotid-femoral pulse wave velocity, aortic pressure, and carotid remodeling and stiffness measures were assessed in 180 patients with established diagnosis of CKD (mean measured GFR, 32 ml/min per 1.73 m2) who were followed prospectively for a mean of 3.1 years. In that study, only PP was associated with higher risk for ESRD, but these results were not adjusted for BP (13). Likewise, in another study of 329 patients with a mean eGFR of 39±18 ml/min per 1.73 m2, baseline PP was the only risk factor associated with decline in eGFRcreat during a 6-month follow-up (12). Similar results have been observed in populations with mild or no evidence of CKD (14–16). In the Framingham Heart Study, markers of arterial stiffness, such as carotid femoral PWV, forward pressure wave amplitude, central PP, augmentation pressure, and augmentation index, were associated with development of albuminuria but not incident CKD (defined by eGFR < 60 ml/min per 1.73 m2) in 2680 individuals (14). In a recent study of 4850 patients from the study of Multiethnic Study of Atherosclerosis, large arterial elasticity (measured by pulse contour analyses of the radial artery) and PP were associated with faster kidney function decline among participants with baseline eGFR > 60 ml/min per 1.73 m2 (15). In a cohort of 2050 Japanese patients with GFR ≥ 60 ml/min per 1.73 m2, followed for 5–6 years, higher baseline brachial-ankle PWV was associated with lower follow-up eGFR and with higher annual rate of decline in eGFR (16). Our study adds to this work by using a large cohort of older adults, having moderate length of follow-up, evaluating both aPWV and PP, and including cystatin C.
There are several potential mechanisms through which arterial stiffness may promote development of kidney disease. First, increased arterial stiffness leads to increased PP, which in turn may give rise to kidney damage through alterations in kidney microcirculation, increased glomerular pressure, and development of proteinuria (6,9). Damage to small arteries induced by increased pulsatile stress may lead to tearing of the endothelial and smooth muscle cells with disruption of the vessel, predisposing to thrombosis and microinfarction (7). In fact, PP can be detected across the kidney glomerular microcirculation in normal and spontaneously hypertensive rats (8). Second, arterial stiffness may result in greater transmission of elevated systemic SP to the glomerular capillaries, thereby exacerbating glomerular hypertension (9). Finally, increased arterial stiffness and PP may lead to atherosclerosis and hypertension and through these pathways promote kidney disease progression.
Our findings suggest that the relationship between PP and aPWV with kidney outcomes, particularly when eGFRcysC is used, may differ. The reason for this is unclear, even though both are markers of arterial stiffness, they may not be measuring the same biologic phenomenon. In our analyses, the correlation between aPWV and PP was weak, which supports this contention. PP is a measure of pulsatility, which is influenced by arterial stiffness but is also influenced by other non–stiffness-related factors, such as left ventricle contractility, pattern of left ventricle ejection, and competence of the aortic valve. It is possible that PP captures more dynamic information through the cardiac cycle than does aPWV alone, which measures distance over time (28). Aortic stiffness measured by aPWV is determined by the impedance of the proximal aorta but is also influenced by other factors, such as heart rate, distending pressure, resistance of the arterial wall, and the presence of atherosclerotic plaques. If we consider PP and aPWV as independent measures of arterial rigidity, we could hypothesize that both pulsatility and aortic stiffness are associated with incident CKD, whereas only pulsatility is associated with kidney function decline.
What are the implications of our results? First, they suggest that both aortic stiffness and pulsatility are risk factors for development of kidney disease, independent of BP. Second, our results also suggest that PP is perhaps a better risk factor for kidney function decline. Because PP is easy to measure, it should be evaluated in addition to proteinuria and BP in clinical prediction tools of incident and progressive kidney disease. Finally, interventions, such as medications known to improve vascular stiffness and pulsatility (e.g., angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, calcium-channel blockers, and β-blockers), should be considered in trials to evaluate their effect on kidney disease progression.
Our study has several strengths. First, in addition to PP, we measured aPWV, the gold standard measure of aortic stiffness. Second, we performed analyses using cystatin C, a potentially more accurate measure of kidney function in the elderly than serum creatinine. Third, Health ABC had detailed ascertainment of risk factors and outcomes, long-term follow-up, and a large sample of older adults who are at increased risk for both kidney disease and arterial stiffness (17).
The study also has some limitations. Albumin excretion rate, an important marker of kidney damage, was not measured during follow-up in Health ABC; therefore, it could not be used as part of the outcome. In addition, because this is an observational study, residual confounding from variables that were included in the analysis and unmeasured confounding from variables not assessed in Health ABC are possible. Finally, our results may not be generalizable to younger populations or populations with advanced CKD.
In conclusion, we found that aortic stiffness assessed by aPWV is associated with incident CKD and that pulsatility assessed by PP is associated with both rapid kidney function decline and incident CKD, independent of BP. Future research should seek to determine whether interventions that target arterial rigidity will prevent development and progression of CKD.
Disclosures
None.
Supplementary Material
Acknowledgments
This research was supported by National Institute on Aging (NIA) contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106; NIA grant R01-AG028050; and National Institute of Nursing Research grant R01-NR012459. This study was also supported in part by the Intramural Research Program of the National Institutes of Health, NIA, National Institute of Diabetes and Digestive and Kidney Disease (K24 DK078204) and CONACYT SALUD-2008-C01-87236
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.07900812/-/DCSupplemental.
References
- 1.Konings CJ, Hermans M, Kooman JP, Meinders JM, Hoeks AP, van der Sande FM, Leunissen KM: Arterial stiffness and renal replacement therapy. Perit Dial Int 24: 318–322, 2004 [PubMed] [Google Scholar]
- 2.Klassen PS, Lowrie EG, Reddan DN, DeLong ER, Coladonato JA, Szczech LA, Lazarus JM, Owen WF, Jr: Association between pulse pressure and mortality in patients undergoing maintenance hemodialysis. JAMA 287: 1548–1555, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Blacher J, Safar ME, Guerin AP, Pannier B, Marchais SJ, London GM: Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int 63: 1852–1860, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Madero M, Peralta C, Wassel-Fyr C, Najjar S, Tyrrell Sutton K, Fried L, Canada R, Newman A, Shlipak M, Sarnak M: Cystatin C associates with arterial stiffness in older adults. J Am Soc Nephrol 20: 1086–1093, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norlund L, Fex G, Lanke J, Von Schenck H, Nilsson JE, Leksell H, Grubb A: Reference intervals for the glomerular filtration rate and cell-proliferation markers: Serum cystatin C and serum beta 2-microglobulin/cystatin C-ratio. Scand J Clin Lab Invest 57: 463–470, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Covic A, Gusbeth-Tatomir, P, Goldsmith, D: Arterial stiffness in renal patients: An update. Am J Kidney Dis 45: 965–977, 2005 [DOI] [PubMed]
- 7.O’Rourke MF, Safar ME: Relationship between aortic stiffening and microvascular disease in brain and kidney: Cause and logic of therapy. Hypertension 46: 200–204, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Yip KP, Holstein-Rathlou NH, Marsh DJ: Chaos in blood flow control in genetic and renovascular hypertensive rats. Am J Physiol 261: F400–F408, 1991 [DOI] [PubMed] [Google Scholar]
- 9.Pedrinelli R, Dell’Omo G, Penno G, Bandinelli S, Bertini A, Di Bello V, Mariani M: Microalbuminuria and pulse pressure in hypertensive and atherosclerotic men. Hypertension 35: 48–54, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Taal MW, Sigrist MK, Fakis A, Fluck RJ, McIntyre CW: Markers of arterial stiffness are risk factors for progression to end-stage renal disease among patients with chronic kidney disease stages 4 and 5. Nephron Clin Pract 107: c177–c181, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Ford ML, Tomlinson LA, Chapman TP, Rajkumar C, Holt SG: Aortic stiffness is independently associated with rate of renal function decline in chronic kidney disease stages 3 and 4. Hypertension 55: 1110–1115, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Arulkumaran N, Diwakar R, Tahir Z, Mohamed M, Kaski JC, Banerjee D: Pulse pressure and progression of chronic kidney disease. J Nephrol 23: 189–193, 2010 [PubMed] [Google Scholar]
- 13.Briet M, Collin C, Karras A, Laurent S, Bozec E, Jacquot C, Stengel B, Houillier P, Froissart M, Boutouyrie P, Nephrotest Study Group : Arterial remodeling associates with CKD progression. J Am Soc Nephrol 22: 967–974, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Upadhyay A, Hwang SJ, Mitchell GF, Vasan RS, Vita JA, Stantchev PI, Meigs JB, Larson MG, Levy D, Benjamin EJ, Fox CS: Arterial stiffness in mild-to-moderate CKD. J Am Soc Nephrol 20: 2044–2053, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peralta CA, Jacobs DR, Jr, Katz R, Ix JH, Madero M, Duprez DA, Sarnak MJ, Criqui MH, Kramer HJ, Palmas W, Herrington D, Shlipak MG: Association of pulse pressure, arterial elasticity, and endothelial function with kidney function decline among adults with estimated GFR >60 mL/min/1.73 m(2): The Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis 59: 41–49, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomiyama H, Tanaka H, Hashimoto H, Matsumoto C, Odaira M, Yamada J, Yoshida M, Shiina K, Nagata M, Yamashina A: Arterial stiffness and declines in individuals with normal renal function/early chronic kidney disease. Atherosclerosis 212: 345–350, 2010 [DOI] [PubMed] [Google Scholar]
- 17.O’Hare AM, Bertenthal D, Covinsky KE, Landefeld CS, Sen S, Mehta K, Steinman MA, Borzecki A, Walter LC: Mortality risk stratification in chronic kidney disease: One size for all ages? J Am Soc Nephrol 17: 846–853, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Health ABC. National Institute on Aging: National Institutes of Health. 2010. Available at: http://www.grc.nia.nih.gov/branches/ledb/healthabc/index.htm Accessed December 4, 2012
- 19.Finney H, Newman DJ, Thakkar H, Fell JM, Price CP: Reference ranges for plasma cystatin C and creatinine measurements in premature infants, neonates, and older children. Arch Dis Child 82: 71–75, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inker LA, Eckfeldt J, Levey AS, Leiendecker-Foster C, Rynders G, Manzi J, Waheed S, Coresh J: Expressing the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) cystatin C equations for estimating GFR with standardized serum cystatin C values. Am J Kidney Dis 58: 682–684, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A, Health ABC Study : Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 111: 3384–3390, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Rifkin DE, Shlipak MG, Katz R, Fried LF, Siscovick D, Chonchol M, Newman AB, Sarnak MJ: Rapid kidney function decline and mortality risk in older adults. Arch Intern Med 168: 2212–2218, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shlipak MG, Katz R, Kestenbaum B, Siscovick D, Fried L, Newman A, Rifkin D, Sarnak MJ: Rapid decline of kidney function increases cardiovascular risk in the elderly. J Am Soc Nephrol 20: 2625–2630, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarnak MJ, Katz R, Stehman-Breen CO, Fried LF, Jenny NS, Psaty BM, Newman AB, Siscovick D, Shlipak MG, Cardiovascular Health Study : Cystatin C concentration as a risk factor for heart failure in older adults. Ann Intern Med 142: 497–505, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C: Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 352: 2049–2060, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adji A, O’Rourke MF, Namasivayam M: Arterial stiffness, its assessment, prognostic value, and implications for treatment. Am J Hypertens 24: 5–17, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.