Abstract
IL-10 contributes to the maintenance of intestinal homeostasis via the regulation of inflammatory responses to enteric bacteria. Loss of IL-10 signaling results in spontaneous colitis in mice and early onset enterocolitis in humans. Nucleotide-binding oligomerization domain (NOD) 2 is an intracellular receptor of bacterial peptidoglycan products, and, although NOD2 mutations are associated with Crohn’s disease, the precise role of NOD2 in the development of intestinal inflammation remains undefined. To determine the role of NOD2 in the development of colitis on the clinically relevant genetic background of IL-10–deficient signaling, we generated mice lacking IL-10 and NOD2 (IL-10−/−NOD2−/−). Loss of NOD2 in IL-10−/− mice resulted in significant amelioration of chronic colitis, indicating that NOD2 signaling promotes the development of intestinal inflammation in IL-10−/− mice. Contrary to previous reports investigating immune function in NOD2−/− mice, T cell proliferative capacity and IL-2 production were not impaired, and immune polarization toward type 1 immunity was not affected. However, loss of NOD2 in IL-10–deficient macrophages reduced IL-6, TNF-α, and IL-12p40 production in response to bacterial stimulation. Further analysis of the intrinsic macrophage response before the onset of inflammation revealed that, in the absence of IL-10, synergistic signaling between various TLRs and NOD2 resulted in hyperresponsive, proinflammatory macrophages, thus providing the appropriate immune environment for the development of colitis. Data presented in this study demonstrate that NOD2 signaling contributes to intestinal inflammation that arises through loss of IL-10 and provides mechanistic insight into the development of colitis in inflammatory bowel disease patients with impaired IL-10 signaling.
Introduction
Inflammatory bowel disease (IBD) is a relapsing remitting disease of the intestinal tract that encompasses two clinically defined forms, ulcerative colitis (UC) and Crohn’s disease (CD). The condition is heterogeneous in nature, and the exact causes are as yet unknown. However, one widely accepted theory is that IBD arises due to inappropriate responses to commensal bacteria in genetically susceptible hosts (1).
Nucleotide-binding oligomerization domain (NOD) 2 belongs to a family of NOD-like receptors that function as intracellular pattern recognition receptors (PRR), sensing bacterial products and contributing to innate immunity (2). NOD2 was originally described as an intracellular receptor for muramyl dipeptide (MDP), a conserved motif present in peptidoglycan from Gram-positive and Gram-negative bacteria (3). Detection of MDP by NOD2 results in the potentiation of the innate inflammatory response to various TLR agonists, resulting in enhanced proinflammatory cytokine production from murine and human cells (4–11). Genome-wide association studies have identified various NOD2 mutations that associate with the incidence of CD (12, 13), implicating NOD2 as a key player in intestinal homeostasis and disease. Although the functional consequences of the various mutations are not fully understood, studies comparing the response of cells from CD patients homozygous for NOD2 mutations and cells bearing wild-type (WT) NOD2 suggest that mutations result in altered synergy with TLRs, thus disrupting intestinal homeostasis (14–17).
IL-10 is a pleiotropic cytokine produced by T cells, B cells, and APCs that has regulatory activity able to control excessive immune responses to proinflammatory stimuli (18). Its role in regulating intestinal inflammation was initially highlighted by the development of spontaneous colitis in IL-10−/− mice. APCs from IL-10−/− mice have been shown to be hyperresponsive to bacterial stimuli (19), resulting in increased production of proinflammatory cytokines such as IL-12 and IL-23, which are central for the development of colitis by promoting colitogenic Th1 and Th17 cells (20). Furthermore, IL-10 produced by APCs can regulate pathogenic T cell responses to commensal flora (21). IL-10 signaling has also been associated with the pathogenesis of human IBD. IL-10 can regulate excessive proinflammatory cytokine production by lamina propria mononuclear cells isolated from IBD patients (22). PBMCs from CD patients homozygous for NOD2 mutations produce lower levels of IL-10 compared with WT cells from healthy volunteers (23, 24), and low mucosal levels of IL-10 in CD patients are associated with severe disease (25). The importance of IL-10 in IBD has recently been highlighted by genome-wide association studies identifying IL-10 as a susceptibility gene for UC and CD (13, 26) and the identification of mutations in the IL-10R gene, resulting in the abrogation of IL-10 signaling, which associate with early onset enterocolitis (27). Apart from mutations in the IL-10R gene, IL-10–deficient signaling similar to IL-10−/− and STAT3−/− mice is not generally observed in IBD patients, although the clinical evidence described above suggests that, in subpopulations of patients, alterations in IL-10 signaling is a factor that contributes to the pathogenesis of IBD.
TLRs have been shown to affect the development of colitis in IL-10−/− mice (28–30) and chemically induced colitis models (31–33). However, the contribution of intracellular pathogen PRR, such as NOD2, toward the development of colitis in IL-10−/− mice has not been reported. Whereas genetic association in humans has implicated NOD2 as a key player in the pathogenesis of IBD, NOD2 signaling in mice is not essential for gut homeostasis as mice deficient in NOD2 do not develop spontaneous colitis or differ from WT mice in the development of acute and chronic dextran sulfate sodium (DSS)–induced colitis (4, 34). However, other studies investigating the role of NOD2 in intestinal inflammation have demonstrated that deletion of NOD2 can impact both positively and negatively on the development of colitis depending on the model system used. For example, MDP administration has been shown to protect mice from 2,4,6-trinitrobenzenesulfonic acid (TNBS) and DSS-induced colitis; the protective role of NOD2 signaling was confirmed as MDP-mediated protection with loss in NOD2−/− mice (35). In contrast, others have demonstrated that NOD2−/− T cells result in reduced chronic colitis following transfer into immunocompromised mice, suggesting that, in the context of T cell–mediated colitis, NOD2 signaling can exacerbate inflammation (36).
In this present study, we use the IL-10−/− mouse model of colitis and demonstrate that mice double deficient in IL-10 and NOD2 are protected from developing severe chronic colitis. Both innate and adaptive immune responses contribute to colitis in IL-10−/− mice. Thus, to elucidate the mechanism by which mice are protected, we investigated T cell and macrophage function and demonstrate that loss of NOD2 signaling in IL-10−/− mice predominantly affects macrophage activity. We show that NOD2 contributes to enhanced proinflammatory activity of macrophages from IL-10−/− mice and that the synergistic activity of MDP with TLR ligands in the absence of IL-10 renders macrophages intrinsically hyperresponsive to bacterial stimulus, thus contributing to the dysregulated immune environment that ultimately results in the development of colitis in IL-10−/− mice.
Materials and Methods
Mice
WT C57/BL6 mice and IL-10−/− mice were purchased from Charles River. NOD2−/− mice have been previously described (4, 37). IL-10−/− mice were crossed with NOD2−/− to generate IL-10−/−NOD2−/− mice. All animals were on a C57BL/6 background, bred, and housed under specific pathogen-free conditions. Animal experiments were approved by the Novartis ethical review process and conducted in accordance with United Kingdom Home Office regulations.
Histopathology
The colon was excised, and a 10-mm piece of distal, mid, and proximal colon was formalin fixed before paraffin embedding. Paraffin sections were H&E stained and blinded before histological scoring was undertaken by an investigator blinded to the groups. For each mouse, distal, mid, and proximal colon was scored for inflammation, as follows: increased immune cell infiltrate in the mucosa predominantly at the base of the crypts; patchy immune cell infiltrate in larger areas of mucosa with occasional, continuous inflammation or immune cell foci; most of the mucosa involved with multifocal infiltrates and submucosal involvement. Sections were scored for mucosal damage, as follows: some crypt elongation/epithelial hyperplasia, no mucus depletion; increased elongation/hyperplasia of crypts with mucus depletion, resulting in marked increase in mucosal thickness; disruption of crypt architecture with crypt abscesses and/or ulceration. The scores for each colon region were combined to give a maximum score of 18 for each animal.
Preparation of colon homogenates
Colonic samples were homogenized (PreCellys 24; Bertin Technologies) in 1 ml lysis buffer (150 mM NaCl, 20 mM Tris [pH 7.5], 1 mM EDTA, 1 mM EGTA, 1% Triton X-100) containing a mixture of protease inhibitors (Roche), and supernatant was collected after centrifugation. Protein content was calculated using the bicinchoninic acid protein assay kit (Pierce), according to manufacturer’s instructions. Cytokine levels were measured using the Meso Scale Discovery Ultra-Sensitive Kit for Mouse Th1/Th2. Cytokine concentrations were calculated using the Meso Scale Discovery Workbench analysis and normalized per mg protein.
Primary cell culture
Spleens were forced through 70-μm cell strainers, and RBCs were lysed. Peritoneal cells were recovered by performing a peritoneal lavage with 10 ml RPMI 1640 supplemented with 5% FBS. Macrophages were isolated from spleen or peritoneal cell suspensions using CD11b microbeads (MACS) according to the manufacturer’s instructions. All cell culture was done in RPMI 1640 supplemented with 10% heat-inactivated FBS (Invitrogen), 50 U/ml penicillin, 50 μg/ml streptomycin, 2 mM l-glutamine (Life Technologies) at 37°C, 5% CO2.
Proliferation assay.
Cells (2 × 105 per well) were cultured in 96-well, U-bottom plates for 72 h at 37°C, 5% CO2, and stimulated by 100 ng/ml soluble anti-CD3ε Ab (clone 145-2C11; BD Pharmingen). Cultures were pulsed with 1 μCi/well methyl-[3H]thymidine (Amersham) for the last 16–18 h of culture, cells were harvested, and methyl-[3H]thymidine incorporation was determined.
Spleen cell culture.
To measure IL-2 production, cells (5 × 106) were stimulated with 100 ng/ml anti-CD3ε Ab and incubated for 24 h, and supernatant was harvested. For other cytokine quantification, cells were stimulated with 500 ng/ml anti-CD3ε Ab and 4 μg/ml anti-CD28 (clone 37.51; BD Pharmingen) and incubated for 72 h before harvesting supernatants.
Macrophage culture.
Macrophages were seeded at 1 × 105 cells/well in 96-well flat-bottom culture plates and stimulated with various PRR ligands, all purchased from Invivogen: LPS (ultrapure), catalog tlrl-3pelps (10 ng/ml); unmethylated CpG oligonucleotide (1 μg/ml), catalog tlrl-1826; PAM3CSK4, catalog tlrl-pms (200 ng/ml); and MDP, catalog tlrl-mdp (10 μg/ml). All PRR ligands were reconstituted in sterile endotoxin-free water. Peritoneal macrophages were stimulated with LPS at a lower dose of 1 ng/ml. In some experiments, 300 pg/ml mouse rIL-10 (PeproTech) was added to macrophage cultures. After 24 h, supernatants were harvested for cytokine quantification.
ELISAs
Levels of serum amyloid A were quantified by ELISA, according to the manufacturer’s instructions (Invitrogen). The following cytokines, IL-12p40, TNF-α, IL-2, IFN-γ, IL-4, and IL-6, were quantified in cell culture supernatants using Duoset ELISA kits (R&D Systems), according to the manufacturer’s instructions.
Flow cytometry
Mouse T regulatory (Treg) cell detection kit was used (MACS). Spleen cell suspensions were prepared, and Treg cells were stained, as per manufacturer’s instructions. The following isotype control Abs were used, and all were purchased from BD Pharmingen: rat IgM PE conjugated, rat IgG2b FITC conjugated, and mouse IgG1 allophycocyanin conjugated.
Statistics and data analysis
All statistical analyses were performed with GraphPad Prism software (version 5). Data are presented as mean ± SEM, and a p value <0.05 was considered significant. We used a one-way or two-way ANOVA and Gehan–Breslow–Wilcoxon test, as appropriate, to compare data sets.
Results
Chronic colitis is ameliorated in IL-10−/−NOD2−/− mice
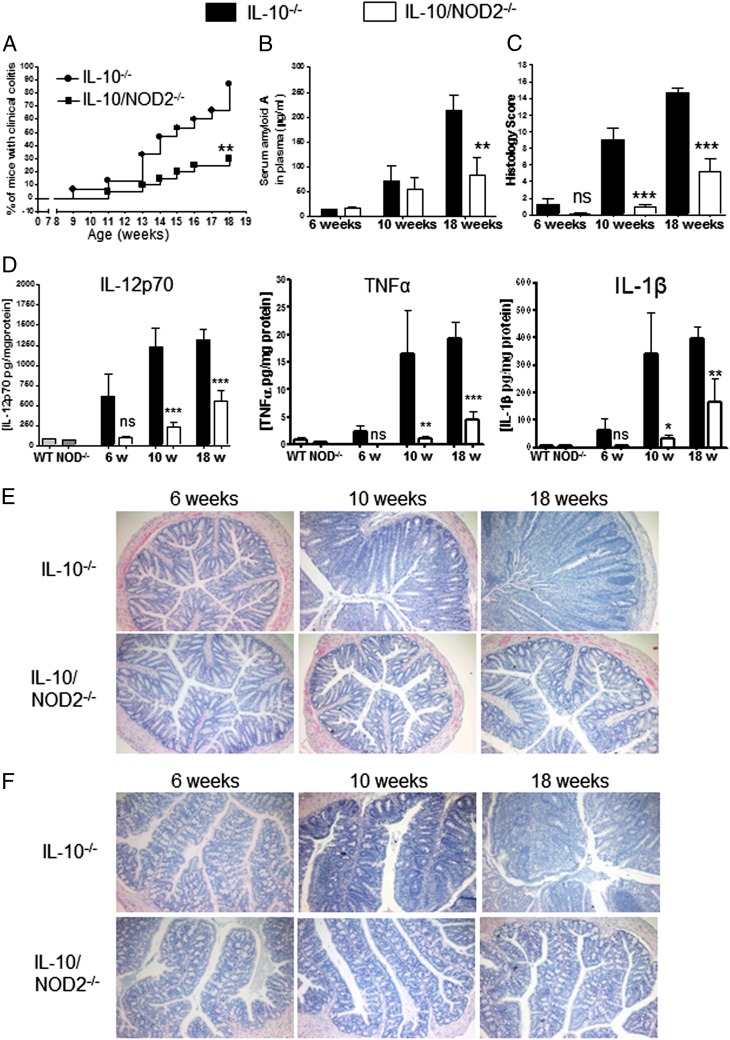
To determine the role of NOD2 on the development of spontaneous colitis, we backcrossed IL-10−/− mice with mice lacking NOD2 to generate IL-10−/−NOD2−/− mice and compared the onset of colitis with IL-10−/− mice. As previously reported, IL-10−/− mice start to show signs of colitis from 10 wk of age (38). In our colony, we defined overt or clinical colitis as weight loss (>4%) that coincided with diarrhea observed for consecutive 2 d or more. The incidence of clinical colitis in IL-10−/− mice continued to increase over time until at 18 wk 13 of 15 mice (87%) had developed clinical colitis. This is in contrast to IL-10−/−NOD−/− mice in which only 6 of 20 (30%) mice had developed colitis (Fig. 1A). At 18 wk of age, the reduction in the incidence of colitis in IL-10−/−NOD2−/− mice was associated with significant reduction in serum amyloid A, a nonspecific marker of inflammation (Fig. 1B).
FIGURE 1.
Chronic colitis is ameliorated in IL-10−/−NOD2−/− mice. The development of colitis was investigated in IL-10−/− and IL-10−/−NOD2−/− mice. (A) The incidence of clinical colitis was defined by weight loss (>4%), which coincided with diarrhea observed for two consecutive days or more and plotted as percentage of mice with clinical colitis. Incidence curves were compared using Gehan–Breslow–Wilcoxon test, **p < 0.01. (B) Mice were bled at indicated times via tail vein puncture, and levels of serum amyloid A were quantified by ELISA. (C) To assess progression of colon inflammation, distal, mid, and proximal colon were formalin fixed and embedded in paraffin at the indicated times, and sections were stained with H&E. Distal, mid, and proximal colon was scored by an investigator blinded to the groups, and a cumulative score was given for each mouse (maximum 18). Histopathology scores of IL-10−/− and IL-10−/−NOD2−/− mice were compared at each time point using one-way ANOVA with Bonferroni's correction. Data are from 11 to 13 mice per group and representative of three separate experiments. (D) At the indicated time points, IL-10−/− and IL-10−/−NOD2−/− mice were euthanized, and colon was removed and homogenized for the quantification of cytokines by ELISA. Colon homogenates from 18-wk-old WT and NOD2−/− mice were used to indicate background levels of colon cytokine. Levels of cytokine are expressed as pg/mg protein. Levels of serum amyloid A and cytokines in IL-10−/− and IL-10−/−NOD2−/− mice were compared using one-way ANOVA with Bonferroni’s posttests. *p < 0.05, **p < 0.01, ***p < 0.001. Data are shown as means + SEM from 4 to 8 mice per group and representative of three separate experiments. Representative images of distal (E) and proximal (F) H&E-stained colons at the indicated age. Original magnification ×200. ns, Not significant.
IBD and colitis in IL-10−/− mice are characterized by increased mucosal inflammatory cytokine levels arising primarily from the immune cell infiltrate. At 6 wk of age, there was no significant difference in colonic cytokine levels in IL-10−/− mice compared with IL-10−/−NOD2−/− mice. However, by 10 wk of age, levels of IL-12p70, TNF-α, and IL-1β were significantly elevated in the colons of IL-10−/− mice when compared with IL-10−/−NOD2−/− mice of the same age. Moreover, proinflammatory colonic cytokine levels remained attenuated in older (18-wk) IL-10−/−NOD2−/− mice, thus confirming amelioration of spontaneous colitis (Fig. 1D).
To look specifically at progression of intestinal inflammation, we analyzed the colon for histopathological changes in 6-, 10-, and 18-wk-old mice. At 6 wk of age, histological analysis revealed a small inflammatory cell infiltrate restricted to the lamina propria at the base of the crypts and elongated crypts (mild damage) in a small number of IL-10−/− mice (3 of 11) and IL-10−/−NOD2−/− mice (1 of 11). However, there was no significant difference in histopathology between IL-10−/− and IL-10−/−NOD2−/− at 6 wk of age (Fig. 1C), concurring with the slight, but nonsignificant, increase in colon cytokines observed in IL-10−/− mice compared with IL-10−/−NOD2−/− mice (Fig. 1D). By 10 wk of age, the incidence (11 of 12) and severity of histopathology had increased in IL-10−/− mice, characterized by increased inflammatory cell infiltrate and crypt elongation associated with goblet cell depletion along the length of the colon (Fig. 1E, 1F). In contrast, 5 of 11 IL-10−/−NOD2−/− mice had no histopathology, and those that did had limited inflammation and epithelial damage, resulting in significantly less histopathology scores (Fig. 1C). At 18 wk, IL-10−/− mice had marked mucosal thickening, epithelial hyperplasia, and crypt abscesses involving distal and proximal colon (Fig. 1E, 1F). Severe epithelial damage was associated with increased immune infiltrate extending into the submucosa and the presence of immune cell foci. This is in contrast to colons from 18-wk-old IL-10−/−NOD2−/− mice that had significantly less mucosal damage and immune cell infiltrate. Taken together these data demonstrate that deletion of NOD2 in IL-10−/− mice ameliorates spontaneous chronic colitis.
The role of effector T cell responses in protection from colitis in IL-10−/−NOD2−/− mice
Type 1 T cell–mediated inflammation, characterized by increased levels of IFN-γ, plays a critical role in the development of colitis in IL-10−/− mice (39). To determine whether loss of NOD2 contributed to colitis in IL-10−/− mice by impacting on T cell activity, we compared the ex vivo T cell responses of IL-10 and IL-10−/−NOD2−/− mice. To account for confounding effect of colonic inflammation in older mice with colitis, we also compared the T cell responses from 6-wk-old (precolitis) mice, thus focusing on the intrinsic T cell response.
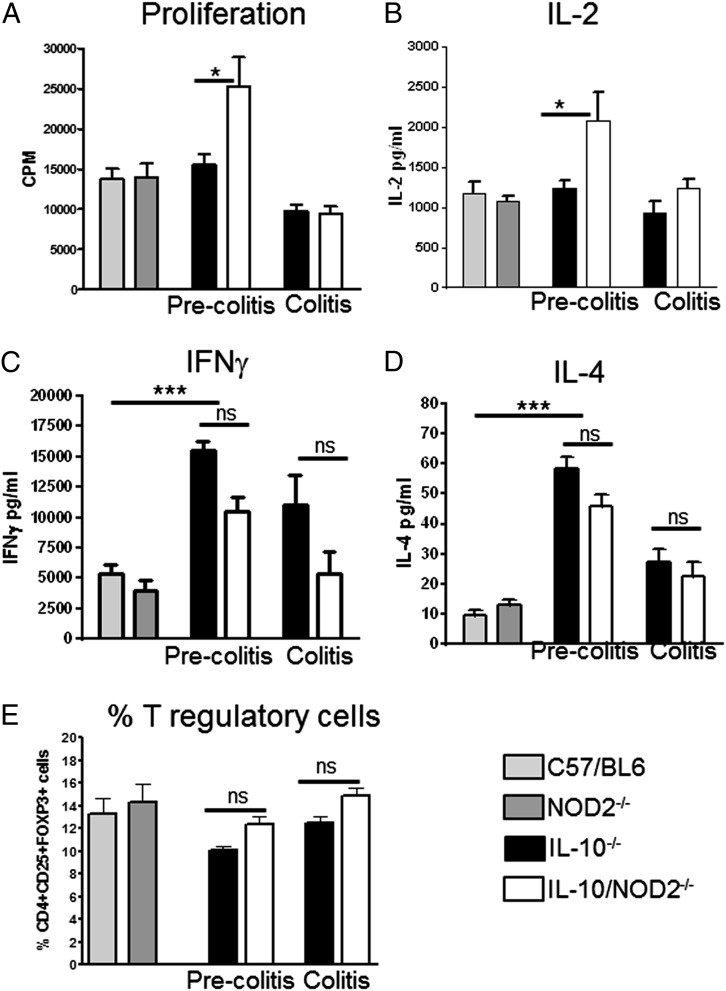
Shaw et al. (36) demonstrated that amelioration of T cell transfer colitis was associated with hyporesponsive phenotype in T cells lacking NOD2, characterized by reduced proliferation and IL-2 secretion and a diminished type 1 response. To investigate whether loss of NOD2 was promoting hyporesponsive T cells in IL-10−/− mice spleen T cells were suboptimally stimulated by soluble anti-CD3 Ab; these stimulation conditions fail to induce proliferation if T cells are rendered hyporesponsive or anergic in vivo (40, 41). However, rather than a reduction in proliferative capacity indicative of a hyporesponsive phenotype, T cells from 6-wk-old IL-10−/−NOD2−/− mice had enhanced proliferative response to anti-CD3 stimulation. In older mice, the enhanced proliferative capacity was lost, possibly due to the indirect effect of colonic inflammation (Fig. 2A). Hyporesponsive T cells are also associated with reduced IL-2 production, which has previously been demonstrated in T cells lacking NOD2 (36). However, significantly more IL-2 was produced by anti-CD3–stimulated splenocytes from precolitic IL-10−/−NOD2−/− mice compared with IL-10−/− mice (Fig. 2B). The significant difference was also lost as mice developed colitis, correlating with changes in proliferative capacity. These data demonstrate that protection from colitis in IL-10−/−NOD2−/− mice was not due to defective IL-2 production or hyporesponsive T cells.
FIGURE 2.
Effector T cell responses in IL-10−/− and IL-10−/−NOD2−/− mice. Effector T cell responses were investigated via ex vivo stimulation of spleen cells from WT (C57BL/6), precolitis IL-10−/− and IL-10−/−NOD2−/− (6-wk-old mice), and mice with colitis (18-wk-old mice). Absence/Presence of colitis was confirmed histologically. To determine proliferative capacity of T cells, spleen cells were suboptimally stimulated with soluble anti-CD3 Ab (100 ng/ml). (A) T cell proliferation was quantified by methyl-[3H]thymidine incorporation and expressed as cpm. (B) IL-2 was measured by ELISA in the supernatants after 24 h of culture. Effector T cell phenotype was determined by stimulating spleen T cells with 500 ng/ml anti-CD3ε Ab and 4 μg/ml anti-CD28; supernatants were harvested after 72 h. (C and D) Th1 (IFN-γ) and Th2 (IL-4) cytokines were measured by ELISA. Graphs show means + SEM from six mice per group and are representative of two separate experiments. The percentage of Treg cells in the spleen was analyzed by flow cytometry. (E) Spleen cell suspensions were stained with Abs to CD4, CD25, and FOXP3. Lymphocytes were gated via forward and side scatter. Data are presented as percentage of CD4+, CD25+, FOXP3+ cells. Graphs show means + SEM from four to six mice per group and are representative of two separate experiments. Differences between groups were analyzed by one-way ANOVA with Bonferroni's correction. *p < 0.05, **p < 0.01, ***p < 0.001. ns, Not significant.
Loss of NOD2 has previously been associated with development of a Th2 response (42), which may account for suppression of Th1-mediated colitis in IL-10−/− mice. To determine whether loss of NOD2 in IL-10−/− mice influenced the polarization of a type 1 or type 2 immune response, we investigated the levels of IL-4 and IFN-γ in spleen culture supernatants. The regulatory effect of IL-10 on Th1/Th2 effector response is clearly demonstrated by the significant increase in IFN-γ and IL-4 production by anti-CD3–stimulated T cells from precolitic IL-10−/− mice compared with WT controls (Fig. 2C, 2D). However, loss of NOD2 in IL-10−/− mice did not significantly alter T cell–derived IFN-γ and IL-4, demonstrating that NOD2 in IL-10−/− mice does not influence effector T cell polarization.
Tregs are a T cell subpopulation central to controlling murine colitis (43, 44), and, although IL-10 contributes to the mechanism by which Tregs control intestinal inflammation, it is not essential (45). Thus, Treg activity may still contribute to protection in IL-10−/−NOD2−/− mice. We have shown that T cells from IL-10−/−NOD2−/− mice produced significantly more IL-2 (Fig. 2B). As IL-2 is a key growth factor for Treg cells (46), we investigated whether loss of NOD2 altered the number of Treg cells in the spleen. The percentage of CD4+ CD25+FOXP3+ cells was not significantly higher in the spleen of IL-10−/−NOD2−/− mice compared with IL-10−/− mice both before inflammation and in mice with colitis (Fig. 2E).
Macrophages from IL-10−/−NOD2−/− have a diminished response to LPS
Macrophages contribute to colitis in IL-10−/− mice by having an enhanced response to bacterial stimuli (19), resulting in the production of proinflammatory cytokines such as IL-12, which in turn favors the differentiation of Th1 cells (47). NOD2 expression was initially demonstrated in monocytes (48) and subsequently shown to be a key mediator of macrophage responses to bacterial stimuli (4). The key roles of IL-10 and NOD2 on macrophage function prompted us to investigate macrophage responses to bacterial stimulus in IL-10−/− and IL-10−/−NOD2−/− mice.
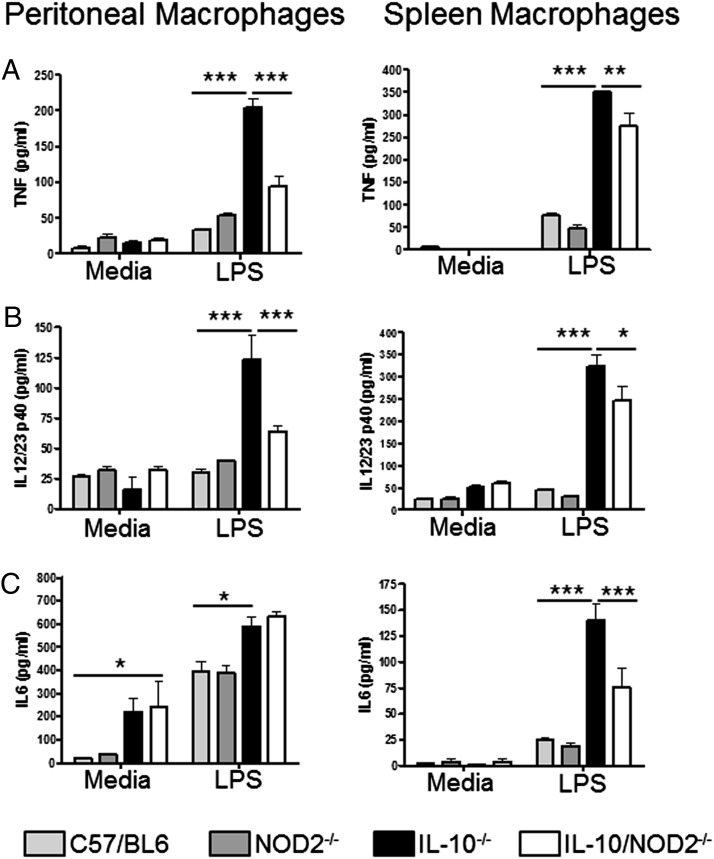
CD11b-positive cells were purified from the spleens and peritoneum of 18-wk-old WT, NOD2−/−, IL-10−/−, and IL-10−/−NOD2−/− mice. Isolated cells were then stimulated with LPS, and proinflammatory cytokine production was measured. In agreement with previous studies using bone marrow–derived macrophages (4), there was no significant difference in response to LPS between peritoneal and spleen macrophages from WT and NOD2−/− mice (Fig. 3). Also, in accordance with previous findings (19), macrophages from colitic IL-10−/− mice have enhanced responsiveness to LPS compared with WT mice, as demonstrated by significantly elevated levels of TNF-α, IL-12p40, and IL-6 (Fig. 3). There was also a significant increase in IL-6 release from unstimulated IL-10−/− peritoneal macrophages, demonstrating the potent regulatory effect that IL-10 has on the basal activity of peritoneal cells (Fig. 3C). Loss of NOD2 in IL-10−/− mice reduced the proinflammatory response of macrophages to LPS when compared with macrophages isolated from IL-10−/− mice with intact NOD2 signaling. Macrophages isolated from the spleen of IL-10−/−NOD2−/− mice produced significantly less IL-12p40, TNF-α, and IL-6 compared with cells from IL-10−/− mice (Fig. 3). This phenotype was not restricted to spleen macrophages as peritoneal macrophages from IL-10−/−NOD2−/− mice also produced significantly less TNF-α and IL-12p40 when stimulated with LPS (Fig. 3A, 3B). However, there was no difference in IL-6 production, possibly reflecting different in vivo phenotypes and activated states of spleen and peritoneal macrophages (Fig. 3C). Taken together, these data suggest that NOD2 signaling contributes to enhanced macrophage activity in response to LPS in cells isolated from IL-10−/− mice with colitis.
FIGURE 3.
Colonic inflammation enhances macrophage activity. Spleen and peritoneal macrophages were isolated from 18-wk-old mice using anti-CD11b magnetic beads and stimulated for 24 h with LPS (10 ng/ml). Levels of TNF-α (A), IL-12p40 (B), and IL-6 (C) were measured in the cell culture supernatant by ELISA. Graphs show means + SEM from three mice per group and are representative of three separate experiments. Differences between groups were analyzed by two-way ANOVA with Bonferroni posttests. *p < 0.05, **p < 0.01, ***p < 0.001.
Colonic inflammation enhances macrophage activity
To address whether the reduced proinflammatory response of macrophages isolated from IL-10−/−NOD2−/− mice reflected the downstream consequence of reduced intestinal inflammation or is an intrinsic difference due to the lack of NOD2 signaling, we investigated the responsiveness of macrophages isolated from young mice before the onset of inflammation. Macrophages isolated from precolitic IL-10−/− mice produced lower levels of cytokines when stimulated with LPS compared with older mice with colitis (Table I). Despite having a lower level of inflammation, this was also observed with macrophages isolated from 18-wk-old IL-10/NOD2−/− mice. Increased macrophage responsiveness to LPS in older mice deficient in IL-10 was most likely due to the downstream consequence of intestinal inflammation and not age per se, as demonstrated by the greater fold increase in cytokine production between age-matched WT and IL-10−/− with colitis compared with the fold increase in age-matched WT and precolitic mice (Table I). These data suggest that increased macrophage sensitivity to LPS stimulation in IL-10−/− mice is, in part, attributable to intestinal inflammation. However, loss of IL-10 in the absence of inflammation still renders macrophages intrinsically hyperresponsive to LPS, resulting in the increased expression of some (IL-6 and TNF-α), but not all (IL-12p40) proinflammatory cytokines (Table I, Fig. 4A). We have shown that IL-10/NOD2−/− mice have reduced colonic inflammation and macrophage-derived cytokines in response to LPS. However, as highlighted above, colonic inflammation can influence macrophage activity. Indeed, in the absence of inflammation in young mice, loss of NOD2 did not significantly alter macrophage response to LPS and other TLR ligands (Fig. 4), suggesting that diminished cytokine production by IL-10−/−NOD2−/− macrophages in response to LPS stimulation shown in Fig. 3 reflects reduced intestinal inflammation in 18-wk-old IL-10−/−NOD2−/− mice and was not directly due to loss of NOD2 signaling in macrophages.
Table I. Colonic inflammation in IL-10−/− mice increases the proinflammatory activity of stimulated macrophages.
| 6 Wk Old (Precolitis) |
18 Wk Old (Colitis) |
||||||
|---|---|---|---|---|---|---|---|
| WT | IL-10−/− | Fold Change | WT | IL-10−/− | Fold Change | ||
| TNF | WT | 52.7 ± 6.9 | 122.7 ± 10.2 | 2.3 | 76.7 ± 5.4 | 350.2 ± 5.5 | 6.0 |
| NOD2−/− | 36.3 ± 4.3 | 82.1 ± 21.09 | 2.3 | 48.4 ± 7.9 | 274.1 ± 30.5 | 5.7 | |
| IL-6 | WT | 32.2 ± 2.9 | 62.7 ± 5.7 | 1.9 | 25.3 ± 1.2 | 140.5 ± 15.2 | 5.6 |
| NOD2−/− | 24.9 ± 4.2 | 64.8 ± 10.1 | 2.6 | 18.8 ± 3.7 | 75.4 ± 18.1 | 4.0 | |
| IL-12 | WT | 123.8 ± 7.0 | 123.6 ± 8.6 | 0.9 | 45.4 ± 2.9 | 324.7 ± 31.3 | 7.6 |
| NOD2−/− | 100.9 ± 6.0 | 81.5 ± 5.1 | 0.81 | 32.3 ± 1.4 | 248 ± 31.25 | 7.7 | |
To compare the proinflammatory activity of macrophages isolated from 6-wk-old mice without colitis and 18-wk-old mice with colitis, the mean cytokine level (±SEM) produced by LPS-stimulated macrophages isolated from WT, NOD2−/−IL-10−/−, and NOD2−/−IL-10−/− is shown. The mean LPS-induced cytokine level from 6-wk-old and 18-wk-old mice deficient in IL-10 was divided by the mean cytokine level produced by macrophages isolated from age-matched controls (WT and NOD2−/−) to give the relative fold change.
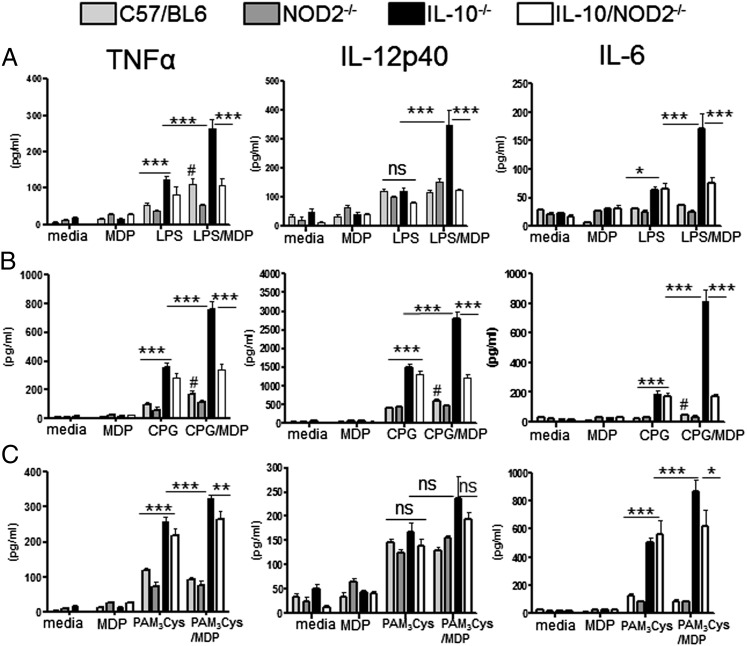
FIGURE 4.
NOD2 signaling acts synergistically with various TLR ligands to promote intrinsically hyperresponsive macrophages in IL-10−/− mice. Spleen macrophages were isolated from 6-wk-old mice using anti-CD11b magnetic beads and stimulated for 24 h. Levels of TNF-α, IL-12p40, and IL-6 were measured in the supernatant by ELISA. (A) Macrophages were stimulated with MDP (10 μg/ml) and/or LPS (10 ng/ml). (B) MDP (10 μg/ml) and/or CpG oligonucleotide (1 μg/ml). (C) MDP (10 μg/ml) and/or PAM3CSK4 (200 ng/ml). Graphs show means + SEM from six mice per group and are representative of two separate experiments. Differences between groups were analyzed by two-way ANOVA with Bonferroni posttest. *p < 0.05, **p < 0.01, ***p < 0.001. Comparison of responses to the different stimulations in WT macrophages was analyzed by one-way ANOVA, #p < 0.05. ns, Not significant.
NOD2 signaling acts synergistically with various TLR ligands to promote intrinsically hyperresponsive macrophages in IL-10−/− mice
MDP, the bacterial ligand for NOD2, has been shown to have synergistic activity both in vivo and in vitro when combined with other bacterial products (4–7, 9, 11, 15, 16, 23). As macrophages in vivo are exposed to multiple TLR/NOD-like receptor ligands, we investigated the impact of NOD2/MDP signaling on macrophage responsiveness in combination with various TLR stimulations. To control for the indirect effect of a heightened inflammatory state in mice with colitis, we isolated macrophages from the spleen of 6-wk-old IL-10−/− and IL-10−/−NOD2−/− mice with no colitis, as confirmed histologically. Preliminary dose-response experiments were undertaken to determine the optimal dose for LPS and MDP in our assay (Supplemental Fig. 1). As has been shown in other studies utilizing murine macrophages (4), 10 ng/ml LPS combined with 10 μg/ml MDP gave maximal synergistic cytokine production in WT mice. Confirming the findings in the preliminary dose-response experiments, stimulation of WT macrophages with MDP and LPS or the TLR9 ligand, unmethylated CpG oligonucleotide (CPG), resulted in synergistic activity in some cytokines, although, in comparison with IL-10−/− mice, overall cytokine induction was low (Fig. 4). However, separate statistical analysis of the responses of WT macrophages revealed that there was significant potentiation of LPS-induced TNF and CPG-induced TNF, IL-6, and IL-12p40 by MDP. There was no synergistic activity on WT macrophages with MDP and TLR2 (Fig. 4).
MDP stimulation alone has been shown to be a relatively poor stimulator of cytokine production in macrophages (6, 11, 49). In agreement with these studies, cytokine levels produced by MDP stimulation alone were comparable to unstimulated cells, and there was no significant difference between genotypes (Fig. 4). When cells from IL-10−/− and IL-10−/−NOD2−/− mice were stimulated with TLR ligand alone, LPS induced significantly more TNF-α and IL-6 compared with WT and NOD2−/− mice (Fig. 4A), and CPG stimulation resulted in significant increase in TNF-α, IL-12p40, and IL-6 production (Fig. 4B), whereas TLR2 stimulation with the synthetic ligand PAM3CSK4 increased TNF-α and IL-6 production compared with cells from mice with intact IL-10 signaling (Fig. 4C). Thus, IL-10 deficiency renders macrophages intrinsically hyperresponsive to stimulation by various bacterial ligands. As with LPS stimulation, the heightened responsive state of IL-10−/− macrophages to a single bacterial stimulus was not influenced by NOD2 signaling, as there was no difference in cytokine production between IL-10−/− and IL-10−/−NOD2−/− when macrophages were stimulated with either LPS, CPG, or PAM3CSK4 alone (Fig. 4). However, when cells from IL-10−/− mice were stimulated with TLR ligands and MDP, there was a large synergistic effect that significantly increased TNF-α, IL-12p40, and IL-6 production compared with TLR stimulation alone (Fig. 4). Importantly, however, the enhanced proinflammatory response was not observed when macrophages deficient in IL-10 and NOD2 were stimulated with TLR ligand and MDP. These data demonstrate that NOD2 signaling acts synergistically with TLR stimulation to promote intrinsically hyperresponsive macrophages in IL-10−/− mice prior to intestinal inflammation. Thus, loss of NOD2 signaling compensates for the hyperresponsive macrophages driven by lack of IL-10 regulatory activity.
The notable exception to these findings was TLR2 (PAM3CSK4)-induced IL-12p40. PAM3CSK4 stimulation did not increase IL-12p40 production in macrophages deficient in IL-10 compared with WT cells; furthermore, MDP did not act synergistically with TLR2 activation. The inability of PAM3CSK4 to induce elevated cytokine production from IL-10−/− cells was restricted to IL-12p40 as TLR2/MDP-driven TNF-α and IL-6 (Fig. 4C) were enhanced in the absence of IL-10, but not in IL-10−/−NOD2−/− macrophages.
IL-10 is potentiated by TLR/NOD2 synergy and can regulate the proinflammatory activity of macrophages
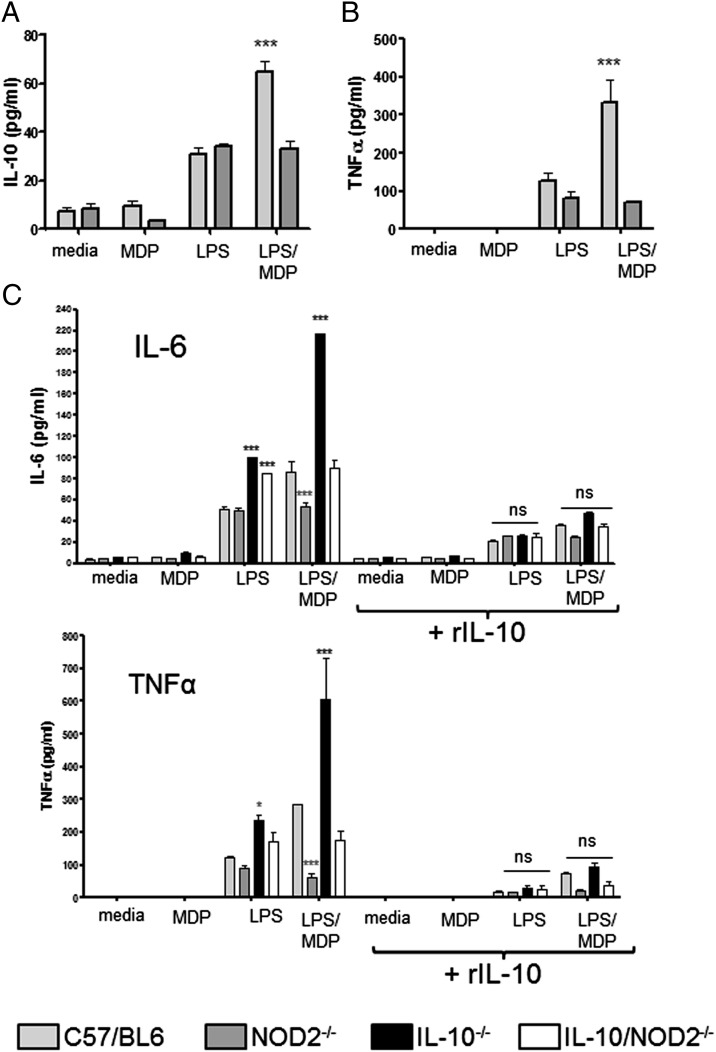
We have shown that macrophages deficient in IL-10 are hyperresponsive to MDP/TLR synergy, resulting in potentiated production of proinflammatory cytokines. NOD2 signaling has also been shown to induce IL-10 in human and mouse cells (16, 50). To determine whether NOD2/TLR activation potentiates regulatory cytokines (IL-10) as well as proinflammatory cytokines, we stimulated macrophages isolated from the spleens of WT and NOD2−/− mice with LPS with or without MDP and quantified IL-10 and TNF-α levels. Both IL-10 (Fig. 5A) and TNF-α (Fig. 5B) levels are potentiated by MDP/TLR4 stimulation. The role of NOD2 was confirmed, as cytokines were not increased in NOD2−/− mice. To investigate further the relationship between NOD2 signaling and the regulatory activity of IL-10 and specifically to determine whether IL-10 can control the proinflammatory synergistic effects of NOD2 signaling, we repeated the assay described in Fig. 4 with and without the addition of exogenous rIL-10. As previously shown in Fig. 4A, LPS and MDP act synergistically in the absence of IL-10 to significantly enhance IL-6 and TNF-α production compared with WT mice, and this effect was dependent on NOD2, as cytokine production from IL-10−/−NOD2−/− cells was not significantly different from WT mice (Fig. 5C). However, when exogenous IL-10 was added to cells, MDP had no significant enhancing effect on LPS stimulation of IL-10–deficient cells when compared with cells from WT mice. These data confirm that NOD2/TLR stimulation potentiates the production of IL-10 in WT mice and that the regulatory activity of IL-10 controls the proinflammatory activity of NOD2/TLR synergy.
FIGURE 5.
IL-10 is potentiated by TLR/NOD2 synergy and can regulate the proinflammatory activity of macrophages. Spleen macrophages were isolated from 6-wk-old WT and NOD2−/− mice using anti-CD11b magnetic beads and stimulated for 24 h with LPS/MDP. Levels of IL-10 (A) and TNF-α (B) were measured in the supernatant by ELISA. Graphs show means + SEM from six mice per group. Differences between WT and NOD2−/− macrophages were analyzed by two-way ANOVA with Bonferroni posttest. (C) Pooled spleen macrophages from six mice per group were stimulated with LPS and MDP with and without rIL-10 (300 pg/ml). Levels of TNF-α and IL-6 were measured by ELISA. Graphs show means + SD of duplicate wells and are representative of two separate experiments. Differences compared with WT mice were analyzed by two-way ANOVA with Bonferroni posttest. *p < 0.05, ***p < 0.001. ns, Not significant.
Discussion
In the current study, we show that deletion of NOD2 ameliorates the development of colitis in IL-10−/− mice. Ex vivo analysis of immune cell function revealed that T effector response and Treg cell numbers were not significantly different. However, IL-10–deficient macrophages were intrinsically hyperresponsive to synergistic stimulation with TLR ligands and MDP before the onset of intestinal inflammation. The potentiation of the inflammatory response to TLR ligands by MDP resulted in significantly increased TNF-α, IL-6, and IL-12p40 production from IL-10−/− macrophages and was not observed in cells from IL-10−/−NOD2−/− mice. We go on to demonstrate that NOD2/TLR stimulation also potentiated IL-10 production from WT cells, and the addition of exogenous IL-10 to macrophage cultures negated the potentiation of cytokine production by MDP, thus confirming that IL-10 can regulate the proinflammatory activity of NOD2/TLR synergy. We conclude that NOD2/TLR synergy potentiates the proinflammatory activity of macrophages in the absence of IL-10, thus providing the immune conditions for the development of colitis.
Although precise mechanisms are unknown, it is generally accepted that IBD arises through disruption of intestinal homeostasis that exists between the host’s mucosal immune response and intestinal microbiota (1). This hypothesis is supported by genome-wide association studies that have identified disease-associated polymorphisms of genes that are involved in bacterial sensing such as NOD2 (12) and immune regulation such as IL-10 (26). The central role of IL-10 signaling in the immune regulation of the intestine is highlighted by the development of colitis in mice lacking IL-10, IL-10R, and STAT3 (a key signaling component of IL-10) (51–53). The clinical relevance of the IL-10–deficient models is supported by the identification of IBD-associated mutations in the IL-10 signaling pathway, including genes encoding IL-10, IL-10Rs, and STAT3, which are associated with UC (26), early onset enterocolitis (54), and CD/UC (55), respectively.
The mechanism by which mice deficient in IL-10 develop spontaneous colitis is thought to involve insufficient regulation of TLR stimulation by commensal flora. In normal conditions, activation of TLRs by commensals is vital for gut homeostasis (56); when these responses are not appropriately regulated (for example, by IL-10), intestinal homeostasis is disrupted and colitis develops. This is supported by the demonstration that mice double deficient in IL-10 and MyD88, a cytosolic adapter protein essential for nearly all TLR signaling, do not develop spontaneous colitis (57). Subsequent studies have shown that unregulated response to specific TLRs such as TLR4 can contribute to colitis in the absence of IL-10. Colitis in IL-10−/−MyD88−/− mice is almost completely abrogated, most likely due to the combined effect of impaired TLR and IL-1β/IL-18R signaling (28). However, IL-10−/−TLR4−/− are similar to IL-10−/−NOD2−/− mice in that they develop ameliorated colitis compared with IL-10−/− and it is possible that lack of the proinflammatory synergy between TLR4 and NOD2 signaling is the common mechanism that results in ameliorated colitis observed in IL-10−/−NOD2−/− mice and IL-10−/−TLR4−/− mice.
In contrast to TLR4, deletion of TLR2 in IL-10−/− mice does not affect the development of colitis (30), suggesting differential effects of TLRs in regulating intestinal inflammation. Interestingly, we show that stimulation with TLR2 ligand and MDP did not potentiate IL-12p40 production in IL-10−/− macrophages, suggesting that the nature of synergy between TLR2 and MDP is different from that observed with TLR4 and TLR9 at least in the production of IL-12p40. This finding concurs with the demonstration that IL-12 is potentiated when human dendritic cells are stimulated with MDP and TLR4 or TLR9, although is absent when cells are stimulated with MDP and TLR2 agonist (7). IL-12p40 (as the subunit of IL-23) is a key driver of colitis in IL-10–deficient mice (20, 58). Thus, lack of TLR2-mediated potentiation of IL-12p40 in IL-10−/− macrophages may explain why deletion of TLR2 does not impact on colitis in IL-10−/− mice.
It has been shown elsewhere that NOD2 and TLR2 synergize negatively, resulting in decreased IL-12 production from murine macrophages with intact IL-10 signaling (37). We did not observe negative synergistic regulation of IL-12 in our assay possibly because we did not use the high levels of MDP (100 μg/ml) used by Watanabe et al. to demonstrate maximal effects (37). Ultimately, the functional nature of NOD2/TLR2 synergy will vary depending on experimental setting and genetic background. However, various studies (7, 16, 23, 37) along with data presented in this study suggest that the interaction between NOD2 and TLR2 differs from that of other TLRs.
Polymorphism of NOD2 was the first of many genetic variants shown to be associated with IBD susceptibility, and remains one of the strongest associations to CD (12, 59). However, how NOD2 and the disease-associated mutations impact on intestinal homeostasis and inflammation remains unclear. Initial findings from a mouse model harboring one of the CD-associated NOD2 mutations suggest a gain-of-function mutation resulting in elevated IL-1β and increase susceptibility to DSS colitis (60). However, a recent study utilizing mice with the same gene knocked in demonstrated a loss-of-function phenotype with reduced levels of cytokine in response to MDP and bacterial infection (61). These later findings concur with studies utilizing cells from CD patients, suggesting a loss-of-function mutation (15, 16, 62–65). When the response of cells from CD patients bearing NOD2 mutations was compared with cells with WT NOD2, the potentiation of cytokine response by MDP was lost in patients with mutated NOD2 (14–16). It seems paradoxical that a disease susceptibility mutation that results in reduced inflammatory response can lead to a condition characterized by increased tissue and systemic inflammation. Several potential mechanisms have been proposed to explain this dichotomy, including impaired epithelial barrier function due to lack of TLR9 and NOD2 synergy (14), defective IL-10 production (23, 24), or loss of cross-tolerance to TLRs (17, 66, 67), in patients with NOD2 mutations.
Although data presented in this study do not shed light on the nature of NOD2 mutations and their role in IBD, the findings highlight the critical role that NOD2 has on macrophage function, intestinal homeostasis, and development of colitis in the absence of IL-10 signaling and provide mechanistic insight into the development of colitis in mice and humans due to reduced or absent IL-10. We provide evidence to suggest that NOD2’s potentiation of the TLR response before the onset of inflammation provides the appropriate immune environment for the development of colitis in IL-10−/− mice. In WT mice with intact NOD2, NOD2/TLR synergy results in the potentiation of proinflammatory cytokines; the functional consequence of this, however, is kept in check with the simultaneous potentiation of IL-10, which, as we demonstrate (Fig. 5), can directly control the proinflammatory effect of TLR/NOD2 synergy. In mice lacking IL-10, NOD2/TLR synergy is biased toward a proinflammatory phenotype, resulting in hyperresponsive macrophages, which ultimately leads to the development of colitis. The key role for NOD2 in this process is demonstrated by the loss of the potentiated macrophage response and ameliorated colitis in IL-10−/−NOD2−/− mice. These findings are consistent with the hypothesis that NOD2 mutations alter the balance between pro- and anti-inflammatory activity via diminished IL-10 signaling and tolerance to TLR activation (16, 17, 23, 24, 66).
Despite strong genetic linkage to disease and evidence to suggest that the mutations are loss of function, NOD2−/− mice do not develop spontaneous colitis. One explanation for this is that NOD2 protein in patients is not deleted, rather mutated, resulting in altered function. In mice, complete deletion of NOD2 would remove both the proinflammatory and anti-inflammatory activity, resulting in little or no net effect on gut/microbiota homeostasis. However, this is not the case for pathogenic bacteria, in which NOD2 deletion has been shown to increase susceptibility to infection with Listeria and Salmonella species; in this study, susceptibility to infection may be due to NOD2’s role of bacterial defense in epithelial cells, as NOD2−/− mice were only susceptible when infected via the oral route (4, 68).
NOD2−/− mice do not differ from WT mice in the development of DSS-induced colitis (4). However, NOD2−/− mice develop exacerbated disease in TNBS-induced colitis (69), and sensitizing mice with MDP or administration of lactobacillus peptidoglycan protects from TNBS-induced inflammation in a NOD2-dependent manner (35, 70). These findings are contrary to the pathogenic role for NOD2 signaling demonstrated in this study. This discrepancy is most likely due to the lack of IL-10 in our model. In studies in which IL-10 signaling was intact, deletion of NOD2 would remove a key regulatory and/or tolerogenic pathway. MDP administration induced tolerance to TLR activation (35), and, although IL-10 was not directly measured, the mechanism involved IFN regulatory factor 4, a transcription factor that potentiates IL-10 production (71, 72). TNBS-colitis was also ameliorated by administration of peptidoglycan from certain strains of lactobacillus, and protection correlated with NOD2-dependent induction of IL-10. These findings together with data presented in this study suggest that the functional consequence of NOD2 activation on intestinal inflammation can vary depending on genetic background and proinflammatory stimulus.
In conclusion, we present data supporting previous studies (4, 5, 7, 14, 15, 17, 23, 37, 73) demonstrating that NOD2 signaling in macrophages/monocytes plays a key role in determining the response to TLR stimulation. As a result of genetic mutation or disease phenotype, subpopulations of IBD patients (CD and UC) have been shown to have diminished IL-10 signaling or low IL-10 production. Thus, on the clinically relevant genetic background of IL-10 deficiency, we show that the predominant effect of NOD2 activation is the potentiation of TLR signaling, resulting in intrinsically hyperresponsive macrophages that are central to the development of colitis in IL-10−/− mice (21, 74). The deletion of NOD2 in IL-10−/− mice abrogates the enhanced, proinflammatory synergy with TLR signaling, thus leading to ameliorated colitis. As alterations in IL-10 signaling are associated with some forms of human IBD, the findings presented in the current study provide preclinical evidence to suggest that patients WT for NOD2 but with impaired IL-10 signaling may benefit from a pharmacological therapy targeting blockade of NOD2 signaling.
Supplementary Material
The online version of this article contains supplemental material.
- CD
- Crohn’s disease
- DSS
- dextran sulfate sodium
- IBD
- inflammatory bowel disease
- MDP
- muramyl dipeptide
- NOD
- nucleotide-binding oligomerization domain
- PRR
- pattern recognition receptor
- TNBS
- 2,4,6-trinitrobenzenesulfonic acid
- Treg
- T regulatory
- UC
- ulcerative colitis
- WT
- wild-type.
Disclosures
J.J., S.P., S.J.P., and P.S. are currently employed by Novartis. N.C. has no conflicts of interest.
References
- 1.Kaser A., Zeissig S., Blumberg R. S. 2010. Inflammatory bowel disease. Annu. Rev. Immunol. 28: 573–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen G., Shaw M. H., Kim Y. G., Nuñez G. 2009. NOD-like receptors: role in innate immunity and inflammatory disease. Annu. Rev. Pathol. 4: 365–398 [DOI] [PubMed] [Google Scholar]
- 3.Girardin S. E., Boneca I. G., Viala J., Chamaillard M., Labigne A., Thomas G., Philpott D. J., Sansonetti P. J. 2003. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278: 8869–8872 [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi K. S., Chamaillard M., Ogura Y., Henegariu O., Inohara N., Nuñez G., Flavell R. A. 2005. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307: 731–734 [DOI] [PubMed] [Google Scholar]
- 5.Yang S., Tamai R., Akashi S., Takeuchi O., Akira S., Sugawara S., Takada H. 2001. Synergistic effect of muramyldipeptide with lipopolysaccharide or lipoteichoic acid to induce inflammatory cytokines in human monocytic cells in culture. Infect. Immun. 69: 2045–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park J. H., Kim Y. G., McDonald C., Kanneganti T. D., Hasegawa M., Body-Malapel M., Inohara N., Núñez G. 2007. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J. Immunol. 178: 2380–2386 [DOI] [PubMed] [Google Scholar]
- 7.Tada H., Aiba S., Shibata K., Ohteki T., Takada H. 2005. Synergistic effect of Nod1 and Nod2 agonists with Toll-like receptor agonists on human dendritic cells to generate interleukin-12 and T helper type 1 cells. Infect. Immun. 73: 7967–7976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferwerda G., Kramer M., de Jong D., Piccini A., Joosten L. A., Devesaginer I., Girardin S. E., Adema G. J., van der Meer J. W., Kullberg B. J., et al. 2008. Engagement of NOD2 has a dual effect on proIL-1beta mRNA transcription and secretion of bioactive IL-1beta. Eur. J. Immunol. 38: 184–191 [DOI] [PubMed] [Google Scholar]
- 9.Uehara A., Yang S., Fujimoto Y., Fukase K., Kusumoto S., Shibata K., Sugawara S., Takada H. 2005. Muramyldipeptide and diaminopimelic acid-containing desmuramylpeptides in combination with chemically synthesized Toll-like receptor agonists synergistically induced production of interleukin-8 in a NOD2- and NOD1-dependent manner, respectively, in human monocytic cells in culture. Cell. Microbiol. 7: 53–61 [DOI] [PubMed] [Google Scholar]
- 10.Abbott D. W., Yang Y., Hutti J. E., Madhavarapu S., Kelliher M. A., Cantley L. C. 2007. Coordinated regulation of Toll-like receptor and NOD2 signaling by K63-linked polyubiquitin chains. Mol. Cell. Biol. 27: 6012–6025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolfert M. A., Murray T. F., Boons G. J., Moore J. N. 2002. The origin of the synergistic effect of muramyl dipeptide with endotoxin and peptidoglycan. J. Biol. Chem. 277: 39179–39186 [DOI] [PubMed] [Google Scholar]
- 12.Hugot J. P., Chamaillard M., Zouali H., Lesage S., Cézard J. P., Belaiche J., Almer S., Tysk C., O’Morain C. A., Gassull M., et al. 2001. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 411: 599–603 [DOI] [PubMed] [Google Scholar]
- 13.Franke A., McGovern D. P. B., Barrett J. C., Wang K., Radford-Smith G. L., Ahmad T., Lees C. W., Balschun T., Lee J., Roberts R., et al. 2010. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat. Genet. 42: 1118–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Heel D. A., Ghosh S., Hunt K. A., Mathew C. G., Forbes A., Jewell D. P., Playford R. J. 2005. Synergy between TLR9 and NOD2 innate immune responses is lost in genetic Crohn’s disease. Gut 54: 1553–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Heel D. A., Ghosh S., Butler M., Hunt K. A., Lundberg A. M., Ahmad T., McGovern D. P., Onnie C., Negoro K., Goldthorpe S., et al. 2005. Muramyl dipeptide and Toll-like receptor sensitivity in NOD2-associated Crohn’s disease. Lancet 365: 1794–1796 [DOI] [PubMed] [Google Scholar]
- 16.Netea M. G., Ferwerda G., de Jong D. J., Jansen T., Jacobs L., Kramer M., Naber T. H., Drenth J. P., Girardin S. E., Kullberg B. J., et al. 2005. Nucleotide-binding oligomerization domain-2 modulates specific TLR pathways for the induction of cytokine release. J. Immunol. 174: 6518–6523 [DOI] [PubMed] [Google Scholar]
- 17.Kullberg B. J., Ferwerda G., de Jong D. J., Drenth J. P. H., Joosten L. A. B., Van der Meer J. W., Netea M. G. 2008. Crohn’s disease patients homozygous for the 3020insC NOD2 mutation have a defective NOD2/TLR4 cross-tolerance to intestinal stimuli. Immunology 123: 600–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray P. J. 2006. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr. Opin. Pharmacol. 6: 379–386 [DOI] [PubMed] [Google Scholar]
- 19.Takakura R., Kiyohara T., Murayama Y., Miyazaki Y., Miyoshi Y., Shinomura Y., Matsuzawa Y. 2002. Enhanced macrophage responsiveness to lipopolysaccharide and CD40 stimulation in a murine model of inflammatory bowel disease: IL-10-deficient mice. Inflamm. Res. 51: 409–415 [DOI] [PubMed] [Google Scholar]
- 20.Yen D., Cheung J., Scheerens H., Poulet F., McClanahan T., McKenzie B., Kleinschek M. A., Owyang A., Mattson J., Blumenschein W., et al. 2006. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Invest. 116: 1310–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu B., Tonkonogy S. L., Sartor R. B. 2011. Antigen-presenting cell production of IL-10 inhibits T-helper 1 and 17 cell responses and suppresses colitis in mice. Gastroenterology 141: 653–662.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreiber S., Heinig T., Thiele H.-G., Raedler A. 1995. Immunoregulatory role of interleukin 10 in patients with inflammatory bowel disease. Gastroenterology 108: 1434–1444 [DOI] [PubMed] [Google Scholar]
- 23.Netea M. G., Kullberg B. J., de Jong D. J., Franke B., Sprong T., Naber T. H., Drenth J. P., Van der Meer J. W. 2004. NOD2 mediates anti-inflammatory signals induced by TLR2 ligands: implications for Crohn’s disease. Eur. J. Immunol. 34: 2052–2059 [DOI] [PubMed] [Google Scholar]
- 24.Noguchi E., Homma Y., Kang X., Netea M. G., Ma X. 2009. A Crohn’s disease-associated NOD2 mutation suppresses transcription of human IL10 by inhibiting activity of the nuclear ribonucleoprotein hnRNP-A1. Nat. Immunol. 10: 471–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Correa I., Veny M., Esteller M., Piqué J. M., Yagüe J., Panés J., Salas A. 2009. Defective IL-10 production in severe phenotypes of Crohn’s disease. J. Leukoc. Biol. 85: 896–903 [DOI] [PubMed] [Google Scholar]
- 26.Franke A., Balschun T., Karlsen T. H., Sventoraityte J., Nikolaus S., Mayr G., Domingues F. S., Albrecht M., Nothnagel M., Ellinghaus D., et al. IBSEN Study Group 2008. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat. Genet. 40: 1319–1323 [DOI] [PubMed] [Google Scholar]
- 27.Glocker E.-O., Kotlarz D., Boztug K., Gertz E. M., Schäffer A. A., Noyan F., Perro M., Diestelhorst J., Allroth A., Murugan D., et al. 2009. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N. Engl. J. Med. 361: 2033–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biswas A., Wilmanski J., Forsman H., Hrncir T., Hao L., Tlaskalova-Hogenova H., Kobayashi K. S. 2011. Negative regulation of Toll-like receptor signaling plays an essential role in homeostasis of the intestine. Eur. J. Immunol. 41: 182–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matharu K. S., Mizoguchi E., Cotoner C. A., Nguyen D. D., Mingle B., Iweala O. I., McBee M. E., Stefka A. T., Prioult G., Haigis K. M., et al. 2009. Toll-like receptor 4-mediated regulation of spontaneous Helicobacter-dependent colitis in IL-10-deficient mice. Gastroenterology 137: 1380–1390.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Messlik A., Schmechel S., Kisling S., Bereswill S., Heimesaat M. M., Fischer A., Göbel U., Haller D. 2009. Loss of Toll-like receptor 2 and 4 leads to differential induction of endoplasmic reticulum stress and proapoptotic responses in the intestinal epithelium under conditions of chronic inflammation. J. Proteome Res. 8: 4406–4417 [DOI] [PubMed] [Google Scholar]
- 31.Saunders S. P., Barlow J. L., Walsh C. M., Bellsoi A., Smith P., McKenzie A. N. J. 2010. C-type lectin SIGN-R1 has a role in experimental colitis and responsiveness to lipopolysaccharide. J. Immunol. 184: 2627–2637 [DOI] [PubMed] [Google Scholar]
- 32.Cario E., Gerken G., Podolsky D. K. 2007. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 132: 1359–1374 [DOI] [PubMed] [Google Scholar]
- 33.Rachmilewitz D., Katakura K., Karmeli F., Hayashi T., Reinus C., Rudensky B., Akira S., Takeda K., Lee J., Takabayashi K., Raz E. 2004. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology 126: 520–528 [DOI] [PubMed] [Google Scholar]
- 34.Smith P., Siddharth J., Pearson R., Holway N., Shaxted M., Butler M., Clark N., Jamontt J., Watson R. P., Sanmugalingam D., Parkinson S. J. 2012. Host genetics and environmental factors regulate ecological succession of the mouse colon tissue-associated microbiota. PLoS One 7: e30273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watanabe T., Asano N., Murray P. J., Ozato K., Tailor P., Fuss I. J., Kitani A., Strober W. 2008. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J. Clin. Invest. 118: 545–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaw M. H., Reimer T., Sánchez-Valdepeñas C., Warner N., Kim Y. G., Fresno M., Nuñez G. 2009. T cell-intrinsic role of Nod2 in promoting type 1 immunity to Toxoplasma gondii. Nat. Immunol. 10: 1267–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watanabe T., Kitani A., Murray P. J., Strober W. 2004. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat. Immunol. 5: 800–808 [DOI] [PubMed] [Google Scholar]
- 38.Spencer D. M., Veldman G. M., Banerjee S., Willis J., Levine A. D. 2002. Distinct inflammatory mechanisms mediate early versus late colitis in mice. Gastroenterology 122: 94–105 [DOI] [PubMed] [Google Scholar]
- 39.Davidson N. J., Leach M. W., Fort M. M., Thompson-Snipes L., Kühn R., Müller W., Berg D. J., Rennick D. M. 1996. T helper cell 1-type CD4+ T cells, but not B cells, mediate colitis in interleukin 10-deficient mice. J. Exp. Med. 184: 241–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwartz R. H. 2003. T cell anergy. Annu. Rev. Immunol. 21: 305–334 [DOI] [PubMed] [Google Scholar]
- 41.Smith P., Walsh C. M., Mangan N. E., Fallon R. E., Sayers J. R., McKenzie A. N., Fallon P. G. 2004. Schistosoma mansoni worms induce anergy of T cells via selective up-regulation of programmed death ligand 1 on macrophages. J. Immunol. 173: 1240–1248 [DOI] [PubMed] [Google Scholar]
- 42.Magalhaes J. G., Fritz J. H., Le Bourhis L., Sellge G., Travassos L. H., Selvanantham T., Girardin S. E., Gommerman J. L., Philpott D. J. 2008. Nod2-dependent Th2 polarization of antigen-specific immunity. J. Immunol. 181: 7925–7935 [DOI] [PubMed] [Google Scholar]
- 43.Powrie F., Correa-Oliveira R., Mauze S., Coffman R. L. 1994. Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for the balance between protective and pathogenic cell-mediated immunity. J. Exp. Med. 179: 589–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mottet C., Uhlig H. H., Powrie F. 2003. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J. Immunol. 170: 3939–3943 [DOI] [PubMed] [Google Scholar]
- 45.Asseman C., Read S., Powrie F. 2003. Colitogenic Th1 cells are present in the antigen-experienced T cell pool in normal mice: control by CD4+ regulatory T cells and IL-10. J. Immunol. 171: 971–978 [DOI] [PubMed] [Google Scholar]
- 46.Josefowicz S. Z., Rudensky A. 2009. Control of regulatory T cell lineage commitment and maintenance. Immunity 30: 616–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fiorentino D. F., Zlotnik A., Vieira P., Mosmann T. R., Howard M., Moore K. W., O’Garra A. 1991. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 146: 3444–3451 [PubMed] [Google Scholar]
- 48.Ogura Y., Inohara N., Benito A., Chen F. F., Yamaoka S., Nunez G. 2001. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J. Biol. Chem. 276: 4812–4818 [DOI] [PubMed] [Google Scholar]
- 49.Fritz J. H., Girardin S. E., Fitting C., Werts C., Mengin-Lecreulx D., Caroff M., Cavaillon J. M., Philpott D. J., Adib-Conquy M. 2005. Synergistic stimulation of human monocytes and dendritic cells by Toll-like receptor 4 and NOD1- and NOD2-activating agonists. Eur. J. Immunol. 35: 2459–2470 [DOI] [PubMed] [Google Scholar]
- 50.Moreira L. O., El Kasmi K. C., Smith A. M., Finkelstein D., Fillon S., Kim Y. G., Núñez G., Tuomanen E., Murray P. J. 2008. The TLR2-MyD88-NOD2-RIPK2 signalling axis regulates a balanced pro-inflammatory and IL-10-mediated anti-inflammatory cytokine response to Gram-positive cell walls. Cell. Microbiol. 10: 2067–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. 1993. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75: 263–274 [DOI] [PubMed] [Google Scholar]
- 52.Spencer S. D., Di Marco F., Hooley J., Pitts-Meek S., Bauer M., Ryan A. M., Sordat B., Gibbs V. C., Aguet M. 1998. The orphan receptor CRF2-4 is an essential subunit of the interleukin 10 receptor. J. Exp. Med. 187: 571–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takeda K., Clausen B. E., Kaisho T., Tsujimura T., Terada N., Förster I., Akira S. 1999. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 10: 39–49 [DOI] [PubMed] [Google Scholar]
- 54.Glocker E., Kotlarz D., Boztug K., Gertz E., Schaffer A., Koletzko S., Shah N., Snapper S., Grimbacher B., Klein C. 2009. Early-onset inflammatory bowel disease caused by loss-of-function mutations in the IL10-receptor genes. Inflamm. Bowel Dis. 15: S56 [Google Scholar]
- 55.Anderson C. A., Massey D. C., Barrett J. C., Prescott N. J., Tremelling M., Fisher S. A., Gwilliam R., Jacob J., Nimmo E. R., Drummond H., et al. Wellcome Trust Case Control Consortium 2009. Investigation of Crohn’s disease risk loci in ulcerative colitis further defines their molecular relationship. Gastroenterology 136: 523–529.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rakoff-Nahoum S., Paglino J., Eslami-Varzaneh F., Edberg S., Medzhitov R. 2004. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 118: 229–241 [DOI] [PubMed] [Google Scholar]
- 57.Rakoff-Nahoum S., Hao L., Medzhitov R. 2006. Role of Toll-like receptors in spontaneous commensal-dependent colitis. Immunity 25: 319–329 [DOI] [PubMed] [Google Scholar]
- 58.Kobayashi M., Kweon M. N., Kuwata H., Schreiber R. D., Kiyono H., Takeda K., Akira S. 2003. Toll-like receptor-dependent production of IL-12p40 causes chronic enterocolitis in myeloid cell-specific Stat3-deficient mice. J. Clin. Invest. 111: 1297–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ogura Y., Bonen D. K., Inohara N., Nicolae D. L., Chen F. F., Ramos R., Britton H., Moran T., Karaliuskas R., Duerr R. H., et al. 2001. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 411: 603–606 [DOI] [PubMed] [Google Scholar]
- 60.Maeda S., Hsu L. C., Liu H., Bankston L. A., Iimura M., Kagnoff M. F., Eckmann L., Karin M. 2005. Nod2 mutation in Crohn’s disease potentiates NF-kappaB activity and IL-1beta processing. [Published erratum appears in 2005 Science 308: 633 and 2011 Science 333: 288.] Science 307: 734–738 [DOI] [PubMed] [Google Scholar]
- 61.Kim Y. G., Shaw M. H., Warner N., Park J. H., Chen F., Ogura Y., Núñez G. 2011. Cutting edge: Crohn’s disease-associated Nod2 mutation limits production of proinflammatory cytokines to protect the host from Enterococcus faecalis-induced lethality. J. Immunol. 187: 2849–2852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Netea M. G., Ferwerda G., de Jong D. J., Girardin S. E., Kullberg B. J., van der Meer J. W. 2005. NOD2 3020insC mutation and the pathogenesis of Crohn’s disease: impaired IL-1beta production points to a loss-of-function phenotype. Neth. J. Med. 63: 305–308 [PubMed] [Google Scholar]
- 63.Inohara N., Ogura Y., Fontalba A., Gutierrez O., Pons F., Crespo J., Fukase K., Inamura S., Kusumoto S., Hashimoto M., et al. 2003. Host recognition of bacterial muramyl dipeptide mediated through NOD2: implications for Crohn’s disease. J. Biol. Chem. 278: 5509–5512 [DOI] [PubMed] [Google Scholar]
- 64.Kramer M., Netea M. G., de Jong D. J., Kullberg B. J., Adema G. J. 2006. Impaired dendritic cell function in Crohn’s disease patients with NOD2 3020insC mutation. J. Leukoc. Biol. 79: 860–866 [DOI] [PubMed] [Google Scholar]
- 65.Wehkamp J., Harder J., Weichenthal M., Schwab M., Schäffeler E., Schlee M., Herrlinger K. R., Stallmach A., Noack F., Fritz P., et al. 2004. NOD2 (CARD15) mutations in Crohn’s disease are associated with diminished mucosal alpha-defensin expression. Gut 53: 1658–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cantó E., Moga E., Ricart E., Garcia-Bosch O., Garcia-Planella E., Juarez C., Vidal S. 2009. MDP-induced selective tolerance to TLR4 ligands: impairment in NOD2 mutant Crohn’s disease patients. Inflamm. Bowel Dis. 15: 1686–1696 [DOI] [PubMed] [Google Scholar]
- 67.Hedl M., Li J., Cho J. H., Abraham C. 2007. Chronic stimulation of Nod2 mediates tolerance to bacterial products. Proc. Natl. Acad. Sci. USA 104: 19440–19445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meinzer U., Esmiol-Welterlin S., Barreau F., Berrebi D., Dussaillant M., Bonacorsi S., Chareyre F., Niwa-Kawakita M., Alberti C., Sterkers G., et al. 2008. Nod2 mediates susceptibility to Yersinia pseudotuberculosis in mice. PLoS One 3: e2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barreau F., Meinzer U., Chareyre F., Berrebi D., Niwa-Kawakita M., Dussaillant M., Foligne B., Ollendorff V., Heyman M., Bonacorsi S., et al. 2007. CARD15/NOD2 is required for Peyer’s patches homeostasis in mice. PLoS One 2: e523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Macho Fernandez E., Valenti V., Rockel C., Hermann C., Pot B., Boneca I. G., Grangette C. 2011. Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide. Gut 60: 1050–1059 [DOI] [PubMed] [Google Scholar]
- 71.Lee C. G., Kang K. H., So J. S., Kwon H. K., Son J. S., Song M. K., Sahoo A., Yi H. J., Hwang K. C., Matsuyama T., et al. 2009. A distal cis-regulatory element, CNS-9, controls NFAT1 and IRF4-mediated IL-10 gene activation in T helper cells. Mol. Immunol. 46: 613–621 [DOI] [PubMed] [Google Scholar]
- 72.Lee C. G., Hwang W., Maeng K. E., Kwon H. K., So J. S., Sahoo A., Lee S. H., Park Z. Y., Im S. H. 2011. IRF4 regulates IL-10 gene expression in CD4(+) T cells through differential nuclear translocation. Cell. Immunol. 268: 97–104 [DOI] [PubMed] [Google Scholar]
- 73.Hedl M., Abraham C. 2011. Secretory mediators regulate Nod2-induced tolerance in human macrophages. Gastroenterology 140: 231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Watanabe N., Ikuta K., Okazaki K., Nakase H., Tabata Y., Matsuura M., Tamaki H., Kawanami C., Honjo T., Chiba T. 2003. Elimination of local macrophages in intestine prevents chronic colitis in interleukin-10-deficient mice. Dig. Dis. Sci. 48: 408–414 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.