Abstract
Objectives
This paper examined whether nebivolol protects the heart via nitric oxide (NO) synthase and NO-dependent signaling in an in vivo model of acute myocardial infarction.
Background
Beta3-adrenergic receptor (AR) activation promotes endothelial nitric oxide synthase (eNOS) activity and NO bioavailability. We hypothesized that specific beta3-AR agonists would attenuate myocardial ischemia-reperfusion (MI/R) injury via eNOS activation and increased NO bioavailability.
Methods
Mice were subjected to 45 min of myocardial ischemia in vivo followed by 24 h of reperfusion (R). Nebivolol (500 ng/kg), CL 316243 (1 μg/kg), BRL-37344 (1 μg/kg), or vehicle (VEH) was administered at the time of R. Myocardial area-at-risk (AAR) and infarct size (INF)/AAR was measured at 24 h of R. Cardiac tissue and plasma were collected to evaluate eNOS phosphorylation, neuronal nitric oxide synthase (nNOS), inducible nitric oxide synthase expression, and nitrite and nitrosothiol levels.
Results
Nebivolol (500 ng/kg) reduced INF/AAR by 37% (p < 0.001 vs. VEH) and serum troponin-I levels from 41 ± 4 ng/ml to 25 ± 4 ng/ml (p < 0.05 vs. VEH). CL 316243 and BRL-37344 reduced INF by 39% and 42%, respectively (p < 0.001 vs. VEH). Nebivolol and CL 316243 increased eNOS phosphorylation at Ser-1177 (p < 0.05 vs. VEH) and increased nitrite and total nitrosylated protein levels. Nebivolol and CL 316243 significantly increased myocardial nNOS expression. Nebivolol failed to reduce INF after MI/R in beta3-AR−/−, eNOS−/−, and in nNOS−/− mice.
Conclusions
Our results indicate that beta3-AR agonists protect against MI/R injury. Furthermore, the cardioprotective effects of beta3-AR agonists are mediated by rapid eNOS and nNOS activation and increased NO bioavailability.
Keywords: beta3 adrenergic receptor, cardiac ischemia, endothelial nitric oxide synthase, neuronal nitric oxide synthase, nitric oxide
Acute myocardial infarction is one of the leading causes of death in the adult population. Myocardial ischemia and reperfusion (MI/R) injury involves excess formation of free radicals and calcium overload, leading to cell death, tissue scarring, and remodeling (1–3). Beta-adrenergic receptor (AR) blockers have been shown to play a key role in the management of cardiovascular disease by improvement of coronary circulation, protection from cardiomyocyte apoptosis, and reduction of infarction (4 – 6). These beta-blockers target the beta1 and beta2 ARs as their primary mode of action (7,8). However, the beta3-AR has recently emerged as a potential target for the treatment of heart disease (9,10).
Nebivolol (alpha,alpha′-[iminodimethylene]bis[6-fluoro-2-chrommethanol]) is a third-generation beta blocker approved by the U.S. Food and Drug Administration for the treatment of hypertension. The selectivity of nebivolol for the beta1-AR is considerably higher than that of bisoprolol, carvedilol, or bucindolol, and it was shown to reduce mortality and morbidity in elderly patients with heart failure (11–13). Nebivolol also exhibits nitric oxide (NO)-mediated vasodilating properties thought to result from the stimulation of the endothelial beta3-AR (14,15). Stimulation of the beta3-AR and activation of endothelial nitric oxide synthase (eNOS) increases NO release after nebivolol treatment to cause peripheral vasodilatation and improved endothelial function (16,17). Nebivolol might also activate the beta2-AR (18 –20).
NO exerts profound cardioprotection in animal models of MI/R injury (21–23). The overexpression of eNOS decreases MI/R injury, whereas eNOS deficiency exacerbates MI/R injury (24 –26). Nitric oxide also inhibits mitochondrial respiration and prevents platelet aggregation and neutrophil adherence to vascular endothelium (27,28).
In the present study, we examined whether nebivolol or highly specific beta3-AR agonists protect the heart via eNOS and NO-dependent signaling in an in vivo murine model of acute myocardial infarction.
Methods
An expanded Methods section is available in the Online Appendix.
Animals
Male C57BL6/J mice, 10 to 12 weeks of age (Jackson Labs, Bar Harbor, Maine), eNOS deficient (eNOS−/−) mice (26) (10 to 12 weeks old), and beta3 adrenergic receptor deficient (beta3-AR−/−) mice (12 to 14 weeks) were used (29,30). All animals received humane care in compliance with the Principles of Laboratory Animal Care formulated by the National Society of Medical Research and the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (Publication No. 85-23, Revised 1996). Procedures were approved by the Emory University Institutional Animal Care and Use Committee.
Materials
Nebivolol was provided by the Forest Research Institute (Jersey City, New Jersey). The beta3 receptor agonists CL 316243 and BRL-37344 were purchased from Sigma-Aldrich (St. Louis, Missouri). Nitro-L-arginine methyl ester (L-NAME) (hydrochloride) and the beta3 AR antagonist L-748,337 were purchased from Cayman Chemical (Ann Arbor, Michigan).
MI/R protocol
Surgical ligation of the left coronary artery (LCA), myocardial INF determination, and troponin I measurements were performed as previously described (25,31).
Western blot analysis
Western blot analysis was performed as previously described (25).
NO metabolites
NO and NO metabolite analysis was performed as previously described (32).
Measurement of nebivolol
Liquid chromatography tandem mass spectrometry was used for determination of d/l-nebivolol in mouse plasma. See the Online Appendix for detailed methods.
Hemodynamic parameters
Mice were anesthetized with isoflurane (1.0 to 2.0 l/min) in 100% oxygen. The right carotid artery was exposed for a length of approximately 5 mm. A 1.0-F Millar pressure catheter (Millar Instruments, Houston, Texas) connected to the computerized data-acquisition system (PowerLab 4/30, AD Instruments, Colorado Springs, Colorado) was advanced into the aorta through the right carotid artery to record heart rate (HR), systolic, diastolic, and mean arterial pressures. These values were recorded with LabChart 6 PRO (AD Instruments).
Echocardiography
Baseline echocardiography images were obtained 1 week before LCA ischemia to avoid cardioprotective effects of the isoflurane, as previously described with slight modification. Mice were anesthetized with isoflurane, and transthoracic echocardiography of the left ventricle (LV) was performed with a 30-MHz RMV scanhead interfaced with a Vevo 2100 (Visualsonics, Toronto, Ontario, Canada) to obtain high-resolution M mode images at the rate of 1,000 frames/s. These were used to measure LV end-diastolic diameter, LV end-systolic diameter, and ejection fraction. Echocardiography images were obtained again 1 week after the MI/R protocol.
Statistical analysis
All data in this study are expressed as the mean ± SEM. Differences in data between the groups were compared with Prism 4 (GraphPad Software, La Jolla, California) with Student unpaired 2-tailed t test when 2 groups were compared or a 1-way analysis of variance, when 3 or more groups were compared. If a significant difference was found with the analysis of variance test, a Tukey’s (Figs. 1 to 5, Online Fig. 2) or Dunnett’s (Online Fig. 3) multiple comparison test was used for post hoc analysis. A p value <0.05 was considered significant.
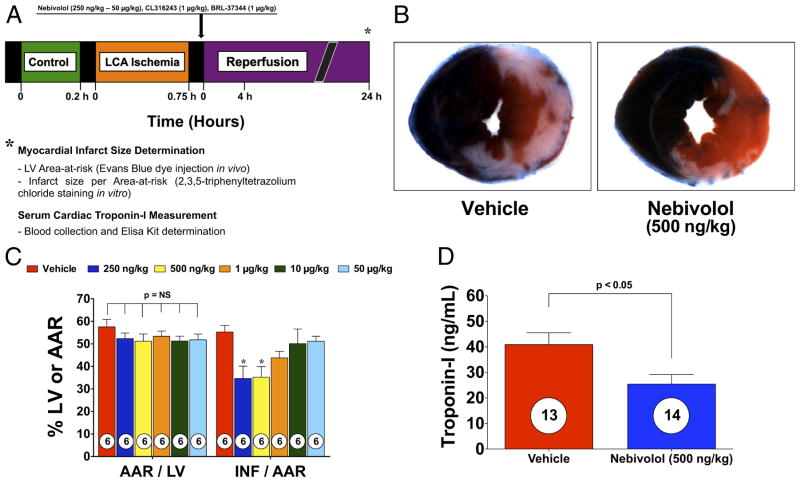
Figure 1. Nebivolol Reduces Myocardial INF and Troponin-I.
(A) Experimental protocol for this study. Mice were subjected to 45 min of left coronary artery (LCA) occlusion followed by 24 h of reperfusion. Myocardial area-at-risk (AAR) and myocardial infarct size (INF) were determined at 24 h of reperfusion. In addition, blood was collected at 24 h of reperfusion, and troponin-I was measured. Nebivolol or saline were injected directly into the left ventricular (LV) cavity just before reperfusion. (B) Representative mid-ventricular photomicrographs of mouse hearts after 45 min of myocardial ischemia and 24 h of reperfusion treated with vehicle (VEH) or nebivolol at the time of reperfusion. (C) Bar graph of myocardial AAR/LV and INF/AAR in mice treated with nebivolol. (D) Troponin levels in VEH and nebivolol-treated mice at 24 h after reperfusion. Nebivolol (500 ng/kg) administered at the time of reperfusion decreased serum troponin I levels, compared with VEH. Values are mean ± SEM. Numbers inside bars indicate the number of animals investigated in each group. *p < 0.05 versus VEH.
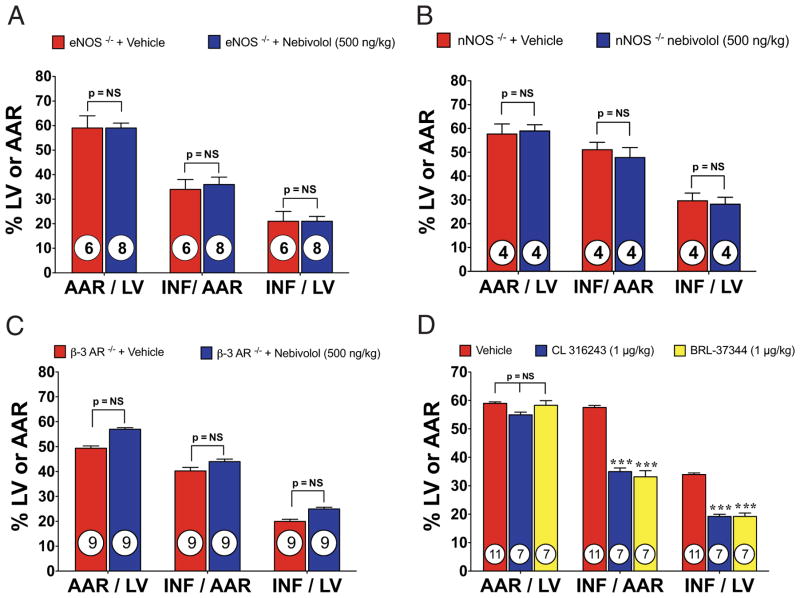
Figure 5. Nebivolol-Mediated Cardioprotection Is Lost in Pathway-Specific KO Mice.
(A) Myocardial INF in eNOS−/− (eNOS knockout [KO]) mice treated with nebivolol (500 ng/kg). (B) Myocardial INF in nNOS−/− mice treated with nebivolol (500 ng/kg) at reperfusion. Bar graph of myocardial AAR/LV and INF/AAR in mice treated with nebivolol. (C) Myocardial INF in beta3-AR−/− mice treated with nebivolol. Treatment with nebivolol (500 ng/kg) at the time of reperfusion failed to reduce INF/AAR or INF/LV. (D) Myocardial INF in mice treated with CL 316243 (1 μg/kg) or BRL-37344 (1 μg/kg). No significant differences were observed with respect to the AAR/LV. Both CL 316243 and BRL 37344 significantly reduced myocardial INF/AAR and/LV. Values are mean ± SEM. ***p < 0.001; numbers inside bars indicate the number of animals/group. Abbreviations as in Figures 1, 2, and 3.
Results
Nebivolol limits myocardial injury
We subjected male C57BL6/J mice to 45 min of LCA ischemia followed by reperfusion. Nebivolol (250 ng/kg to 50 μg/kg) or vehicle (VEH) was administered into the LV lumen at reperfusion (Fig. 1A). We evaluated myocardial INF at 24 h of reperfusion. Representative mid-ventricular cross-sections of VEH- and nebivolol-treated (500 ng/kg) mice are shown in Figure 1B. The area-at-risk (AAR)/LV was similar (p = NS) in all groups (Fig. 1C). Evaluation of INF revealed a dose-response curve, with 250 and 500 ng/kg displaying the most cytoprotection, as assessed by 2,3,5-triphenyltetrazolium chloride staining (Fig. 1C). Mice receiving 250 ng/kg displayed an INF/AAR of (34.7 ± 5.5%) compared with VEH (55.3 ± 2.8%), a 37% reduction. Mice receiving 500 ng/kg displayed an INF/AAR of (35.2 ± 4.7%), a 36% reduction. The dose of 500 ng/kg was used in all subsequent in vivo studies.
We evaluated circulating plasma levels of the cardiac-specific isoform of troponin-I as a marker of myocardial injury (Fig. 1D). The cardiac-specific isoform of troponin-I levels were significantly reduced in nebivolol-treated animals, containing 25.5 ± 3.8 ng/ml versus the VEH level of 40.9 ± 4.6 ng/ml.
Effects of nebivolol on hemodynamic status
We measured mean arterial blood pressure (MABP) and HR after administration of nebivolol to determine the hemodynamic effects (Table 1). The MABP and HR were measured at baseline and at increasing doses to 500 ng/kg nebivolol. There was no significant change in MABP or HR at 500 ng/kg versus baseline. The dose was then increased to 1 mg/kg, which significantly decreased MABP and slightly decreased HR.
Table 1.
Hemodynamic Effects of Nebivolol on Anesthetized Mice
| Group (n = 4) | Mean Arterial Blood Pressure (mm Hg) | Heart Rate (beats/min) |
|---|---|---|
| Baseline | 78.03 ± 0.70 | 492 ± 22 |
| 500 ng/kg | 76.56 ± 0.49 | 495 ± 25 |
| 50 μg/kg | 72.22 ± 0.61 | 489 ± 18 |
| 500 μg/kg | 72.70 ± 1.19 | 477 ± 16 |
| 1 mg/kg | 67.63 ± 3.66* | 459 ± 4 |
Values are mean ± SE.
p < 0.01 versus baseline.
Nebivolol activates NO synthase
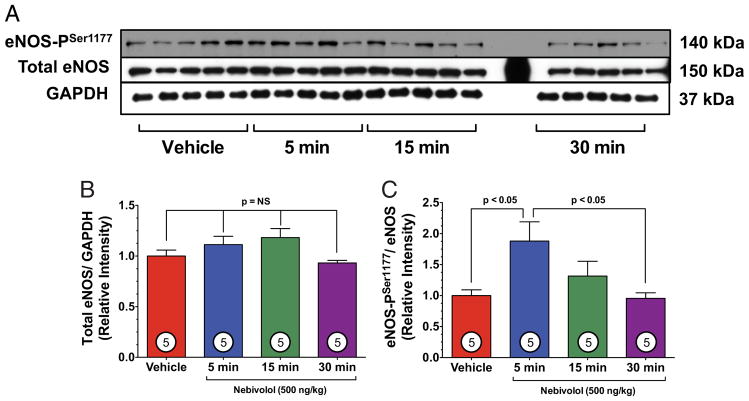
We investigated the effects of acute nebivolol on expression and phosphorylation of eNOS at serine residue-1177 (eNOS-PSer1177) to determine eNOS activation (Fig. 2). Total eNOS expression remained constant (Figs. 2A and 2B). However, nebivolol significantly increased eNOS-PSer1177 at 5 min after administration and returned to basal levels after 30 min (Figs. 2A and 2C).
Figure 2. Nebivolol Increases eNOS-PSer1177.
(A) Representative immunoblots of phosphorylation of endothelial nitric oxide synthase at serine residue 1177 (eNOS-PSer1177) and total endothelial nitric oxide synthase (eNOS) from the left ventricle of sham-operated mice receiving either vehicle (VEH) or nebivolol (500 ng/kg) via intracardiac injection at various time points. (B) Relative intensity of total eNOS remained constant compared with VEH. (C) Nebivolol increased the eNOS-PSer1177 at 15 min, compared with VEH (p < 0.05) and compared with 30 min (p < 0.05). Numbers inside bars indicate the number of animals studied in each group. GAPDH = glyceraldehyde-3-phosphate dehydrogenase.
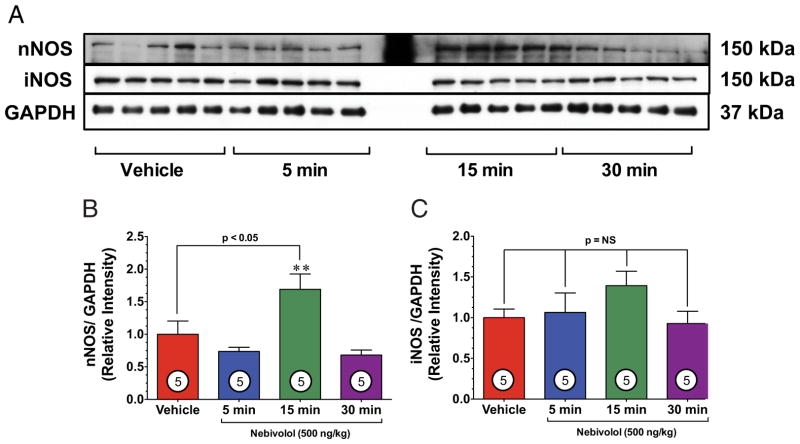
We also evaluated neuronal nitric oxide synthase (nNOS) and inducible nitric oxide synthase (iNOS) (Fig. 3). Interestingly, we observed a 1.7-fold increase in the expression of nNOS in the heart 15 min after treatment, which returned to baseline levels by 30 min (Figs. 3A and 3B). Cardiac levels of iNOS remained unchanged (Fig. 3C).
Figure 3. Nebivolol Increases nNOS Expression.
(A) Representative immunoblots of neuronal nitric oxide synthase (nNOS) and inducible nitric oxide synthase (iNOS) from the left ventricle of sham-operated mice receiving either VEH or nebivolol (500 ng/kg) via intracardiac injection at various time points. (B) Relative intensity of total nNOS increases after 15 min, compared with VEH (p < 0.05). **p < 0.01 versus 5 min and 30 min time points. (C) Relative intensity of total iNOS remained constant compared with VEH. Numbers inside bars indicate the number of animals studied in each group. Abbreviations as in Figure 2.
Nebivolol increases NO
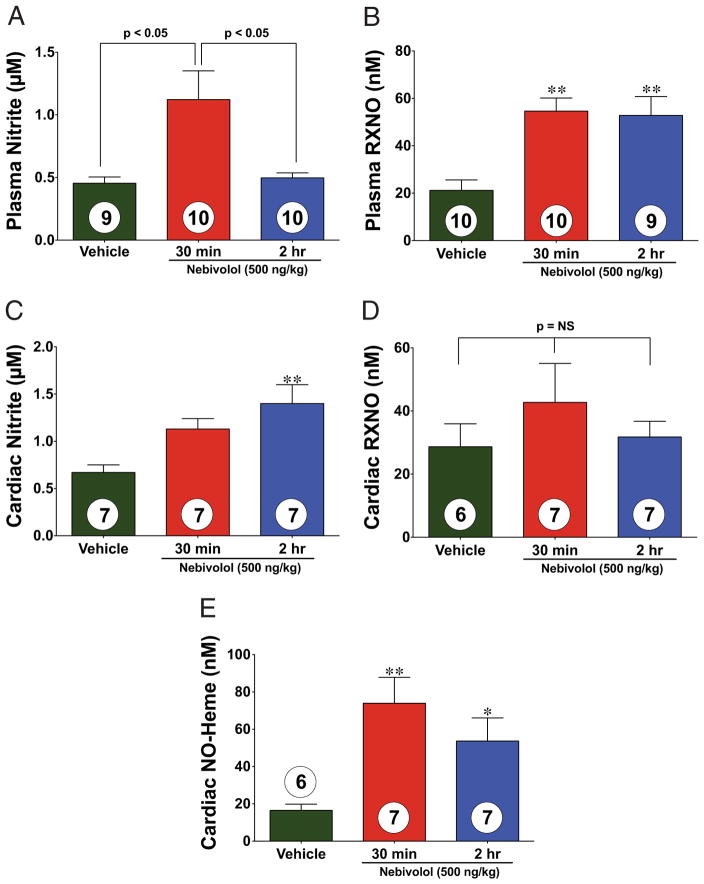
We next investigated plasma and cardiac NO levels (Fig. 4). Mice treated with nebivolol displayed a 2.5-fold increase in plasma nitrite (an NO storage molecule) 30 min after intracardiac injection (Fig. 4A). Additionally, plasma total nitrosylated protein (RXNO) levels were significantly increased (p < 0.01) at 30 min and 2 h after administration (2.6-and 2.5-fold, respectively) (Fig. 4B). Cardiac nitrite levels significantly increased at 2 h (2.1-fold) (Fig. 4C). Cardiac RXNO levels did not show a significant difference after nebivolol administration (Fig. 4D). In contrast, cardiac NO-Heme levels significantly increased (4.5-fold) after 30 min (Fig. 4E).
Figure 4. Nebivolol Increases NO Metabolites.
Plasma and cardiac nitric oxide (NO) metabolites (nitrite and plasma total nitrosylated protein [RXNO]) measured after intracardiac injection of either vehicle (VEH) or nebivolol in sham-operated control mice. (A) Nitrite levels (μmol/l) increased in the plasma of nebivolol-treated mice. (B) The RXNO levels (nmol/l) increased after 30 min of nebivolol administration and remained elevated after 2 h. (C) Nitrite levels (μmol/l) increased in the heart of nebivolol-treated mice. (D) The RXNO levels in the heart did not significantly increase after 30 min of nebivolol administration. (E) The NO-Heme levels (nmol/l) significantly increased after 30 min of nebivolol administration and slightly decreased after 2 h. Values are mean ± SEM. *p < 0.05; **p < 0.01; numbers inside bars indicate the number of animals/group.
Nebivolol protects through beta3-AR and NO synthase
We investigated the effects of nebivolol therapy in eNOS−/− (Fig. 5A) and nNOS−/− mice (Fig. 5B). Myocardial INF was not reduced in eNOS−/− mice after administration of nebivolol. Similar results were found in nNOS−/− mice. We also investigated nebivolol treatment in beta3-AR−/− mice. Myocardial INF was not reduced in beta3-AR−/− mice after administration of nebivolol (Fig. 5C).
Inhibition of NO synthase and beta3-AR abolishes nebivolol-mediated cardioprotection
The L-NAME inhibits the 3 nitric oxide synthase (NOS) isoforms. Therefore, we evaluated myocardial INF in wild-type mice treated with both L-NAME (25 mg/ml) and nebivolol (Fig. 6A). When L-NAME was co-administered, the cardioprotective effects of nebivolol were lost. Subsequently, we studied nebivolol co-administered with a specific beta3-AR antagonist, L-748,337 (33). When L-748,337 (100 μg/kg) was co-administered, the cardioprotective effects of nebivolol were also lost (Fig. 6B).
Figure 6. Nebivolol-Mediated Cardioprotection Is Lost With Pathway-Specific Pharmacological Inhibition.
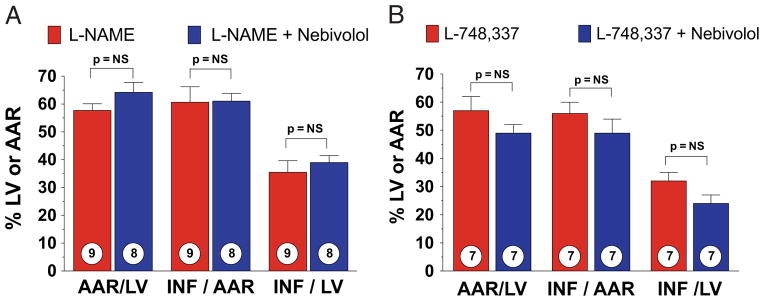
(A) Myocardial INF in wild-type mice treated with nitro-L-arginine methyl ester (L-NAME) (25 mg/kg) and mice treated with L-NAME (25 mg/kg) and nebivolol (500 ng/kg) at reperfusion. (B) Myocardial INF in wild-type mice treated with L-748,337 (100 μg/kg) and mice treated with L-748,337 (100 μg/kg) and nebivolol (500 ng/kg). Values are mean ± SEM. Numbers inside bars indicate the number of animals/group. Abbreviations as in Figure 1.
Beta3-AR specific agonists limit injury after MI/R
We investigated 2 beta3-AR specific agonists, CL 316243 (34) and BRL-37344 (35), to confirm the effects of beta3-AR stimulation on MI/R injury (Fig. 5D). Mice receiving 1 μg/kg of CL 316243 displayed an INF/AAR of (35.0 ± 3.3%) compared with VEH (59.0 ± 1.8%), a 39% reduction. Mice receiving 1 μg/kg of BRL-37344 displayed an INF/AAR of (33.1 ± 4.7%), a 42% reduction.
CL 316243 mediates NOS activation
We also evaluated the effect of CL 316243 on the expression and phosphorylation levels of eNOS-PSer1177 in sham-operated mice. Total eNOS expression remained constant (Online Fig. 2A). However, 2 h after CL 316243 administration, eNOS-PSer1177 increased significantly (Online Fig. 2A), whereas eNOS-PThr495 remained constant (Online Fig. 2A). The expression of nNOS and iNOS were also measured. There was a significant increase in nNOS protein expression after 15 min of CL 316243 administration (Online Fig. 2B). The expression levels of iNOS were not significantly different (Online Fig. 2B). We also measured plasma and cardiac nitrite and RXNO levels and NO-Heme levels (Online Fig. 3). We observed an apparent increase in plasma nitrite levels and a significant increase in cardiac nitrite levels after CL 316243 injection that persisted to 2 h (1.8-fold in the heart) (Online Figs. 3A and 3C). The RXNO values also exhibited a similar trend with a 3.1-fold increase in plasma RXNO after 2 h, when compared with VEH (Online Fig. 3B). Heart RXNO and NO-Heme values also increased significantly after 30 min, showing a 1.9- and 3.6-fold increase, respectively.
Circulating levels of nebivolol
We measured the circulating levels of nebivolol at different time points (1, 5, and 15 min) after intracardiac injection, to evaluate the pharmacokinetics. Nebivolol was administered to 3 different groups of mice (n = 3), and blood samples were taken to isolate plasma. The concentration of nebivolol was 1.2 ± 0.29 ng/ml after 1 min, then 0.4 ± 0.1 ng/ml after 5 min, and below detection after 15 min.
Nebivolol and LV function
We measured LV function and cardiac dimensions of mice treated with nebivolol and VEH (Table 2). Wild-type mice were subjected to 45-min myocardial ischemia and received nebivolol or VEH at reperfusion. No significant difference was observed in the ejection fraction, LV end diastolic diameter, LV end systolic diameter, and fractional shortening.
Table 2.
Effects of Nebivolol on Left Ventricular Function and Dimensions
| Baseline
|
7-Day Post-MI/R
|
|||
|---|---|---|---|---|
| VEH (n = 10) | Nebivolol (n = 9) | VEH (n = 10) | Nebivolol (n = 9) | |
| EF (%) | 66.0 ± 1.87 | 64.4 ± 2.43 | 41.7 ± 3.49 | 41.3 ± 4.11 |
|
| ||||
| LVEDD (mm) | 3.4 ± 0.04 | 3.5 ± 0.06 | 4.0 ± 0.14 | 4.1 ± 0.14 |
|
| ||||
| LVESD (mm) | 2.2 ± 0.06 | 2.3 ± 0.08 | 3.1 ± 0.16 | 3.3 ± 0.20 |
|
| ||||
| FS | 35.6 ± 1.39 | 34.6 ± 1.92 | 20.3 ± 1.89 | 20.3 ± 2.28 |
Values are mean ± SE. p = NS (nebivolol vs. vehicle [VEH]).
EF = ejection fraction; FS = fractional shortening; LVEDD = left ventricular end-diastolic diameter; LVESD = left ventricular end-systolic diameter; MI/R = myocardial ischemia-reperfusion.
Discussion
The effects of beta1- and beta2-ARs are well-established in mammals, because their stimulation mediates an increase in HR, myocardial contraction force, and acceleration of relaxation (36). The beta3-AR has been shown to produce a negative inotropic effect that antagonizes the activity of beta1- and beta2-ARs (37,38). The beta3-AR is activated at higher concentrations of catecholamines than required for beta1- and beta2-ARs and is thought to act as a counter-mechanism during sympathetic overstimulation (39). The beta3-AR–mediated signaling also results in NO generation from eNOS (40).
Previous studies suggest that beta3-AR attenuates obesity (41). The beta3-AR agonists such as BRL 37344 (34) and CL 316243 (35) were developed as a treatment for obesity but failed in clinical trials. The involvement of the beta3-AR in cardiac function is primarily explained by the activation of eNOS, but the precise signaling mechanism has yet to be elucidated.
We investigated the cardioprotective actions of nebivolol and 2 other beta3-AR specific agonists (i.e., BRL 37344 and CL 316243) in the setting of a murine model of acute MI/R injury. Here we show that nebivolol treatment results in significant cardioprotection in the setting of MI/R via the stimulation of the beta3-AR. Nebivolol stimulates NO production in endothelial cells by activation of the beta3-AR (17,33). Both exogenous NO therapy and actions that promote endogenous NO production from eNOS protect the ischemic myocardium (10,42). We have demonstrated that activation of the beta3-AR in the heart by nebivolol, CL 316243, and BRL-37344 significantly reduces INF/AAR through an NO-dependent mechanism.
Several reports show inconsistencies with potency and specificity of beta3-AR agonists (43–45); however, we provide evidence with multiple agonists that supports beta3-AR stimulation as a novel approach to address cardiovascular disease. We also show that L-NAME, a known NOS inhibitor, abolishes the cardioprotective actions of nebivolol treatment in MI/R. Previous evidence suggests L-748,337, a specific beta3-AR antagonist, is effective in reducing the effects of nebivolol (33). We also observed this in our MI/R studies.
Our study provides evidence that stimulation of the beta3-AR acts as a dual activator of eNOS and nNOS. Some studies have shown that the beta3-AR modulates nNOS or iNOS (46,47), whereas the role of eNOS has been confirmed (40,48). We show a rapid increase in the activation of eNOS and the expression of nNOS after administration of nebivolol or CL 316243. We also report the absence of cardioprotection by nebivolol in eNOS knockout mice, nNOS knockout mice, and beta3-AR knockout mice.
Nebivolol has been shown to provide beneficial effects on LV dysfunction after myocardial infarction (49). In the present study we failed to demonstrate significant improvements in LV function at 7 days after reperfusion, which is in contrast to the Sorrentino et al. (49) study. There are, however, clear differences in methodology, because our experiments are based on a single dose of nebivolol (500 ng/kg) before reperfusion as opposed to treatment of 10 mg/kg/day for 30 days (49).
Nitric oxide is well-established as a trigger and mediator of cardioprotection and is involved in many protective signaling cascades (22,23). Activation of the beta3-AR likely stimulates many cytoprotective signaling cascades, which ultimately result in the cardioprotection we have observed. Our findings demonstrate that a single dose of nebivolol confers significant cardioprotection against MI/R injury in mice when administered at the time of reperfusion. We show for the first time that a beta1-blocker/beta3-AR agonist and a specific beta3-AR agonist attenuates myocardial INF by an NO-mediated mechanism involving both eNOS and nNOS. We also demonstrate that the beta1-blocker properties of nebivolol do not seem to be part of this cardioprotection, because hemodynamic measurements at the doses investigated in this study did not affect MABP or HR. At higher doses (twice that used in our experiments) we did observe a significant decrease in MABP but did not observe a reflex increase in HR. This might be because NO can attenuate the baroreflex HR increase (50,51). We also present data that acute administration just before reperfusion “preconditions” the heart against subsequent myocardial infarction (Online Fig. 1), whereas treatment with nebiviolol 24 h before reperfusion fails to protect. These data suggest that nebivolol can promote an “early” preconditioning state.
Taken together, our results indicate that an acute administration of nebivolol might have practical clinical use after myocardial ischemia. Future research will be aimed at further elucidation of the signaling mechanisms responsible for these cardioprotective actions. The involvement of nNOS as a redundant and additive signal for NO generation after acute administration of beta3-agonists also requires additional investigation.
Supplementary Material
Acknowledgments
Forest Research Institute provided nebivolol and an Investigator-Initiated Grant for this study. Additional funding was provided by the National Institutes of Health, National Heart, Lung, and Blood Institute (2R01HL-060849-09, 5R01HL-092141-01, and 1R01HL093579-01 to Dr. Lefer, and 5Ro1HL098481-02 to Dr. Calvert); the American Heart Association (09BGIA2250379 to Dr. Barouch); the American Diabetes Association (7-09-BS-26 to Dr. Calvert and 1-10-BS-11 to Dr. Barouch), and the Carlyle Fraser Heart Center. Dr. Wright is an employee of Forest Research Institute, a subsidiary of Forest Laboratories. Dr. Lefer has received grant support from Forest Research Labs to perform studies with nebivolol.
The authors thank Henry Li, PhD, and Julie Wangsa, PhD, from the Forest Research Institute, for their technical help.
Abbreviations and Acronyms
- AAR
area-at-risk
- AR
adrenergic receptor
- eNOS
endothelial nitric oxide synthase
- eNOS-PSer1177
phosphorylation of endothelial nitric oxide synthase at serine residue 1177
- INF
infarct size
- iNOS
inducible nitric oxide synthase
- HR
heart rate
- LCA
left coronary artery
- L-NAME
nitro-L-arginine methyl ester
- LV
left ventricle/ventricular
- MABP
mean arterial blood pressure
- MI/R
myocardial ischemia-reperfusion
- nNOS
neuronal nitric oxide synthase
- NOS
nitric oxide synthase
- RXNO
total nitrosylated proteins
- VEH
vehicle
APPENDIX
For supplementary figures and text, please see the online version of this article.
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Bolli R. Mechanism of myocardial “stunning. Circulation. 1990;82:723–38. doi: 10.1161/01.cir.82.3.723. [DOI] [PubMed] [Google Scholar]
- 2.Ovize M, Baxter GF, Di Lisa F, et al. Postconditioning and protection from reperfusion injury: where do we stand? Position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2010;87:406–23. doi: 10.1093/cvr/cvq129. [DOI] [PubMed] [Google Scholar]
- 3.Heusch G. Postconditioning: old wine in a new bottle? J Am Coll Cardiol. 2004;44:1111–2. doi: 10.1016/j.jacc.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Hjalmarson A, Herlitz J, Holmberg S, et al. The Goteborg Metoprolol Trial. Effects on mortality and morbidity in acute myocardial infarction. Circulation. 1983;67:I26–32. [PubMed] [Google Scholar]
- 5.Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- 6.Reiken S, Wehrens XH, Vest JA, et al. Beta-blockers restore calcium release channel function and improve cardiac muscle performance in human heart failure. Circulation. 2003;107:2459–66. doi: 10.1161/01.CIR.0000068316.53218.49. [DOI] [PubMed] [Google Scholar]
- 7.Communal C, Singh K, Sawyer DB, Colucci WS. Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis: role of a pertussis toxin-sensitive G protein. Circulation. 1999;100:2210–2. doi: 10.1161/01.cir.100.22.2210. [DOI] [PubMed] [Google Scholar]
- 8.Morisco C, Zebrowski DC, Vatner DE, Vatner SF, Sadoshima J. Beta-adrenergic cardiac hypertrophy is mediated primarily by the beta(1)-subtype in the rat heart. J Mol Cell Cardiol. 2001;33:561–73. doi: 10.1006/jmcc.2000.1332. [DOI] [PubMed] [Google Scholar]
- 9.Gauthier C, Tavernier G, Charpentier F, Langin D, Le Marec H. Functional beta3-adrenoceptor in the human heart. J Clin Invest. 1996;98:556–62. doi: 10.1172/JCI118823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rozec B, Gauthier C. beta3-adrenoceptors in the cardiovascular system: putative roles in human pathologies. Pharmacol Ther. 2006;111:652–73. doi: 10.1016/j.pharmthera.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Bristow MR, Nelson P, Minobe W, Johnson C. Characterization of β1-adrenergic receptor selectivity of nebivolol and various other beta-blockers in human myocardium (abstr) Am J Hypertens. 2005:51A–2A. [Google Scholar]
- 12.Flather MD, Shibata MC, Coats AJ, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26:215–25. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 13.Ignarro LJ. Different pharmacological properties of two enantiomers in a unique beta-blocker, nebivolol. Cardiovasc Ther. 2008;26:115–34. doi: 10.1111/j.1527-3466.2008.00044.x. [DOI] [PubMed] [Google Scholar]
- 14.Kuroedov A, Cosentino F, Luscher TF. Pharmacological mechanisms of clinically favorable properties of a selective beta1-adrenoceptor antagonist, nebivolol. Cardiovasc Drug Rev. 2004;22:155–68. doi: 10.1111/j.1527-3466.2004.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 15.Maffei A, Lembo G. Nitric oxide mechanisms of nebivolol. Ther Adv Cardiovasc Dis. 2009;3:317–27. doi: 10.1177/1753944709104496. [DOI] [PubMed] [Google Scholar]
- 16.Balligand JL. beta(3)-Adrenoceptor stimulation on top of beta(1)-adrenoceptor blockade “Stop or Encore? J Am Coll Cardiol. 2009;53:1539–42. doi: 10.1016/j.jacc.2009.01.048. [DOI] [PubMed] [Google Scholar]
- 17.Dessy C, Saliez J, Ghisdal P, et al. Endothelial beta3-adrenoreceptors mediate nitric oxide-dependent vasorelaxation of coronary microvessels in response to the third-generation beta-blocker nebivolol. Circulation. 2005;112:1198–205. doi: 10.1161/CIRCULATIONAHA.104.532960. [DOI] [PubMed] [Google Scholar]
- 18.Broeders MA, Doevendans PA, Bekkers BC, et al. Nebivolol: a third-generation beta-blocker that augments vascular nitric oxide release: endothelial beta(2)-adrenergic receptor-mediated nitric oxide production. Circulation. 2000;102:677–84. doi: 10.1161/01.cir.102.6.677. [DOI] [PubMed] [Google Scholar]
- 19.Georgescu A, Pluteanu F, Flonta ML, Badila E, Dorobantu M, Popov D. The cellular mechanisms involved in the vasodilator effect of nebivolol on the renal artery. Eur J Pharmacol. 2005;508:159–66. doi: 10.1016/j.ejphar.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 20.Quang TT, Rozec B, Audigane L, Gauthier C. Investigation of the different adrenoceptor targets of nebivolol enantiomers in rat thoracic aorta. Br J Pharmacol. 2009;156:601–8. doi: 10.1111/j.1476-5381.2009.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duranski MR, Greer JJ, Dejam A, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–40. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulz R, Kelm M, Heusch G. Nitric oxide in myocardial ischemia/reperfusion injury. Cardiovasc Res. 2004;61:402–13. doi: 10.1016/j.cardiores.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 23.Heusch G, Boengler K, Schulz R. Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation. 2008;118:1915–9. doi: 10.1161/CIRCULATIONAHA.108.805242. [DOI] [PubMed] [Google Scholar]
- 24.Bryan NS, Calvert JW, Gundewar S, Lefer DJ. Dietary nitrite restores NO homeostasis and is cardioprotective in endothelial nitric oxide synthase-deficient mice. Free Radic Biol Med. 2008;45:468–74. doi: 10.1016/j.freeradbiomed.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones SP, Girod WG, Palazzo AJ, et al. Myocardial ischemia-reperfusion injury is exacerbated in absence of endothelial cell nitric oxide synthase. Am J Physiol. 1999;276:H1567–73. doi: 10.1152/ajpheart.1999.276.5.H1567. [DOI] [PubMed] [Google Scholar]
- 26.Sharp BR, Jones SP, Rimmer DM, Lefer DJ. Differential response to myocardial reperfusion injury in eNOS-deficient mice. Am J Physiol Heart Circ Physiol. 2002;282:H2422–6. doi: 10.1152/ajpheart.00855.2001. [DOI] [PubMed] [Google Scholar]
- 27.Loke KE, Laycock SK, Mital S, et al. Nitric oxide modulates mitochondrial respiration in failing human heart. Circulation. 1999;100:1291–7. doi: 10.1161/01.cir.100.12.1291. [DOI] [PubMed] [Google Scholar]
- 28.Walter U, Gambaryan S. Roles of cGMP/cGMP-dependent protein kinase in platelet activation. Blood. 2004;104:2609. doi: 10.1182/blood-2004-06-2389. [DOI] [PubMed] [Google Scholar]
- 29.Moens AL, Leyton-Mange JS, Niu X, et al. Adverse ventricular remodeling and exacerbated NOS uncoupling from pressure-overload in mice lacking the beta3-adrenoreceptor. J Mol Cell Cardiol. 2009;47:576–85. doi: 10.1016/j.yjmcc.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Susulic VS, Frederich RC, Lawitts J, et al. Targeted disruption of the beta 3-adrenergic receptor gene. J Biol Chem. 1995;270:29483–92. doi: 10.1074/jbc.270.49.29483. [DOI] [PubMed] [Google Scholar]
- 31.Jones SP, Greer JJ, Kakkar AK, et al. Endothelial nitric oxide synthase overexpression attenuates myocardial reperfusion injury. Am J Physiol Heart Circ Physiol. 2004;286:H276–82. doi: 10.1152/ajpheart.00129.2003. [DOI] [PubMed] [Google Scholar]
- 32.McKnight GM, Smith LM, Drummond RS, Duncan CW, Golden M, Benjamin N. Chemical synthesis of nitric oxide in the stomach from dietary nitrate in humans. Gut. 1997;40:211–4. doi: 10.1136/gut.40.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rozec B, Erfanian M, Laurent K, Trochu JN, Gauthier C. Nebivolol, a vasodilating selective beta(1)-blocker, is a beta(3)-adrenoceptor agonist in the nonfailing transplanted human heart. J Am Coll Cardiol. 2009;53:1532–8. doi: 10.1016/j.jacc.2008.11.057. [DOI] [PubMed] [Google Scholar]
- 34.Dolan JA, Muenkel HA, Burns MG, et al. Beta-3 adrenoceptor selectivity of the dioxolane dicarboxylate phenethanolamines. J Pharmacol Exp Ther. 1994;269:1000–6. [PubMed] [Google Scholar]
- 35.Muzzin P, Seydoux J, Giacobino JP, Venter JC, Fraser C. Discrepancies between the affinities of binding and action of the novel beta-adrenergic agonist BRL 37344 in rat brown adipose tissue. Biochem Biophys Res Commun. 1988;156:375–82. doi: 10.1016/s0006-291x(88)80851-9. [DOI] [PubMed] [Google Scholar]
- 36.Brodde OE. Cardiac beta-adrenergic receptors. ISI Atlas of Science. 1987:107–12. [Google Scholar]
- 37.Emorine LJ, Marullo S, Briend-Sutren MM, et al. Molecular characterization of the human beta 3-adrenergic receptor. Science. 1989;245:1118–21. doi: 10.1126/science.2570461. [DOI] [PubMed] [Google Scholar]
- 38.Nahmias C, Blin N, Elalouf JM, Mattei MG, Strosberg AD, Emorine LJ. Molecular characterization of the mouse beta 3-adrenergic receptor: relationship with the atypical receptor of adipocytes. EMBO J. 1991;10:3721–7. doi: 10.1002/j.1460-2075.1991.tb04940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nantel F, Bonin H, Emorine LJ, et al. The human beta 3-adrenergic receptor is resistant to short term agonist-promoted desensitization. Mol Pharmacol. 1993;43:548–55. [PubMed] [Google Scholar]
- 40.Brixius K, Bloch W, Pott C, et al. Mechanisms of beta 3-adrenoceptor-induced eNOS activation in right atrial and left ventricular human myocardium. Br J Pharmacol. 2004;143:1014–22. doi: 10.1038/sj.bjp.0705983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arch JR. beta(3)-adrenoceptor agonists: potential, pitfalls and progress. Eur J Pharmacol. 2002;440:99–107. doi: 10.1016/s0014-2999(02)01421-8. [DOI] [PubMed] [Google Scholar]
- 42.Loscalzo J, Welch G. Nitric oxide and its role in the cardiovascular system. Prog Cardiovasc Dis. 1995;38:87–104. doi: 10.1016/s0033-0620(05)80001-5. [DOI] [PubMed] [Google Scholar]
- 43.Candelore MR, Deng L, Tota L, et al. Potent and selective human beta(3)-adrenergic receptor antagonists. J Pharmacol Exp Ther. 1999;290:649–55. [PubMed] [Google Scholar]
- 44.Hoffmann C, Leitz MR, Oberdorf-Maass S, Lohse MJ, Klotz KN. Comparative pharmacology of human beta-adrenergic receptor subtypes—characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:151–9. doi: 10.1007/s00210-003-0860-y. [DOI] [PubMed] [Google Scholar]
- 45.Niclauss N, Michel-Reher MB, Alewijnse AE, Michel MC. Comparison of three radioligands for the labelling of human beta-adrenoceptor subtypes. Naunyn Schmiedebergs Arch Pharmacol. 2006;374:99–105. doi: 10.1007/s00210-006-0104-z. [DOI] [PubMed] [Google Scholar]
- 46.Amour J, Loyer X, Le Guen M, et al. Altered contractile response due to increased beta3-adrenoceptor stimulation in diabetic cardiomyopathy: the role of nitric oxide synthase 1-derived nitric oxide. Anesthesiology. 2007;107:452–60. doi: 10.1097/01.anes.0000278909.40408.24. [DOI] [PubMed] [Google Scholar]
- 47.Maffei A, Di Pardo A, Carangi R, et al. Nebivolol induces nitric oxide release in the heart through inducible nitric oxide synthase activation. Hypertension. 2007;50:652–6. doi: 10.1161/HYPERTENSIONAHA.107.094458. [DOI] [PubMed] [Google Scholar]
- 48.Gauthier C, Leblais V, Kobzik L, et al. The negative inotropic effect of beta3-adrenoceptor stimulation is mediated by activation of a nitric oxide synthase pathway in human ventricle. J Clin Invest. 1998;102:1377–84. doi: 10.1172/JCI2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sorrentino SA, Doerries C, Manes C, et al. Nebivolol exerts beneficial effects on endothelial function, early endothelial progenitor cells, myocardial neovascularization, and left ventricular dysfunction early after myocardial infarction beyond conventional beta1-blockade. J Am Coll Cardiol. 2011;57:601–11. doi: 10.1016/j.jacc.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 50.Matsumura K, Abe I, Tsuchihashi T, Fujishima M. Central nitric oxide attenuates the baroreceptor reflex in conscious rabbits. Am J Physiol. 1998;274:R1142–9. doi: 10.1152/ajpregu.1998.274.4.R1142. [DOI] [PubMed] [Google Scholar]
- 51.Paton JF, Deuchars J, Ahmad Z, Wong LF, Murphy D, Kasparov S. Adenoviral vector demonstrates that angiotensin II-induced depression of the cardiac baroreflex is mediated by endothelial nitric oxide synthase in the nucleus tractus solitarii of the rat. J Physiol. 2001;531:445–58. doi: 10.1111/j.1469-7793.2001.0445i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.