Abstract
Eastern equine encephalitis virus (EEEV) is an arthropod-borne virus associated with life-threatening encephalitis in humans, equines, birds and many other domestic animals. To investigate the suitability of the Aotus nancymaae New World owl monkey as a viable animal model for EEE candidate vaccine testing we used clinical presentation, serology, viral isolation and PCR to evaluate pathogenesis and immunity in infected animals. Monkeys were inoculated subcutaneously (SQ) or intranasally (IN) with 104 pfu of virulent EEEV and were initially followed for 45 days. While none of the animals displayed clinical signs of disease, all of the SC inoculated animals (n = 6) manifested a viremia averaging 3.2 days (±0.8 days). Likewise, serologic responses (IgM, IgG and PRNT) were observed in all SC infected animals. Interestingly, none of the IN inoculated animals (n = 6) became viremic or mounted an antibody response and no pathological abnormalities were observed in two animals that were necropsied on day 6 post-infection (p.i.) from each group. To determine if the antibodies produced by the SC inoculated animals were protective against homologous challenge, three animals from the SC group were serologically evaluated on day 253 p.i. and were administered an inoculum identical to initial challenge on day 270 p.i. A positive control group of four naïve animals was also infected as before. All of the naïve positive control animals manifested a similar viremia as observed initially, averaging 2.75 days (±0.5 days) while none of the previously challenged animals became viremic. On days 45 and 253 p.i. geometric mean PRNT titers in the SC group were 453 and 101, respectively. This study demonstrates that the Aotus nancymaae can be reproducibly infected with EEE virus and can serve as a suitable model for infection and immunogenicity for the evaluation of candidate vaccines against EEEV.
Keywords: Aotus nancymaae, Eastern equine encephalitis (EEE), Animal model
1. Introduction
Eastern equine encephalitis virus (EEEV) is a member of the family Togaviridae and belongs in the genus Alphavirus. EEEV is maintained in a zoonotic transmission cycle between birds and ornithophilic mosquitoes, and can spread to humans, pigs, and horses through the bite of bridge mosquito vectors, however these tangential hosts fail to produce sufficient viremia for subsequent transmission and are therefore considered dead-ends [1]. EEEV occurs in the eastern United States and South American countries although different antigenic varieties circulate in each hemisphere leading to widely variable outcomes of infection. Outbreaks involving North American strains of EEEV are associated with high morbidity and mortality in humans and other mammals, with death resulting in about 70% of symptomatic human cases. Those individuals that survive often experience severe residual neurologic sequelae and the financial burden of infection is significant, where medical care can exceed $1M per patient [2,3]. There is no treatment for human infection other than supportive therapy and vaccination remains the most promising method of prevention.
While a vaccine for horses has been successfully used for years [4] and recent attempts to vaccinate wild birds has shown some success [5] there is no currently licensed vaccine for humans. In order to adequately evaluate human vaccine candidates and strategies it is necessary to develop an animal model where efficacy and outcome of vaccine treatments can be assessed. Current animal models for EEEV infection include the mouse, hamster, macaque, and various bird species [5–11]. While rodents and birds exhibit varying degrees of susceptibility to EEEV infection, primates display severe disease development following aerosol infection, as seen in humans [8].
Here we present the development of the Aotus nancymaae owl monkey animal model for EEEV infection and demonstrate that subcutaneous delivery of virus results in a measurable viremia and protective immune response in a non-lethal model.
2. Materials and methods
2.1. Animals
Animal studies were approved by the Naval Medical Research Center Detachment (NMRCD) Institutional Animal Care and Use Committee (NMRCD06-3) and the Department of the Navy Bureau of Medicine and Surgery. Captive-born Aotus nancymaae were purchased from the Instituto Veterinario de Investigaciones Tropicales y de Altura (IVITA), University of San Marcos, Peru. Sixteen A. nancymaae, weighing between 1 and 1.5 kg and ranging from 2- to 4-year, were randomly selected from the non-human primate issue pool at NMRCD. This facility is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, International; therefore all husbandry and experimental procedures were performed in compliance with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals. Animals were pair-housed in standard metal cages with nest boxes and perches. A commercially formulated monkey diet (New World Primate Diet 8794N, Harlan Teklad, Madsion, WI) was fed daily. The diet was supplemented with a variety of fresh fruits and monkey biscuits purchased from IVITA. Distilled water was provided ad libitum.
2.2. Virus
EEEV strain FL93-939 was used for all infections and assays. This strain was isolated from a Florida mosquito pool in 1993, and passaged once in cell culture before cDNA clone construction [12]. It is wild-type in all known phenotypes, including high virulence for mice [12], horses and marmosets (SCW, unpublished). Virus stocks were prepared from baby hamster cell cultures electroporated with transcribed RNA as described previously [12].
2.3. Animal challenge
In an initial infection study, 16 animals were randomly assigned to three groups: two study groups of six animals each and one control group of four animals to be used as controls for clinical signs and immune responses. One study group received 104 pfu of EEEV in 100 μl PBS via the intranasal route, administered by pipette (~50 μl in each nare). A second study group received 104 pfu of EEEV in 1.0 ml PBS via the subcutaneous route with a 26-gauge needle in the nape of the neck. Animals were initially evaluated twice daily for 10 days to assess clinical manifestations of disease. Blood was drawn from each animal on days 0–10, 14, 30, 45 and 250 post-infection for serological testing (IgM/IgG ELISA, PRNT), viral isolation, and PCR. On day 6 post-infection, two animals were randomly chosen from the IN and SC inoculated groups and euthanized to assess gross pathology and to collect organs for histopathology.
To assess protection afforded by previous exposure to EEEV, three animals from the SC inoculated group and three naïve animals were each inoculated SC with 104 pfu of EEEV in 1.0 ml PBS SQ. A control group of two animals was inoculated with 1.0 ml PBS SQ. Blood was drawn from each animal on days 0–9, 30, 45, and 60 for assessment of serological response and viremia, as above.
2.4. Virus isolation
Sera were diluted 1:5 in minimum essential medium containing 2% heat-inactivated fetal bovine serum and antibiotics. African Green Monkey Vero (37 °C) cell cultures were each inoculated with 200 μl of the diluted serum in 25 ml flasks. The cells were checked daily for viral cytopathic effect (CPE). If CPE was detected then the cells were harvested and the presence of EEE virus was detected via IFA. If no CPE was observed the cells were removed from the flasks and placed on 12-well glass spot-slides for examination by immunofluoresence assay (IFA) on day 10 post-infection using EEEV-specific polyclonal antibodies.
2.5. IgM and IgG ELISA
Sera were assayed for IgG and IgM antibodies against EEEV by ELISA as previously described [13,14]. Briefly, IgM antibodies against EEEV were detected using a capture ELISA as follows: 96-well microtiter plates were sensitized with goat F(ab′)2 anti-human IgM overnight at 4 °C. Monkey sera samples were diluted to 1/100 and incubated at 37 °C for 1 h, followed by serial addition of EEEV strain FL 93-939 antigen, anti-EEE FL 93-939 hyperimmune mouse ascites fluid, horseradish peroxidase conjugated anti-mouse IgG + IgM and ABTS peroxidase substrate, followed by spectrophotometric analysis at 405 nm. IgG antibodies to EEEV were similarly assayed: 96-well microtiter plates were sensitized with EEEV strain FL 93-939 antigen overnight at 4 °C. Monkey sera samples were diluted to 1/100 and incubated at 37 °C by 1 h, followed by addition of horseradish peroxidase conjugate anti-human IgG and developed using ABTS peroxidase substrate. The positive cutoff OD value was determined by the mean adjusted OD of non-infected negative control monkey sera, plus 3 standard deviations.
2.6. PRNT
The PRNT protocol was modified from Morens et al. [15]. Briefly, test sera was diluted twofold in media (EMEM + pen/strep) from 1:4 to 1:26,400. 200 μl media containing 40–80 pfu of EEEV FL 93-939 was mixed with 200 μl diluted test serum and incubated at 4 °C for 15 h. In triplicate, 100 μl virus-serum mixture was added to 0.5 ml media containing 1.5 × 105 Vero cells and then added to a well of a 24-well tissue culture plate and incubated at 37 °C with 5% CO2 for 3 h. 0.5 ml overlay media (0.6% carboxymethyl cellulose, MEM, w/o phenol red, 10% FBS, 0.075% NaHCO3 and pen/strep) was added to the cell and was incubated at 37 °C with 5% CO2 for 3 days. The media was removed, and the cells rinsed with PBS and stained with 0.5 ml/well stain solution (0.1% (w/v) naphthol blue black, 1.36% (w/v) sodium acetate, and 6% (v/v) glacial acetic acid) for 30 min. The stain was then removed and the plaques where counted. The results were expressed as the serum dilution, determined by probit analysis that reduced the number of plaques by 70% compared to that of normal human serum at the same dilution.
2.7. Q-RT-PCR
TaqMan RT-PCR was performed using the QIAGEN Quantitect Probe RT-PCR kit. Each reaction was made mixing 25 μl of 2X quantitect probe RT-PCR master mix; 0.5 μl of quantitect RT mix, 0.5 μl (100 μM) of each one EEE-F1 and EEE-R1 (North American) primers; 0.3 μl (25 uM) of the EEE-P1 probe, 3.2 μl of nuclease free water and 20 μl of the RNA template. The amplification started with a RT incubation step of 50 °C for 30 min followed by a denaturing temperature of 95 °C for 10 min then 45 cycles of 95 °C for 15 s and 60°C for 1 min.
The above procedure was made for the standard curve (constructed using a 10-fold dilution of the EEEV strain FL93-939 of known pfu concentration) and all unknown samples. Amplification fragment spans the E2 glycoprotein and the primers and probe sequences were EEE-F1 (9391-9411/ACA CCG CAC CCT GAT TTT ACA), EEE-R1 (9566-9545/CTT CCA AGT GAC CTG GTC GTC) and EEE-P1 (9390-9414/Fam-TGC ACC CGG ACC ATC CGA CCT-Tam).
3. Results
3.1. Serum antibody responses in Aotus monkeys after inoculation
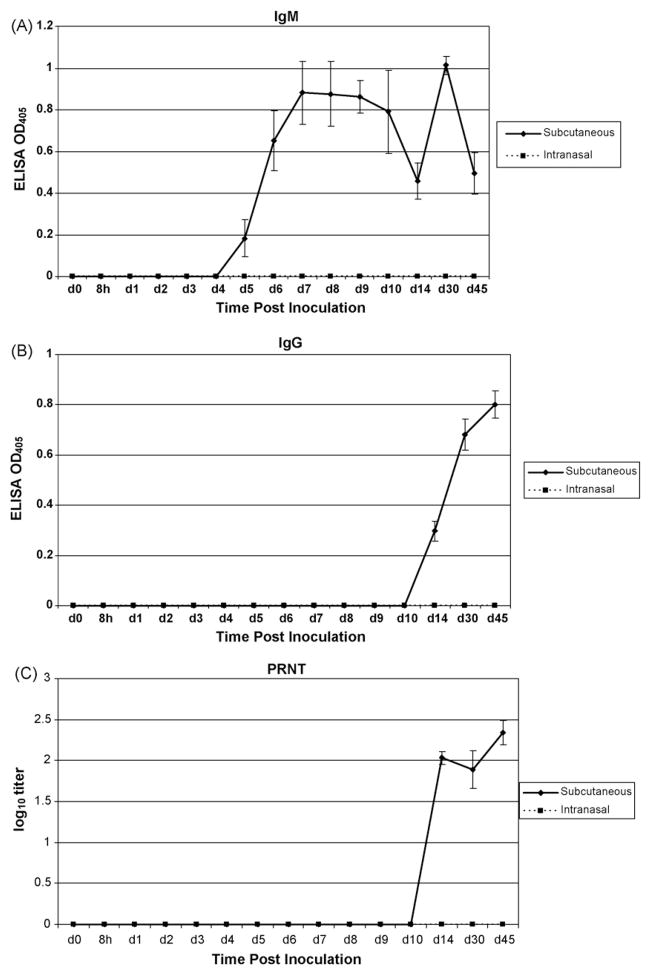
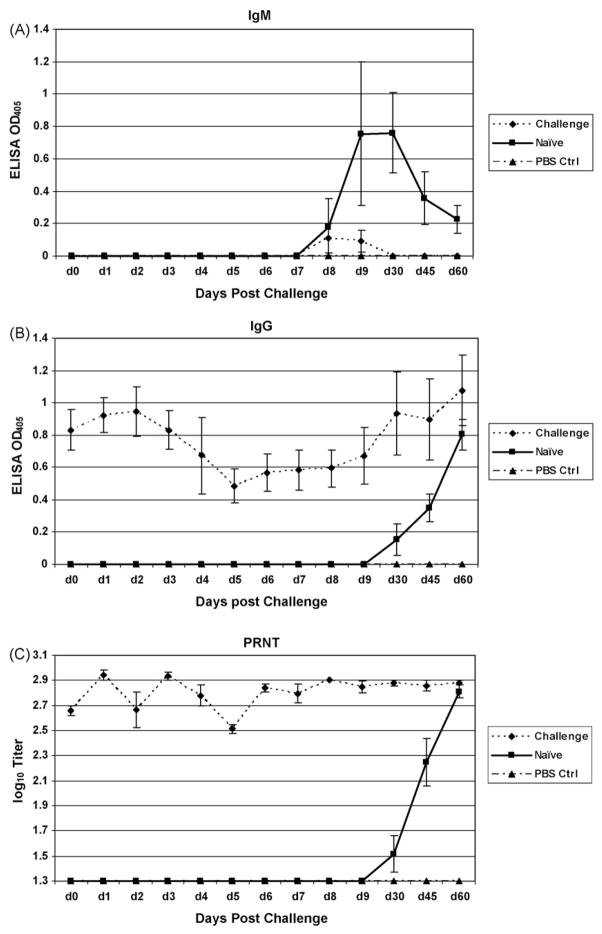
Two groups of six animals each were inoculated with EEE virus at 104 pfu via the intranasal (IN) and subcutaneous (SQ) routes. A control group of four monkeys was sham infected SC with PBS. IN inoculated monkeys did not exhibit any immune response as measured by IgM and IgG titers in the sera throughout a 6-month evaluation period. In contrast, animals inoculated with virus by the SC route showed increased IgM and IgG antibody response beginning 5 and 14 days after inoculation, respectively (Fig. 1a and b). Eight weeks after the initial infection, IgM titers in all animals in the SC group returned to basal levels. Neutralizing antibody was also exclusively limited to the SQ-infected group, observed on days 14–45 post-inoculation (Fig. 1c). The IN inoculated group exhibited no neutralizing antibody titers.
Fig 1.
IgM/IgG ELISA and PRNT of sera from Aotus nancymaae inoculated with EEE. Subcutaneous injection with 104 pfu EEE resulted in an IgM response [A] beginning on day 5 post-infection and an IgG [B] and PRNT [C] response by day 14. Intranasal inoculation with the same dose did not result in any measurable IgM or IgG response. Neutralizing antibodies are likewise observed by day 14 in the SQ challenge group but absent in the IN group.
3.2. Viremia and pathophysiology after inoculation
Animals infected by the SC route developed viremia within 24 h post-inoculation, lasting 3.3 days on average as assessed by RT-PCR and isolation in Vero cells (Table 1). No animals in the IN inoculated group were positive by RT-PCR or viral isolation. Physical appearance, behavior and body temperature of the animals were monitored for 10 days post-infection. Each animal was scored twice daily by a veterinarian to detect clinical signs of disease, including fever, behavior changes, food intake, respiration rate and other neurological signs associated with encephalitis. Based on these criteria, no clinical disease was evident in any of the groups throughout the study. On day 6 post-infection, two animals from each infected group were euthanized for histologic examination. No gross pathological presentations were observed and histology was non-remarkable (data not shown).
Table 1.
Viremia in Aotus nancymaae monkeys.
| Phasea | Group | Monkey | Days of viremiab
|
Mean viremia (days) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 8 h/0c | 1 | 2 | 3 | 4 | 5 | 6 | ||||
| A | SC | T-1800 | - | 3+/+ | 3+/+ | - | - | - | - | 3.3 |
| T-1573 | - | 2+/+ | 3+/+ | −/+ | - | - | - | |||
| T-1864 | 3+/− | 3+/+ | 3+/− | 3+/− | 3+/+ | - | - | |||
| T-1816 | - | 3+/+ | 3+/+ | 3+/+ | −/+ | - | - | |||
| T-1934 | 3+/− | 2+/− | 3+/+ | 3+/+ | - | - | - | |||
| T-1868 | - | 2+/− | 3+/+ | 3+ | −/+ | - | - | |||
| IN | T-1858 | - | - | - | - | - | - | - | 0 | |
| T-1611 | - | - | - | - | - | - | - | |||
| T-1789 | - | - | - | - | - | - | - | |||
| T-1882 | - | - | - | - | - | - | - | |||
| T-1732 | - | - | - | - | - | - | - | |||
| T-1917 | - | - | - | - | - | - | - | |||
| PBS | T-1098 | - | - | - | - | - | - | - | 0 | |
| T-1804 | - | - | - | - | - | - | - | |||
| T-1810 | - | - | - | - | - | - | - | |||
| T-1812 | - | - | - | - | - | - | - | |||
|
| ||||||||||
| B | SC infected | T-1864 | - | - | - | - | - | - | - | 0 |
| T-1934 | - | - | - | - | - | - | - | |||
| T-1868 | - | - | - | - | - | - | - | |||
| Naïve | T-1905 | - | - | - | −/+ | 3+/− | 3+/− | 2 | ||
| T-1974 | - | 3+/+ | 3+/+ | - | - | - | - | |||
| T-1982 | - | 3+/+ | 3+/+ | - | - | - | - | |||
| T-1929 | - | −/+ | 3+/+ | - | - | - | - | |||
| PBS | N-73 | - | - | - | - | - | - | - | 0 | |
| N-63 | - | - | - | - | - | - | - | |||
A: initial infection with 104 EEEV, B: challenge infection with 104 EEEV.
Viremia results are presented as the isolation of virus in Vero cells (3+, >75% cells; 2+, 50–75% cells)/Taqman PCR detection of viral RNA in animal sera (+/−).
Phase A: blood draw 8 h after inoculation, Phase B: blood draw previous to challenge.
3.3. Immune response after homologous challenge
To determine the level of protection afforded by previous exposure to EEEV, three of the SQ-infected monkeys and four naïve monkeys were SC challenged 9.5 months after the first inoculation with 104 pfu of EEEV. The IN-inoculated animals were dropped from the study and were not followed up for the challenge due to the fact that there was no indication of infection or immunity following initial inoculation. Two monkeys were sham infected with PBS as a negative control. Sera obtained each day for 9 days, then weekly for 2 months, were analyzed for IgM, IgG and PRNT antibody levels (Fig. 2). Protection was assessed by reduction in the level and duration of viremia in the animals as quantified by RT-PCR and direct plaque assay on C6/36 cells. Previously infected animals exhibited IgG and PRNT titers prior to challenge (Fig. 2b and c). On day 8 post-infection, measurable IgM levels in both groups were apparent, with the naïve group displaying a more robust response in comparison to the re-infected animals (Fig. 2a). IgG and PRNT titers were observed to increase after day 9 in the naïve group, which parallel initial infection as seen in the previous inoculation of naïve animals (Fig. 2b and c).
Fig 2.
IgM/IgG ELISA and PRNT of sera from Aotus rechallenged with EEE. Challenge stimulated IgM production [A] in both groups, but at higher levels and longer duration in the naïve animals. Animals (n = 3) inoculated 9 months previously with EEEV showed elevated IgG [B] and protective antibody [C] throughout the study, whereas naïve animals (n = 3) produced neutralizing antibody and IgG after day 9 post-challenge.
Viremia after challenge. All of the naïve challenged monkeys exhibited viremia by day 1 (n = 3) or day 4 (n = 1) post-challenge by viral isolation and/or RT-PCR for an average of 2 days (Table 1). Of note, none of the animals that were previously infected with EEE were viremic during the challenge study. As with the first infection study, none of the animals developed clinical signs of encephalopathy or infection. All body temperatures and behavioral indices were within normal parameters. A negative control group (n = 2) was sham inoculated with PBS and showed no immune response or viremia.
4. Discussion
In the present study, we investigated whether the Aotus nancymaae New World owl monkey could become infected with subcutaneous or intranasal administration of EEEV. A SC administration was capable of infecting the owl monkey at a rate of 100% as assessed by viral isolation, RT-PCR and serological titers, while IN inoculation resulted in no infected animals based on RT-PCR and seroconversion. Viremia in the SC animal group lasted an average of 3.3 days and IgG and PRNT serology remained elevated for at least 9 months post-infection suggesting a long-lived humoral immunity to the virus. IN infected animals exhibited no such antibody response to inoculation. These results imply that, in this host, a transmission route more typical of natural mosquito infection permits invasion and induction of an immune response. Introduction of the infection through the skin may also serve to target APCs, specifically resident dendritic cells, resulting in the cell-mediated induction of protective humoral immunity observed. In contrast, this animal model does not appear to be susceptible to IN introduction of virus, perhaps through lack of specific viral receptors in the nasal mucosa. While the only study to compare old- and new-world monkey olfactory epithelium noted no differences in cellular makeup, no analysis of alphavirus receptors has been conducted. In the macaque, the most closely related animal model developed to that tested here, aerosol infection of the same EEEV strain resulted in a febrile response within 4 days with a rapid onset of neurological manifestations followed by death resulting within 10 days PI [8]. Murine species are also highly susceptible to EEEV infection resulting in rapid onset of neural pathogenesis [10]. While divergent in clinical disease, the Aotus did exhibit viral replication within the first 24 h PI, similar to the murine model. It is possible that a higher initial SC dose of EEEV or use of a more virulent strain may have resulted in pathogenesis and clinical outcomes as seen in these other animal models, or even infection through the IN route.
None of the infected animals exhibited any of the clinical signs associated with EEEV infection notable in natural human or equine infection, such as neurological manifestations characteristic of encephalitis or multiple organ pathologies. The survival rate of all animals regardless of infection route was 100% at 6 months post-infection, except for those animals euthanized for histopathology.
We also demonstrated that previous infection with EEEV is protective against subsequent homologous challenge using viremia as a measure of productive infection. Previous to challenge, IgG antibody titers were evaluated and appear to have remained constant after initial inoculation, while PRNT levels were slightly decreased from earlier levels but were still elevated above pre-infection levels. Within 24 h post-challenge, viremia was observed in 100% of naïve control animals lasting 2 days on average. IgM, IgG and PRNT serologic responses in this group paralleled data seen earlier; an initial IgM response began 8 days post-infection followed by increases in IgG and PRNT titers beginning after day 9. In contrast, none of the animals that had been previously infected with EEEV developed viremia as assessed by viral isolation or PCR, suggesting serologic antibody levels sufficient to afford protection against homologous challenge. Neutralizing antibody response has been successfully used as a marker for alphavirus vaccine efficacy previously [5,16,17]. The observed PRNT titers (100–400 gmt) could, therefore, prove useful as a predictor of vaccine efficacy in the evaluation of EEEV vaccine candidates.
The Aotus was chosen as a primate model for evaluation based on its convenience and safety and our experience in working with this animal. Old world monkeys, such as rhesus and cynomolgus, are increasingly more difficult and costly for researchers to work with and can be carriers of the lethal Herpes B virus [18], whereas the owl monkey is non-aggressive, small in size (adults weighing 1.0–1.5 kg) and is easily handled. While this model does not provide for study into the pathogenesis of EEE, the susceptibility of Aotus to EEEV infection when inoculated by the SC route is a promising step toward development of this non-terminal animal model to evaluate candidate EEEV vaccines. In this regard, the development of a humoral immune response that affords protection against homologous infection is a significant tool that should be considered where duration or absence of viremia can be used as a measure of vaccine efficacy.
Acknowledgments
We thank Carolina Guevarra for specimen management and Cristopher Cruz, Vidal Felices, Cecilia Rivera, and the animal caretaker staff for excellent technical assistance.
The views, opinions, and findings contained in this report are those of the authors and do not reflect official policy or positions of the Department of the Navy, Department of Defense, or the United States Government. We are military service members. This work was prepared as part of our official duties. Title 17 USC § 105 provides that “Copyright protection under this title is not available for any work of the United States Government” Title 17 USC § 101 defines a US Government work as a work prepared by a military service member or employee of the US Government as part of that person’s official duties. This work was supported by a grant from NIAID through the Western Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research, NIH grant number U54 AI057156.
References
- 1.Morris CD. Eastern equine encephalomyelitis. In: Monath TP, editor. The arboviruses: epidemiology and ecology. III. Boca Raton, Florida: CRC Press, Inc; 1989. pp. 1–20. [Google Scholar]
- 2.Villari P, Spielman A, Komar N, McDowell M, Timperi RJ. The economic burden imposed by a residual case of eastern encephalitis. Am J Trop Med Hyg. 1995;52(1):8–13. doi: 10.4269/ajtmh.1995.52.8. [DOI] [PubMed] [Google Scholar]
- 3.Deresiewicz RL, Thaler SJ, Hsu L, Zamani AA. Clinical and neuroradiographic manifestations of eastern equine encephalitis. N Engl J Med. 1997;336(26):1867–74. doi: 10.1056/NEJM199706263362604. [DOI] [PubMed] [Google Scholar]
- 4.Maire LF, 3rd, McKinney RW, Cole FE., Jr An inactivated eastern equine encephalomyelitis vaccine propagated in chick-embryo cell culture I. Production and testing. Am J Trop Med Hyg. 1970;19(1):119–22. doi: 10.4269/ajtmh.1970.19.119. [DOI] [PubMed] [Google Scholar]
- 5.Tengelsen LA, Bowen RA, Royals MA, Campbell GL, Komar N, Craven RB. Response to and efficacy of vaccination against eastern equine encephalomyelitis virus in emus. J Am Vet Med Assoc. 2001;218(9):1469–73. doi: 10.2460/javma.2001.218.1469. [DOI] [PubMed] [Google Scholar]
- 6.McLean RG, Crans WJ, Caccamise DF, McNelly J, Kirk LJ, Mitchell CJ, et al. Experimental infection of wading birds with eastern equine encephalitis virus. J Wildl Dis. 1995;31(4):502–8. doi: 10.7589/0090-3558-31.4.502. [DOI] [PubMed] [Google Scholar]
- 7.Komar N, Dohm DJ, Turell MJ, Spielman A. Eastern equine encephalitis virus in birds: relative competence of European starlings (Sturnus vulgaris) Am J Trop Med Hyg. 1999;60(3):387–91. doi: 10.4269/ajtmh.1999.60.387. [DOI] [PubMed] [Google Scholar]
- 8.Reed DS, Lackemeyer MG, Garza NL, Norris S, Gamble S, Sullivan LJ, et al. Severe encephalitis in cynomolgus macaques exposed to aerosolized Eastern equine encephalitis virus. J Infect Dis. 2007;196(3):441–50. doi: 10.1086/519391. [DOI] [PubMed] [Google Scholar]
- 9.Paessler S, Aguilar P, Anishchenko M, Wang HQ, Aronson J, Campbell G, et al. The hamster as an animal model for eastern equine encephalitis—and its use in studies of virus entrance into the brain. J Infect Dis. 2004;189(11):2072–6. doi: 10.1086/383246. [DOI] [PubMed] [Google Scholar]
- 10.Vogel P, Kell WM, Fritz DL, Parker MD, Schoepp RJ. Early events in the pathogenesis of eastern equine encephalitis virus in mice. Am J Pathol. 2005;166(1):159–71. doi: 10.1016/S0002-9440(10)62241-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guy JS, Siopes TD, Barnes HJ, Smith LG, Emory WH. Experimental transmission of eastern equine encephalitis virus and Highlands J virus via semen of infected tom turkeys. Avian Dis. 1995;39(2):337–42. [PubMed] [Google Scholar]
- 12.Aguilar PV, Adams AP, Wang E, Kang W, Carrara AS, Anishchenko M, et al. Structural and nonstructural protein genome regions of eastern equine encephalitis virus are determinants of interferon sensitivity and murine virulence. J Virol. 2008;82(10):4920–30. doi: 10.1128/JVI.02514-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Innis BL, Nisalak A, Nimmannitya S, Kusalerdchariya S, Chongswasdi V, Suntayakorn S, et al. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am J Trop Med Hyg. 1989;40(4):418–27. doi: 10.4269/ajtmh.1989.40.418. [DOI] [PubMed] [Google Scholar]
- 14.Ansari MZ, Shope RE, Malik S. Evaluation of vero cell lysate antigen for the ELISA of flaviviruses. J Clin Lab Anal. 1993;7(4):230–7. doi: 10.1002/jcla.1860070408. [DOI] [PubMed] [Google Scholar]
- 15.Morens DM, Halstead SB, Repik PM, Putvatana R, Raybourne N. Simplified plaque reduction neutralization assay for dengue viruses by semimicro methods in BHK-21 cells: comparison of the BHK suspension test with standard plaque reduction neutralization. J Clin Microbiol. 1985;22(2):250–4. doi: 10.1128/jcm.22.2.250-254.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fine DL, Roberts BA, Teehee ML, Terpening SJ, Kelly CL, Raetz JL, et al. Venezuelan equine encephalitis virus vaccine candidate (V3526) safety, immunogenicity and efficacy in horses. Vaccine. 2007;25(10):1868–76. doi: 10.1016/j.vaccine.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 17.Clark GG, Dein FJ, Crabbs CL, Carpenter JW, Watts DM. Antibody response of sandhill and whooping cranes to an eastern equine encephalitis virus vaccine. J Wildl Dis. 1987;23(4):539–44. doi: 10.7589/0090-3558-23.4.539. [DOI] [PubMed] [Google Scholar]
- 18.Elmore D, Eberle R, Monkey B. Virus (Cercopithecine herpesvirus 1) Comp Med. 2008;58(1):11–21. [PMC free article] [PubMed] [Google Scholar]