Abstract
Background
11β-hydroxysteroid dehydrogenase type 1 (HSD11B1), 11β-hydroxysteroid dehydrogenase type 2 (HSD11B2), glucocorticoids (GC) and their receptor (GR) play a key role in tissue-specific regulation of GC action.
Objectives
The expression of genes coding HSD11B1, HSD11B2 and GR and their protein products, and levels of cortisol were determined in human skin explants and/or co-cultured keratinocytes/melanocytes after treatment with ultraviolet A (UVA), B (UVB) or C (UVC) wavebands.
Methods
Skin from foreskins and/or co-cultured human keratinocytes/melanocytes were irradiated with UVA, UVB or UVC (skin) and incubated for 12 and 24 h. Methods of RT-PCR, western blot (WB), ELISA and immunohistochemistry (IHC) were used to determine expression and localization of corresponding genes or antigens, respectively.
Results
UVB enhanced the HSD11B1 gene and protein expression in a dose dependent manner, while UVA was without effect. Similarly, UVC increased HSD11B1 protein product as measured by IHC. UVB and UVC enhanced cortisol production and decreased epidermal GR expression with UVA having no detectable effects. Although both UVA and UVB stimulated HSD11B2 gene expression, only UVA increased HSD11B2 protein product levels with UVB and UVC being without an effect.
Conclusions
We suggest that these differential, waveband dependent effects of UVR on cutaneous HSD11B1, HSD11B2, GR gene and corresponding protein products expression and cortisol production are both to protect and/or restore the epidermal barrier homeostasis against disruption caused by elevated cortisol level induced by UVB and UVC.
Introduction
Glucocorticoids (GC) are widely used in dermatology in a large number of diseases that require anti-inflammatory activity and immunosuppression 2,3. Two key enzymes that regulate the local cortisol (F) availability for glucocorticoid receptor (GR) are 11β-HSD1 and 11β-HSD2 4–7. 11β-HSD appears as a single protein that may either increase or decrease F availability to GR and is under multifactorial regulation including down-regulation by F itself4,5. At the high NADPH/NADP+ ratio, 11β-HSD1 switches to ketoreductase activity with the transformation of inactive cortisone (E) to F 4,7. 11β-HSD2 is a high affinity NADP+−dependent enzyme that acts exclusively as a dehydrogenase to inactivate F to E 6,7. The expression of 11β-HSD1/2 has been shown in human placenta, kidney and liver 6,8, also fibroblasts and adipocytes have the potential to transform E to F 5,6,9. Recent studies have demonstrated that intracellular conversion, performed by these two enzymes, together with GR activity, represent a key mechanism of tissue-specific regulation of GC action contributing to GC deficiency, which constitutes a big challenge in dermatology 10,11.
Although there is a significant amount of information on the mechanism of UVA and UVB action in the skin at the molecular level12, there is a shortage of information on UVR regulation of the local steroidogenesis. Therefore, we have investigated changes in expression of the HSD11B1/11β-HSD1 and HSD11B2/11β-HSD2 as well as GR at the gene and protein levels after exposure to UVA, UVB or UVC. Skin from foreskins and/or co-cultured human keratinocytes/melanocytes were irradiated and further analyzed for expression of corresponding genes and antigens and their cellular localization.
Materials and Methods
Human protocols
Use of human tissue was approved by the local (University of Tennessee Health Science Center, Memphis TN) Institutional Review Board (IRB).
Co-cultures
Comprehensive description is provided in 13. Briefly, second passage of human epidermal neonatal keratinocytes (HEKn) and human epidermal neonatal melanocytes (HEMn) were seeded in a ratio 5:1 in a 60 mm Petri dish or chamber slides (Lab-Tek®, Rochester, NY) and maintained with the serum/cortisol−free medium (to remove all exogenous sources of POMC-derived peptides and GC) which consists of KBM-2/MBM-4 in a ratio 1:1, from Lonza, Walkersville, MD. When cells achieved 90% of confluence, media were replaced with PBS and cells were irradiated with UVA or UVB according to the irradiation protocol, described below. After treatment, PBS was replaced to the same media and after 6, 12, and 24 h cells were harvested by trypsinization, and frozen separately at −80 C or processed to double immunofluorescence (IF) performed in chamber slides.
Organ cultures
The detailed description of organ culture preparation is given in Supplementary files.
UV Dosimetry
The spectrum of each UV source (Fig. 1) was measured using a scanning double monochromater spectroradiometer (model OL 756; Optronic Laboratories, Orlando, FL) scanning at 1-nm increments from 250 to 800 nm. The instrument was configured with 0.125/0.5/0.125-mm slits interfaced to a 4-in-diameter integrating sphere with a 20 mm entrance aperture by a 1 meter quartz fiber-optic bundle. The spectroradiometer was calibrated by scanning a NIST traceable tungsten-halogen spectral irradiance standard (model 752-10E, Optronic laboratories) with a precision current source (model 65, Optronic Laboratories). This system also used a small portable dual-source calibration check module (model 756-150, Optronic Laboratories) to check both the photometric gain and the wavelength offset, using Hg lines. Before each calibration or measurement, the wavelength calibration and gain are established or verified. All measurements are made at the use position of the source and all doses are given is standard erythema dose (SED) units (Table 1) or in MEDs (Supplementary Table 1). 1 SED is equivalent to an erythemal effective radiant exposures of 100 J/m2 (MKS) or 0.01 J/cm2 (CGS) 14,15. An erythemic weighted spectrum is obtained by multiplying at each wavelength the measured spectrum of a source by the standard erythemic action spectrum. The human MED determined for many skin type I and II individuals is approximately 2 SEDs, see Suppl. Table 1.
Fig. 1.
Emission spectra of UVA, UVB and UVC sources.
For UVB and UVA the spectra are shown with and without Kodacel filter.
Table 1.
Doses in SED of UV wavebands administered in this study.
| Waveband | Sham & null doses |
Erythemic or Absolute Doses |
|---|---|---|
| UVA | 0.0 |
0.7 SED or 20 J/cm2 and 1.8 SED or 50 J/cm2 |
| UVB | 0.0 |
1.3 SED or 0.05 J/cm2 and 2.5 SED or 0.1 J/cm2 |
| UVC | 0.0 |
0.9 SED or 0.0093 J/cm2) and 4.7 SED or 0.0465 J/cm2) |
Detailed specifications are provided in Supplemental Table 1. For UVA and UVB radiation Kodacel filter was used.
Irradiation protocol
The UV irradiation was performed under biological hood (Supplementary Fig. 1) with Spectroline XX-15A lamp (Spectronics Corp., Westbury, NY) equipped with different (UVA, UVB and UVC) waveband bulb sets specified in Supplementary Table 1 in Supplementary file. The experimental irradiation was performed from the same distance (2.5 inch) as the dosimetry which measured the wavelength spectra and intensity (Suppl. Fig. 1). During UVA and UVB irradiation the bulbs were covered with Kodacel filter (Kodacel™, Eastman Kodak, Rochester, NY) which acts as a cut-off filter allowing transmission of wavelengths longer than 290 nm, eg. UVA and UVB, excluding UVC (Fig. 1). The times of irradiation were calculated upon formula Time (s) = Dose (J/cm2)/Intensity (W/cm2). Doses of irradiations: UVA: (sham=control =0, 20, 50 J/cm2); UVB: (C=0, 0.05, 0.1 J/cm2); UVC: (C=0, 0.0093, 0.0465 J/cm2). Doses are conveniently expressed in SED, see Table 1 and in MED, see Suppl. Table 1.
RNA isolation and Real time RT-PCR (RT-PCR) assay
The detailed description of RT-PCR procedure and primer sequences are provided in Supplementary files.
ELISA assays
Skin fragments maintained in organ culture, after 24 h of irradiation, were lysed (TissueLyse LT, Qiagen, Valencia, CA) with ice-cold RIPA buffer [PBS containing 1% Nonidet P-40, 0.1% Triton X-100 supplemented with 1% proteinase inhibitor cocktail (PIC; 10μl/1ml, Sigma, St. Louis, MO)]. The homogenates were kept on ice for 20 min then centrifuged for 25 min (12,000 g, 4 C). Cortisol concentrations were measured with ELISA kits (R&D Systems, UK) from the standard curve, according to the manufacturer instruction and presented as ng/ml of tissue extract according and normalized to the total protein content in lysates (2.5 μg/μL, Bradford assay).
Western Blot (WB) analyses
The detailed description of SDS/PAGE and WB procedure is provided in Supplementary files.
Double Immunofluorescent (dIF) histochemistry
Following the 24 h incubation with appropriate UV wavelengths, the skin was fixed in 4% buffered PFA (12 h, 4 C), rinsed several times in PBS, cryoprotected with 18% sucrose in PBS for 5 days and cryosectioned (Leica, Bannockburn, IL). Ten micrometres sections were mounted onto silanized slides (Dako, Carpinteria, CA) rinsed several times in PBS and maintained for 1 h in a blocking solution (5% donkey serum, 0.1% BSA, 0.2% Triton X-100 in PBS), rinsed in PBS and incubated for 16 h with primary antibodies mixture raised in different species: sheep 11β-HSD1/rabbit GR and sheep 11β-HSD2/rabbit GR; listed in Table 2. To visualize the immunocomplexes, the secondary donkey anti-rabbit biotinylated IgG were applied for all slides (1 h, RT). After extensive washing, the mixture of CY3–conjugated streptavidin (0.2 μg/ml) and donkey anti-sheep IgG conjugated−FITC (1.6 ng/ml) as a fluorophores were utilized for 1 h, RT (Jackson Immunores., West Grove, PA). All slides were finally counterstained with DAPI (0.5 μg/ml) for 10 min. At least six slides from each specimen were stained and assessed. The immunoreactive (IR) signals were examined under fluorescent microscope (Leica Digital DM4000B) equipped with a digital camera. In addition, the intensity of fluorescent signals inside epidermal layer was evaluated using ImageJ software (NIH, USA) and statistically compared between conditions to the controls. The positive control staining was performed on human adrenals and uterus. The negative controls consisted of tissues incubated without primary antibody or with non-immune rabbit and sheep serum. A dIF staining on chamber slides was performed similarly to that described above, except the cells were fixed with buffered PFA for 30 min.
Table 2.
Specification of antibody and reagents used for WB and dIF.
| Primary antibody | Host | Vendor |
|---|---|---|
| Hydroxysteroid dehydrogenase type 1, 11β-HSD1 | sheep | Binding Site, Birmingham, UK |
| Hydroxysteroid dehydrogenase type 2, 11β-HSD2 | sheep | |
| Glucocorticoid receptor, GR | rabbit | Santa Cruz Biotech., CA |
| Secondary antibody and reagents | ||
| Biotinylated anti rabbit IgG | donkey | Jackson ImmunoRes., West Grove, PA |
| Anti sheep IgG−FITC | donkey | |
| Streptavidin−CY3 | − | |
| Anti sheep IgG−HRP | donkey | Santa Cruz Biotech., CA |
| Anti rabbit IgG−HRP | donkey | |
| β-actin IgG−HRP | mouse | Sigma, St. Louis, MO |
Statistical evaluation
Data are presented as means ± SD and analyzed with Student’s t-test (for two groups, asterisk sign) or with one-way analysis of variance ANOVA Dunnett's Multiple Comparison post hoc test (for more than two groups, pound sign) using Prism 4.00 (GraphPad Software, San Diego, CA). Statistically significant differences are denoted by asterisks/pounds, where ***/###P≤0.001, **/##P≤0.005, */#P≤0.05.
Results
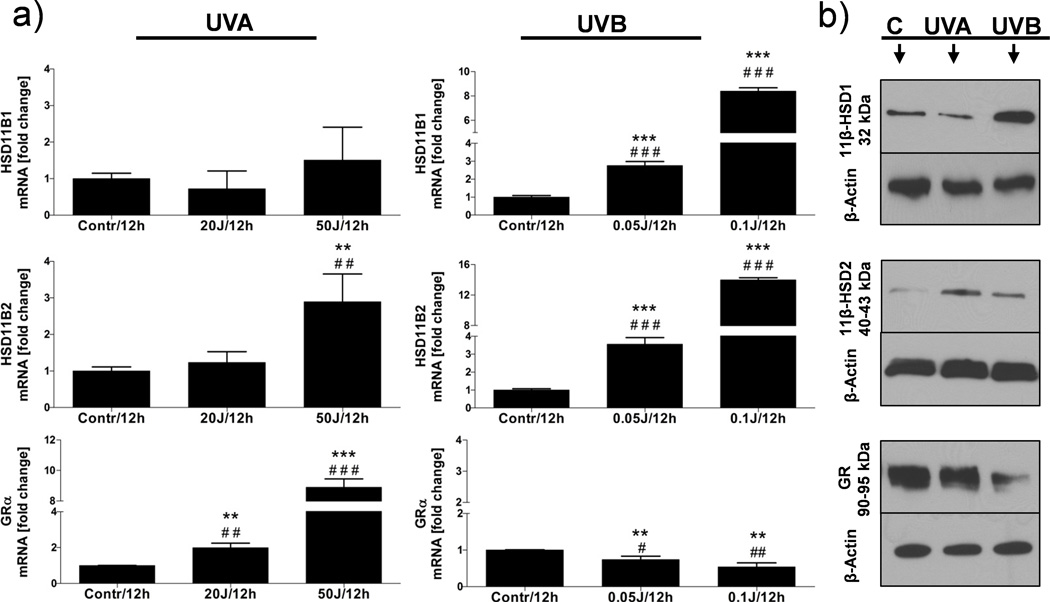
UVR significantly changed expression pattern of the cortisol activity regulating enzymes at the gene (HSD11B1, HSD11B2, GRα) and protein (11β-HSD1, 11β-HSD2, GR) levels in human skin explants and co-cultured HEKn/HEMn. The changes were dose and time related. The highest impact on gene expression had 12 h time point, while for protein levels – 24 h of incubation. The most pronounced induction of the HSD11B1 gene expression was observed after UVB with the peak at 0.1 J/cm2. UVA had no significant effects on HSD11B1 mRNA expression (Fig. 2a). The UVB and UVC (but not UVA) significantly increased the 11β-HSD1 protein expression as shown by WB (Fig. 2b) and by quantitative dIF (Fig. 3a, b).
Fig. 2.
The influence of different UV wavebands and doses on gene (a), and protein (b) expressions.
Twelve hours (12h) after treatment, co-cultured melanocytes and keratinocytes were extracted for RNA and proteins and processed as described in M&M. HSD11B1/11β-HSD1 expression is stimulated after UVB in a dose-dependent manner. HSD11B2 gene was up-regulated after both UVA and UVB; however the protein 11β-HSD2 expression was enhanced only after UVA. The GRα gene expression increased after UVA and decreased after UVB with similar pattern observed at the protein (GR) level. The statistical differences between groups were marked with asterisks (t-student) and pounds (Anova) tests.
Fig. 3.
The influence of different UV wavebands radiation on cortisol activity−regulating enzymes and GR expressions is shown by double IF performed on skin explants (a) and co-cultured HEKn/HEMn (b).
Tissues and cells were collected 24 h after exposure. The intensity of green (FITC; 11β-HSD1 or 11β-HSD2) or red (CY3; GR) signals is proportional to the expression levels of analyzed antigens and showed on inserted graphs to Fig. 3b. The statistical (one-way Anova Dunnett’s test) differences between conditions were marked with red (for GR) and green (for 11β-HSD1 or 11β-HSD2) asterisks on inserted graphs. Double headed arrows indicate the nuclear localization of GR while single headed arrows show cytoplasmic localization of 11β-HSD1/D2 antigens.
The HSD11B2 gene expression was enhanced by both UVA and UVB in a dose related pattern (Fig. 2a), while 11β-HSD2 protein levels increased mainly after high UVA dose (50 J/cm2) with only a small increase after 0.1 J/cm2 of UVB (Fig. 3a, b). Lower doses of UVA (20 J/cm2) and UVB (0.05 J/cm2) and tested spectrum of UVC had no effect on 11β-HSD2 protein levels (Fig. 3a).
The in situ double immunofluorescent (dIF) study showed the cytoplasmic localization of both 11β-HSD1 and 11β-HSD2 antigens in the epidermis of which expression pattern was selectively modified by UVR. For example, the 11β-HSD1 immunoreactivity (IR) increased after UVB and UVC, while for 11β-HSD2 only after UVA (Fig. 3a, b).
The main form of GR gene (GRα) was highly expressed in non-treated co-cultured HEKn/HEMn. The UVA significantly increased, while UVB decreased the mRNA expression of GRα (Fig. 2a). At the protein levels, GR expression decreased after UVB and UVC with UVA having no effect (Fig. 2b). dIF confirmed the nuclear expression of GR antigen (Fig 3a, b).
The cultured skin explants produced cortisol at the basic level (7–8 ng/ml) and only UVB and UVC (but not UVA) enhanced its production (Table 3).
Table 3.
Cortisol levels measured by ELISA.
| Cortisol (ng/ml) |
UVA J/cm2 | UVB J/cm2 | UVC J/cm2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ctr=0 | 20 | 50 | P | Ctr=0 | 0.05 | 0.1 | P | Ctr=0 | 0.0093 | 0.0465 | P | |
| 7.53 ±0.31 | 6.39 ±0.72 | 6.73 ±0.33 | − | 7.35 ±0.07 | 8.23 ±0.87 | 10.7 ±1.12 | ** | 7.43 ±0.37 | 10.5 ±0.76 | 9.21 ±2.01 | ** | |
UVB and UVC stimulate cortisol production in human skin incubated ex-vivo after 24 h of irradiation. Data are presented as means ± SD and analyzed with Student’s t-test. Statistically significant differences are denoted by asterisks, where **P≤0.005.
Discussion
The above data clearly show differential effects of UVB and UVC vs. UVA on HSD11B1/11β-HSD1 and HSD11B2/11β-HSD2 expression with attendant cortisol production and GR expression in human skin. Non-treated and UVA irradiated skin samples produced the cortisol at the basal (low) levels retaining the GR nuclear expression. Recent studies demonstrated the constitutive production of cortisol 10,16–19 and GR and HSD11B1/2 expression in keratinocytes and dermal fibroblasts 9,20,21. This local regulation of cortisol activity at the pre- or receptor level can help maintaining the local homeostasis and can potentially protect epidermis against autoimmune reaction 2,3,22,23. The increased expression of cortisol-activating enzyme HSD11B1 mRNA after UVB and protein after UVB/UVC is consistent with earlier observation that highly energetic wavelengths trigger the local hypothalamic-pituitary-adrenal (HPA) axis with UVA having no or lesser impact 13,23–25. Also, our data are consistent with increased expression of 11β-HSD1 observed in dermal fibroblasts grown from the photoexposed skin biopsies in comparison to donor-matched photoprotected skin, and the reported general expression of HSD11B1 in epidermal and follicular keratinocytes 9. The enhanced activation of HSD11B2 mRNA was observed after both UVA and UVB, while the 11β-HSD2 protein level was significantly stimulated only after UVA but not UVB or UVC. This may explain stronger anti-inflammatory properties of UVB than UVA, e.g., by regulating of local cortisol production and stimulation of the local cutaneous HPA axis 13,23,26. In the light of recent paper by Tewari et al. 12 on the different chromophores and mechanism inducing erythema in humans by UVB and UVA, we would like to speculate that similar mechanism may be responsible for the observed effects on local cortisol activity. Thus, this local endocrine activity may be attributed to UVB excitation of DNA chromophores resulting in production of pyrimidine photoproducts with lesser or different role assigned to excitation of non-DNA chromophores or production of thymidine dimers as discussed by Tewari et al. 12. These issues open new exciting areas of mechanism oriented research since 7-dehydrocholesterol, precursor to vitamin D3, serves as chromophore for UVB27,28, adding another layer of regulation that may separate UVA from UVB, and UVB from UVC.
Interestingly, UVA while inducing 11β-HSD2 sustains the GR expression and cortisol levels remain unchanged, which is in contrast to UVB and UVC. On the other hand, UVB and UVC enhance the 11β-HSD1 expression and cortisol production with simultaneous decrease in GR expression. The observed different mechanism of UVR action on epidermal expression of 11β-HSD1/2 and GR can be best explained by a requirement to maintain the epidermal barrier build by keratinocytes against disruption caused by elevated cortisol levels. On the other hand, cortisol predominantly acts on immune cells, protecting from autoimmune response triggered by UVB/C induced epidermal damage 29,30. Although the phenomenon of decreased GC efficiency in chronic inflammatory diseases and its molecular mechanism is still unclear, the described modulatory effects of different UV wavelengths on 11β-HSD1/2 and GR may contribute to our understanding of context dependent modulation of GC action in the human skin.
Supplementary Material
Statements.
UVB and UVC radiation stimulates the HSD11B1/11β-HSD1 expression and cortisol production in the skin
UVB and UVC radiation inhibits epidermal glucocorticoid receptor (GR) expression
UVA radiation stimulates HSD11B2/11β-HSD2 expression in the skin
UVA radiation has minor effects on GR expression
Differential UV wavebands effects on steroidogenesis are to maintain skin homeostasis
Acknowledgements
This study was supported in part by grants from National Science Foundation (# IOS-0918934) and National Institutes of Health (#1R01AR056666-01A2) to AS.
Footnotes
The authors’ disclosure: No conflict of interest.
References
- 1.Skobowiat C, Dowdy JC, Sayre RM, et al. Cutaneous hypothalamic-pituitary-adrenal axis homolog: regulation by ultraviolet radiation. Am J Physiol Endocrinol Metab. 2011;301:E484–E493. doi: 10.1152/ajpendo.00217.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stahn C, Lowenberg M, Hommes DW, et al. Molecular mechanisms of glucocorticoid action and selective glucocorticoid receptor agonists. Mol Cell Endocrinol. 2007;275:71–78. doi: 10.1016/j.mce.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Wikstrom AC. Glucocorticoid action and novel mechanisms of steroid resistance: role of glucocorticoid receptor-interacting proteins for glucocorticoid responsiveness. J Endocrinol. 2003;178:331–337. doi: 10.1677/joe.0.1780331. [DOI] [PubMed] [Google Scholar]
- 4.Draper N, Stewart PM. 11beta-hydroxysteroid dehydrogenase and the pre-receptor regulation of corticosteroid hormone action. J Endocrinol. 2005;186:251–271. doi: 10.1677/joe.1.06019. [DOI] [PubMed] [Google Scholar]
- 5.Bujalska IJ, Walker EA, Hewison M, et al. A switch in dehydrogenase to reductase activity of 11 beta-hydroxysteroid dehydrogenase type 1 upon differentiation of human omental adipose stromal cells. The Journal of clinical endocrinology and metabolism. 2002;87:1205–1210. doi: 10.1210/jcem.87.3.8301. [DOI] [PubMed] [Google Scholar]
- 6.Bujalska I, Shimojo M, Howie A, et al. Human 11 beta-hydroxysteroid dehydrogenase: studies on the stably transfected isoforms and localization of the type 2 isozyme within renal tissue. Steroids. 1997;62:77–82. doi: 10.1016/s0039-128x(96)00163-8. [DOI] [PubMed] [Google Scholar]
- 7.Tomlinson JW, Walker EA, Bujalska IJ, et al. 11beta-hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr Rev. 2004;25:831–866. doi: 10.1210/er.2003-0031. [DOI] [PubMed] [Google Scholar]
- 8.Ricketts ML, Verhaeg JM, Bujalska I, et al. Immunohistochemical localization of type 1 11beta-hydroxysteroid dehydrogenase in human tissues. The Journal of clinical endocrinology and metabolism. 1998;83:1325–1335. doi: 10.1210/jcem.83.4.4706. [DOI] [PubMed] [Google Scholar]
- 9.Tiganescu A, Walker EA, Hardy RS, et al. Localization, age- and site-dependent expression, and regulation of 11beta-hydroxysteroid dehydrogenase type 1 in skin. The Journal of investigative dermatology. 2011;131:30–36. doi: 10.1038/jid.2010.257. [DOI] [PubMed] [Google Scholar]
- 10.Hannen RF, Michael AE, Jaulim A, et al. Steroid synthesis by primary human keratinocytes; implications for skin disease. Biochem Biophys Res Commun. 2011;404:62–67. doi: 10.1016/j.bbrc.2010.11.059. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald P, O'Brien SM, Scully P, et al. Cutaneous glucocorticoid receptor sensitivity and proinflammatory cytokine levels in antidepressant-resistant depression. Psychol Med. 2006;36:37–43. doi: 10.1017/S003329170500632X. [DOI] [PubMed] [Google Scholar]
- 12.Tewari A, Sarkany RP, Young AR. UVA1 induces cyclobutane pyrimidine dimers but not 6-4 photoproducts in human skin in vivo. The Journal of investigative dermatology. 2012;132:394–400. doi: 10.1038/jid.2011.283. [DOI] [PubMed] [Google Scholar]
- 13.Skobowiat C, Dowdy JC, Sayre RM, et al. Cutaneous hypothalamic pituitary adrenal (HPA) axis homologue - regulation by ultraviolet radiation. Am J Physiol Endocrinol Metab. 2011 doi: 10.1152/ajpendo.00217.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CIE Technical Committee 6-40. CIE Standard 007/E:1998, Erythema Reference Action Spectrum and Standard Erythema Dose. Vienna, Austria: Commission Internationale de l'Eclairage (CIE) Central Bureau; 1998. [Google Scholar]
- 15.International Commission on Illumination (CIE) International Standard ISO 17166:1999(E) - CIE007/E:1998, Erythema Reference Action Spectrum and Standard Erythema Dose. 1st edn. Geneva, Switzerland: International Organization for Standardization (ISO); 1999. [Google Scholar]
- 16.Cirillo N, Prime SS. Keratinocytes synthesize and activate cortisol. J Cell Biochem. 2011;112:1499–1505. doi: 10.1002/jcb.23081. [DOI] [PubMed] [Google Scholar]
- 17.Ito N, Ito T, Kromminga A, et al. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J. 2005;19:1332–1334. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- 18.Slominski A, Zbytek B, Semak I, et al. CRH stimulates POMC activity and corticosterone production in dermal fibroblasts. J Neuroimmunol. 2005;162:97–102. doi: 10.1016/j.jneuroim.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Slominski A, Zbytek B, Szczesniewski A, et al. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol Endocrinol Metab. 2005;288:E701–E706. doi: 10.1152/ajpendo.00519.2004. [DOI] [PubMed] [Google Scholar]
- 20.Farman N, Maubec E, Poeggeler B, et al. The mineralocorticoid receptor as a novel player in skin biology: beyond the renal horizon? Experimental dermatology. 2010;19:100–107. doi: 10.1111/j.1600-0625.2009.01011.x. [DOI] [PubMed] [Google Scholar]
- 21.Vukelic S, Stojadinovic O, Pastar I, et al. Cortisol synthesis in epidermis is induced by IL-1 and tissue injury. J Biol Chem. 2011;286:10265–10275. doi: 10.1074/jbc.M110.188268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taves MD, Gomez-Sanchez CE, Soma KK. Extra-adrenal glucocorticoids and mineralocorticoids: evidence for local synthesis, regulation, and function. Am J Physiol Endocrinol Metab. 2011;301:E11–E24. doi: 10.1152/ajpendo.00100.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slominski A, Wortsman J, Tuckey RC, et al. Differential expression of HPA axis homolog in the skin. Mol Cell Endocrinol. 2007;265-266:143–149. doi: 10.1016/j.mce.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mormede P, Foury A, Barat P, et al. Molecular genetics of hypothalamic-pituitary-adrenal axis activity and function. Ann N Y Acad Sci. 2011;1220:127–136. doi: 10.1111/j.1749-6632.2010.05902.x. [DOI] [PubMed] [Google Scholar]
- 25.Slominski A, Wortsman J, Luger T, et al. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiological reviews. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 26.Miolo G, Caffieri S, Dalzoppo D, et al. Photoactivation of corticosteroids in UVB-exposed skin. J Photochem Photobiol B. 2011;103:35–41. doi: 10.1016/j.jphotobiol.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Holick MF. Vitamin D: A millenium perspective. Journal of cellular biochemistry. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 28.Bikle DD. Vitamin D: an ancient hormone. Experimental dermatology. 2011;20:7–13. doi: 10.1111/j.1600-0625.2010.01202.x. [DOI] [PubMed] [Google Scholar]
- 29.Moneib HA, Salem SA, Younis RM. Comparison of turbo-PUVA and conventional American-style PUVA in the treatment of psoriatic patients. Photodermatology, photoimmunology & photomedicine. 2010;26:205–210. doi: 10.1111/j.1600-0781.2010.00523.x. [DOI] [PubMed] [Google Scholar]
- 30.Slominski A, Baker J, Ermak G, et al. Ultraviolet B stimulates production of corticotropin releasing factor (CRF) by human melanocytes. FEBS Lett. 1996;399:175–176. doi: 10.1016/s0014-5793(96)01315-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.