Abstract
We describe a 54-year-old diabetic woman who developed ischemic optic neuropathy followed by acute retinal necrosis and multiple areas of focal venous beading. Vitreous fluid contained amplifiable VZV DNA but not HSV-1, CMV or toxoplasma DNA. The clinical presentation was remarkable for jaw claudication and intermittent scalp pain, prompting a temporal artery biopsy that was pathologically negative for giant cell arteritis, but notable for VZV antigen. The current case adds to the clinical spectrum of multifocal VZV vasculopathy. The development of acute VZV retinal necrosis after ischemic optic neuropathy supports the notion that vasculitis is an important additional mechanism in the development of VZV retinal injury.
Keywords: varicella zoster virus, ischemic optic neuropathy, temporal arteritis
1. Introduction
In two recent clinical-virological correlative studies in elderly patients with VZV multifocal vasculopathy, arterial infection was restricted to the eye and ipsilateral temporal artery [1, 2]. Importantly, one of the patients had no history of zoster [2]. Herein, we present another case of VZV multifocal vasculopathy without rash in which VZV infection was restricted to the eye and ipsilateral temporal artery. Ischemic optic neuropathy was followed by acute retinal necrosis, with VZV DNA recoverable from the vitreous fluid. Vasculitis may be an important additional mechanism in the development of acute VZV retinal necrosis.
2. Case Report
A 54 year-old diabetic woman complained of blurred vision in the left eye for 5 days without scalp tenderness or headache. Visual acuity (VA) was 20/20 right eye and 20/30 left eye. Intraocular pressures were normal. Funduscopic examination revealed left optic nerve edema and a small disc margin hemorrhage. WBC count was 12,700, ESR was 7 (normal 0–20 mm/hr) and CRP was 2.4 (normal 0–1.0 mg/dL). Three days later, she noted new left eye pain and her vision declined to counting fingers. There was no pain on eye movement. The right pupil was 4 mm and reacted to light; the left pupil was 4 mm, and there was a relative left afferent pupillary defect. The left optic nerve was swollen with a few peripapillary nerve fiber layer hemorrhages along with a cherry red spot and mild macular edema consistent with central retinal artery occlusion; there were no posterior pole or peripheral retinal hemorrhages. Trace cells were seen in the anterior chamber, but not in the vitreous fluid. Repeat WBC, ESR and CRP were normal. Treponemal and Bartonella antibodies, the QuantiFERON-TB test, blood cultures, EKG and carotid Doppler ultrasound were unremarkable. Brain contrast MRI demonstrated no optic nerve enhancement. Twelve days later, left eye pain and vision worsened, and the patient complained of jaw claudication and scalp pain. VA declined to no light perception in the left eye with 1+ cells and flare in anterior chamber and 2+ vitreous cells and haze. Funduscopic examination revealed extensive retinal necrosis and diffuse hemorrhages with multiple areas of focal venous beading (Fig.1), consistent with acute retinal necrosis. Vitreous fluid was examined by PCR for amplifiable HSV, VZV, CMV and toxoplasma sequences and returned positive for VZV DNA. She was treated with a one-time intravitreal injection of ganciclovir 2000 mcg/0.5 ml in the left eye, oral acyclovir 800 mg 5 times daily for 14 days and oral prednisone 60 mg daily for 7 days followed by 20 mg for 7 days. Eleven days after starting treatment, because she had recently developed jaw claudication and intermittent scalp pain, a temporal artery biopsy was performed. Histological examination was normal (Fig. 2) while immunohistochemical analysis demonstrated VZV antigen in the arterial adventitia; evaluation of adjacent sections for HSV-1 antigen and infiltrating leukocytes (CD45, not shown) was negative (Fig. 3). Antiviral therapy was altered to intravenous acyclovir, 10 mg/kg every 8 h for 14 days, and steroids were discontinued.
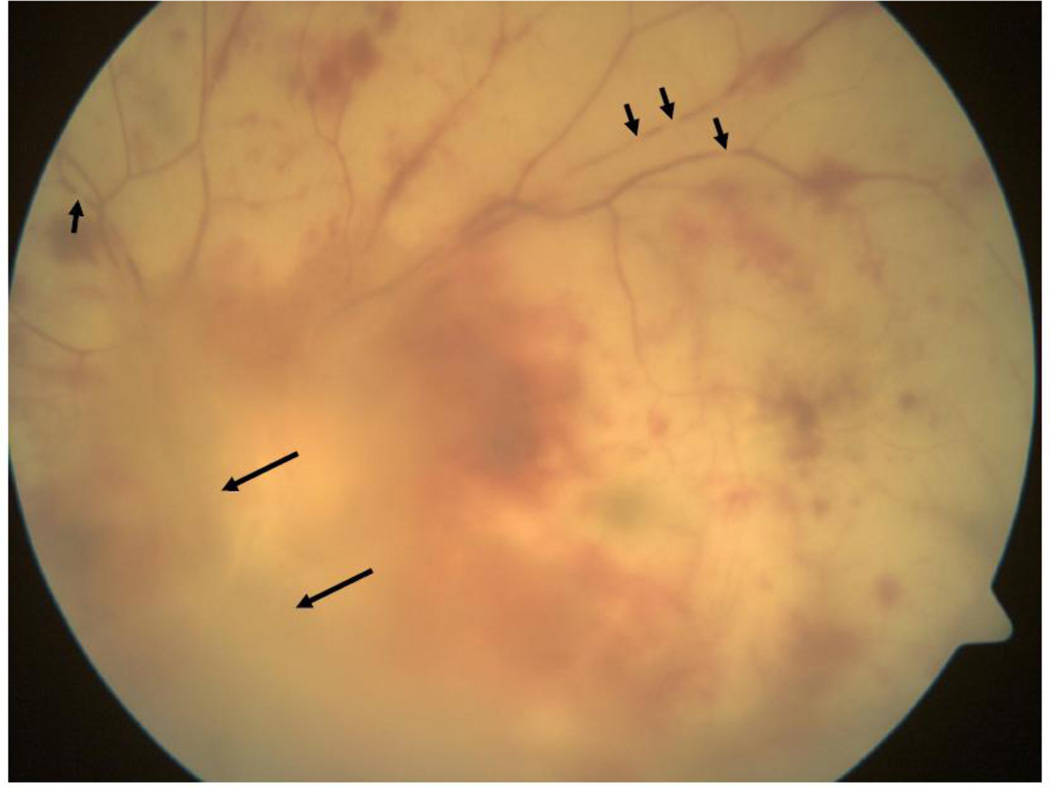
Fig. 1.
Fundus photo of the left eye demonstrates extensive retinal necrosis with intraretinal hemorrhage, overlying vitreitis (long arrows) and focal beading in multiple retinal veins (short arrows).
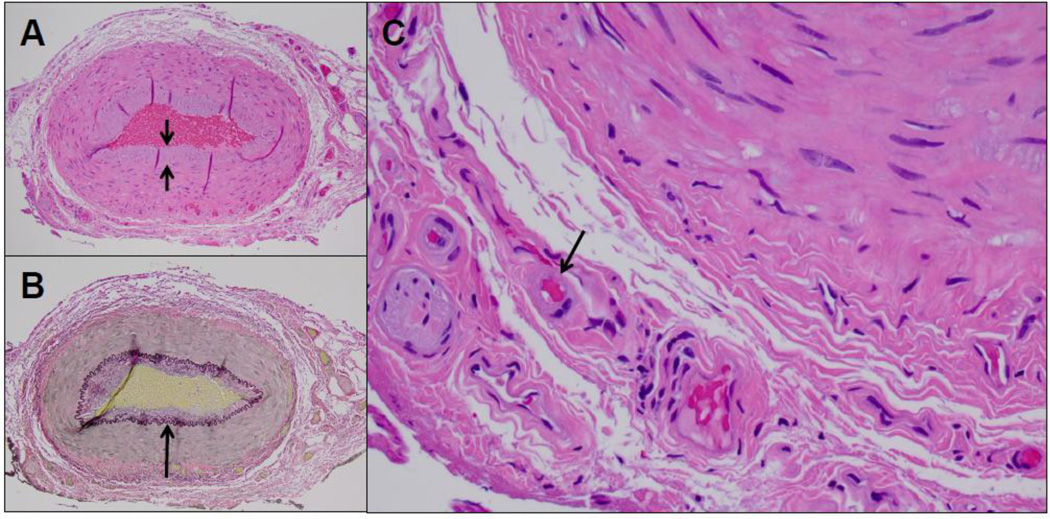
Fig. 2.
Cross-sections of the temporal artery showed mild intimal thickening (short arrows) and an intact internal elastic lamina (long arrow) (A, H&E; B, Van Gieson stain, respectively; magnification 100X). Hyalinazation of vasa vasorum arterioles was noted (C, long arrows), consistent with diabetic vasculopathy. No evidence of inflammation was noted, particularly in the adventitial connective tissue or in association with vasa vasorum or vasa nervosum structures (H&E, magnification 400X. CD45 immunohistochemistry, as previously described [11], confirmed the absence of an inflammatory cell infiltrate (not shown).
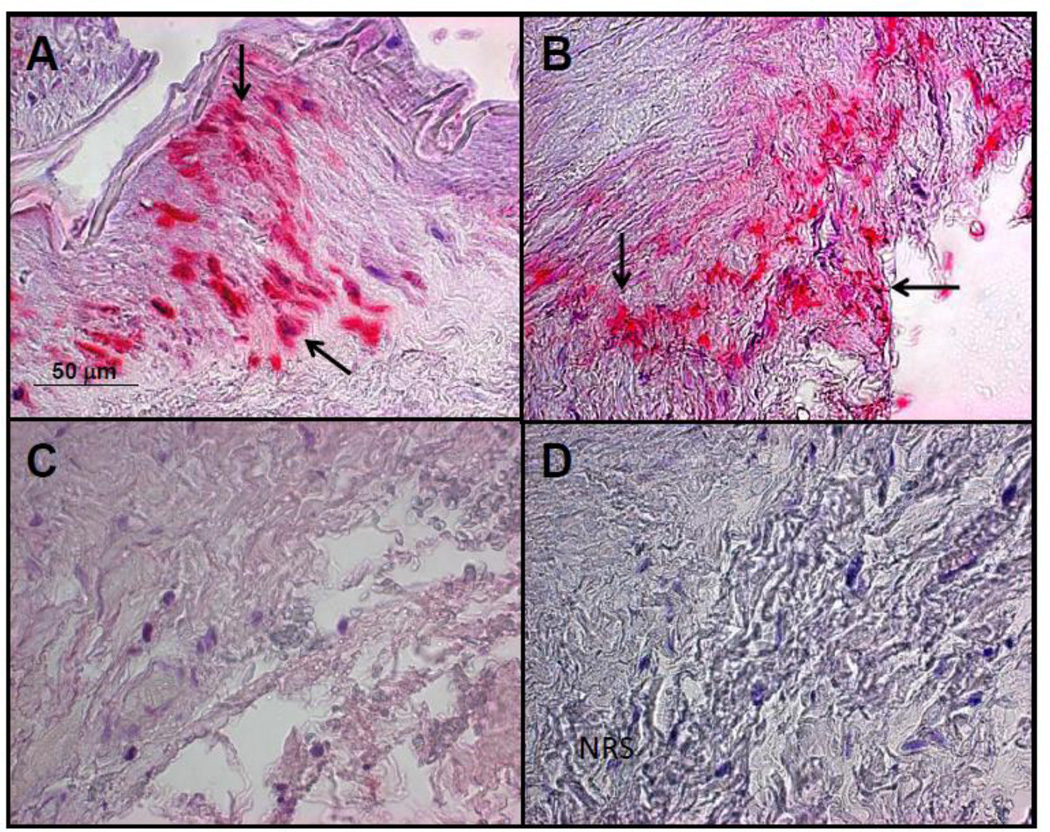
Fig. 3.
The left temporal artery in a patient with multifocal vasculopathy was analyzed for the presence varicella zoster virus (VZV) antigen as previously described [1]. A positive control cadaveric cerebral artery 14 days after VZV infection in vitro (A, pink color, arrows). VZV antigen was seen in the adventitia of the left temporal artery of the subject after staining with anti-VZV antibody (B, pink color, arrows), but not after staining adjacent sections with anti-HSV antibody (C) or normal rabbit serum (D). Magnification 200X.
3. Discussion
We describe a remarkable case of multifocal VZV vasculopathy in a diabetic woman that began with ischemic optic neuropathy followed by acute VZV retinal necrosis. Her clinical course was notable for jaw claudication, scalp pain and an elevated WBC count and CRP suggestive of giant cell arteritis (GCA). Temporal artery biopsy showed no patholgical changes characteristic of GCA, but immunohistochemistry revealed abundant VZV antigen in the arterial adventitia. The lack of inflammation is likely due to treatment with oral acyclovir and prednisone for 11 days before the biopsy; furthermore, VZV is capable of evading the immune system and it is possible that only minimal inflammation was present before initiation of corticosteroids and antiviral therapy.
Three cases of virologically-verified multifocal VZV vasculopathy that began with ischemic optic neuropathy have been reported [1–3]. Two of those patients did not have scalp pain or a painful nodular temporal artery, but the temporal artery was biopsied due to clinical concern for GCA. As in our patient, the temporal arteries were negative for granulomas, but positive for VZV antigen by immunohistochemistry. These cases reveal the diagnostic value of temporal artery biopsy, as a proxy for evaluation of the ophthalmic artery, to demonstrate VZV temporal arteritis in elderly patients who present with ischemic optic neuropathy, and highlight the overlapping clinical (loss of vision) and laboratory features (high ESR or CRP) of patients with GCA and VZV temporal arteritis.
Importantly, temporal arteries from patients with pathologically-verified GCA do not contain VZV antigen [4] or VZV DNA [5]. Moreover, the primary site of pathology in GCA is the arterial media which is highly inflammed and necrotic, while inflammation in the few reported cases of patients with VZV temporal arteritis before antiviral treatment was primarily in the arterial adventitia [1, 2]. Thus, GCA and VZV temporal arteritis appear to be distinct entities.
Note that ischemic optic neuropathy, VZV acute retinal necrosis and VZV temporal artery infection in our patient all developed in the absence of zoster rash. Because each of these disorders, as well as multiple other serious ocular and neurological complications of VZV reactivation, cam occur without rash [6], clinicians must be alert to the possibility that patients with ischemic optic neuropathy may have treatable VZV temporal arteritis as confirmed by temporal artery biopsy. Although a definitive clinical picture of VZV temporal arteritis has not yet emerged, it is eminently clear that patients with both GCA and VZV temporal arteritis manifest many of the same symptoms, signs and laboratory abnormalities. Thus, pathological and virological analysis of the temporal artery is essential for definitive diagnosis and appropriate treatment.
Finally, the severity of VZV multifocal vasculopathy in our patient may in part be due to diabetes, which increases the risk for VZV reactivation. Compared to the well-known decline in VZV-specific cell-mediated immunity (CMI) in healthy individuals with increasing age, diabetics stratified by decade have even lower CMI responses to VZV [7] and are at significantly higher risk for zoster [8], as recently illustrated by persistent multi-dermatomal pain even after oral antiviral treatment in a diabetic patient with chronic VZV radiculopathy [9]. Overall, the current case extends the clinical spectrum of VZV temporal arteritis presenting with ischemic optic neuropathy in the absence of zoster rash and supports the notion that vasculitis is an important additional mechanism in the development of acute VZV retinal necrosis [10]. Further, it underscores the essential nature of performing immunohistochemical evaluation for VZV in temporal arteries, particularly in the setting of pre-biopsy treatment with corticosteroids, as the presence of VZV is otherwise impossible to discern.
Acknowledgments
This work was supported in part by Public Health Service grants NS067070, AG006127 and AG032958 from the National Institutes of Health and the Guthy Jackson Charitable Foundation. The authors thank Marina Hoffman for editorial assistance and Lori DePriest for word processing and formatting the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
All authors report no conflicts of interest.
References
- 1.Salazar R, Russman AN, Nagel MA, et al. VZV ischemic optic neuropathy and subclinical temporal artery involvement. Arch Neurol. 2011;68:517–520. doi: 10.1001/archneurol.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagel MA, Russman A, Feit H, Traktinskiy I, Khmeleva N, Schmid DS, Skarf B, Gilden D. VZV ischemic optic neuropathy and subclinical temporal artery infection without rash. Neurology. 2013 doi: 10.1212/WNL.0b013e31827b92d1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silver B, Nagel MA, Mahalingam R, Cohrs R, Schmid DS, Gilden D. VZV vasculopathy: A treatable form of rapidly progressive multi-infarct dementia after 2 years' duration. J Neurol Sci. 2012;323:245–247. doi: 10.1016/j.jns.2012.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordborg C, Nordborg E, Petursdottir V, LaGuardia J, Mahalingam R, Wellish M, et al. Search for VZV in giant cell arteritis. Ann Neurol. 1998;44:413–414. doi: 10.1002/ana.410440323. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy PG, Grinfeld E, Esiri MM. Absence of detection of varicella-zoster virus DNA in temporal artery biopsies obtained from patients with giant cell arteritis. J Neurol Sci. 2003;215:27–29. doi: 10.1016/s0022-510x(03)00167-9. [DOI] [PubMed] [Google Scholar]
- 6.Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Neurological disease produced by varicella zoster virus reactivation without rash. Curr Top Microbiol Immunol. 2010;342:243–253. doi: 10.1007/82_2009_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okamoto S, Hata A, Sadaoka K, Yamanishi K, Mori Y. Comparison of varicella-zoster virus-specific immunity of patients with diabetes mellitus and healthy individuals. J Infect Dis. 2009;200:1606–1610. doi: 10.1086/644646. [DOI] [PubMed] [Google Scholar]
- 8.Heymann AD, Chodick G, Karpati T, Kamer L, Kremer E, Green MS, et al. Diabetes as a risk factor for herpes zoster infection: results of a population-based study in Israel. Infection. 2008;36:226–230. doi: 10.1007/s15010-007-6347-x. [DOI] [PubMed] [Google Scholar]
- 9.Wolf J, Nagel MA, Mahalingam R, Cohrs RJ, Schmid DS, Gilden D. Chronic active varicella zoster virus infection. Neurology. 2012;79:828–829. doi: 10.1212/WNL.0b013e3182661ffa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Culbertson WW, Blumenkranz MS, Pepose JS, Stewart JA, Curtin VT. Varicella zoster virus is a cause of the acute retinal necrosis syndrome. Ophthalmology. 1986;93:559–569. doi: 10.1016/s0161-6420(86)33701-1. [DOI] [PubMed] [Google Scholar]
- 11.Nagel MA, Traktinskiy I, Choe A, Rempel A, Gilden D. Varicella-zoster virus expression in the cerebral arteries of diabetic subjects. Arch Neurol. 2012;69:142–144. doi: 10.1001/archneur.69.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]