Abstract
This study investigated how acute alcohol intake affects contrast processing mediated by inferred magnocellular (MC) and parvocellular (PC) pathways. Achromatic contrast discrimination thresholds were measured in 16 young healthy participants using a steady-pedestal, pulsed-pedestal or pedestal-Δ-pedestal paradigm designed to favor the inferred MC or the PC pathway. Each participant completed two randomized sessions that included consumption of either 0.8 g/kg alcohol or a placebo beverage, with each session consisting of contrast discrimination measurements at baseline and at 60 min following beverage consumption. The results showed that, compared to placebo, alcohol significantly reduced MC contrast sensitivity and PC contrast gain but had no effect on PC contrast sensitivity for the majority of the participants; and did not alter MC contrast gain consistently across participants. The decrease in contrast gain in the PC pathway can be interpreted as a degradation of the postretinal signal-to-noise ratio, whereas the decrease of sensitivity in the MC pathway likely results from a change of cortical processing.
Keywords: contrast sensitivity, contrast gain, parvocellular, magnocellular, alcohol
Introduction
It is well known that alcohol intake strongly impairs various aspects of visual processing, cognitive function, and behavior that can increase the risk for alcohol-related accidents. Given worldwide alcohol usage and its possible harmful consequences, understanding how alcohol interferes with the ability to perform a wide range of tasks has become an important and widely studied topic (Zoethout, Delgado, Ippel, Dahan, & van Gerven, 2011). The effects of alcohol on higher order cognitive and visual processing, such as memory and attention, have been well documented (Crego et al., 2009; George, Rogers, & Duka, 2005; Kirchner & Sayette, 2003; Saults, Cowan, Sher, & Moreno, 2007). However, the possible underlying mechanisms leading to deleterious alcohol effects on cognitive and visual functions are not well understood (Khan & Timney, 2007a). To better identify the mechanisms underlying alcohol effects on higher order processing, it is necessary to understand how alcohol may alter early visual processing. For instance, alcohol reduces the binocular rivalry alternation rate, but this decrease can be attributed to the reduction in contrast sensitivity caused by alcohol (Donnelly & Miller, 1995). In this study, we examined the effects of acute alcohol administration on contrast processing, an important aspect of visual function associated with object detection and recognition and perceptual decision-making.
Contrast sensitivity and contrast gain are two essential properties of contrast processing. Contrast sensitivity is a measure of the contrast detection threshold, that is, the minimal contrast for a target to be detected against a background of certain luminance (Campbell & Kulikowski, 1966; Kingdom & Whittle, 1996). Contrast gain initially was introduced in physiological studies to indicate how rapidly cell responses increase with increases in stimulus contrast (Kaplan & Shapley, 1986) and, for some cell types, contrast gain can be modified by the contrast distribution in the stimulus (Shapley & Victor, 1979). Psychophysically, contrast gain can be assessed by how rapidly contrast discrimination threshold (e.g., Foley & Legge, 1981) or perceived contrast (Swanson, Wilson, & Giese, 1984; Whittle, 1992) increases with increasing stimulus contrast, that is, the slope of discrimination threshold or perceived contrast as a function of reference contrast.
Many studies have evaluated the effects of alcohol on contrast sensitivity (Andre, 1996; Andre, Tyrrell, Leibowitz, Nicholson, & Wang, 1994; Nicholson, Andre, Tyrrell, Minqi, & Leibowitz, 1995; Pearson & Timney, 1998, 1999b; Roquelaure et al., 1995; Watten & Lie, 1996; Zulauf, Flammer, & Signer, 1988). These studies employed a wide range of measurements protocols, including various manipulations in alcohol dosage, postbeverage measurement time, visual stimuli, or tasks. The majority of the studies reported that alcohol intake impaired contrast sensitivity, particularly for high spatial and/or temporal frequency stimuli. We are aware of only two studies that claimed to investigate alcohol's effect on contrast gain (Khan & Timney, 2007a; Pearson & Timney, 1999a). First, Pearson and Timney (1999a) observed that when the contrast of a grating was 1 log unit above the contrast detection threshold, the discrimination threshold was unaltered by alcohol intake. From these results, the authors concluded that alcohol did not alter contrast gain and that an observed increase in discrimination thresholds at low contrast of high spatial-frequency gratings was likely due to an alcohol-induced shift in detection thresholds. However, Pearson & Timney defined contrast gain not as the slope of the contrast discrimination function, but as the change in discrimination threshold for grating with contrast that is a multiple of the detection threshold. More recently, Khan and Timney (2007a) also reported that alcohol did not change contrast gain since the measured luminance increment thresholds on various background luminances did not change after moderate alcohol consumption. However, increment detection threshold is not equivalent to contrast gain. Contrast gain cannot be determined by a discrimination threshold at one reference contrast. Moreover, none of the studies of contrast sensitivity and contrast gain considered potential differences in an alcohol effect on contrast processing in different visual pathways.
In primates, visual information is represented and processed in parallel retinogeniculate pathways. The magnocellular (MC) and parvocellular (PC) pathways, named after the Lateral Geniculate Nucleus layer to which they project, convey visual information from the retina to the striate cortex (Dacey, 2000; Kaplan, 2004; Livingstone & Hubel, 1987, 1988). Physiological studies show that primate MC and PC cells have different contrast response characteristics (Kaplan, Lee, & Shapley, 1990; Lee, Martin, & Valberg, 1989). For achromatic stimuli, cells in the MC pathway show higher contrast gain than cells in the PC pathway (Kaplan & Shapley, 1986; Lee, Pokorny, Smith, Martin, & Valberg, 1990). Following adaptation to a steady background luminance, cells in the MC pathway show large increases in response rates to small increases in stimulus contrast, but the cell responses saturate with large stimulus contrast steps. In comparison, cells in the PC pathway show relatively linear increases in response rate to increasing stimulus contrast. The goal of the current study was to investigate how acute alcohol consumption affects visual processing of contrast sensitivity and contrast gain in the MC and PC pathways. Given the distinctive contrast processing characteristics in the MC and PC pathways, this study anticipated that alcohol might have different effects on the two pathways.
It has been hypothesized that alcohol may selectively impair the MC but not the PC pathway (Hill & Toffolon, 1990; Khan & Timney, 2007b). This selective impairment hypothesis is partially supported by alcohol resulting in a greater reduction in temporal contrast sensitivity at a high temporal frequency (Pearson & Timney, 1998) and a slowed neural processing and transmission speed (Khan & Timney, 2007b). In this study, we tested the selective hypothesis using the psychophysical paradigms that can separately evaluate the contrast sensitivity and contrast gain of the MC and PC pathway (Pokorny & Smith, 1997). The steady-pedestal paradigm assesses steady-state MC pathway sensitivity, the pulsed-pedestal paradigm reveals PC contrast sensitivity and contrast gain, and the pedestal-Δ-pedestal paradigm is used to measure MC contrast gain. Detailed rationales and theories underlying these paradigms can be reviewed in prior publications (Cao et al., 2011; Pokorny, 2011; Pokorny & Smith, 1997; Smith & Pokorny, 2003; Zele, Wood, & Girgenti, 2010). The contrast gain parameters derived from these paradigms are comparable to those measured from single unit primate retina recordings in MC and PC cells (Kaplan & Shapley, 1986). We used a within-subjects placebo-controlled design to investigate how the parameters derived from these paradigms were altered by alcohol.
Methods
Participants
Sixteen healthy, young non-alcoholic social drinkers (nine males and seven females, age 27.1 ± 5.6 years) with normal or corrected-to-normal visual acuity participated in the experiment. After providing written informed consent, each participant completed demographic background, alcohol history, and health questionnaires to ensure participants were social drinkers (i.e., neither abstainers nor alcohol dependent). The screening included questions about current medical history and medications that might interact with study procedures. Participants were compensated for their time. The study protocols were approved by the Institutional Review Board at The University of Chicago and were in compliance with the Declaration of Helsinki.
Beverage administration procedure
The study employed a within-subjects design with each participant attending two sessions on two separate days during which either an alcohol (0.8 g/kg) or a placebo beverage was administered in a random order. To reduce potential alcohol expectancy effects, participants were informed that they might receive either alcohol or placebo during each experimental session. Beverages consisted of a mixture of water, grape flavored drink mix, a sucralose-based sugar substitute, and ethanol. The quantity of ethanol was based on participant body weight, with an appropriate dose of 16% volume, 190-proof ethanol for the alcohol session (0.8 g/kg, equivalent of 4–5 standard alcohol drinks). For the placebo sessions, 1% ethanol was included as a taste mask to reduce expectancy effects. Women received 85% of the dose of men as a correction for body water differences (Watson, Watson, & Batt, 1980).
Participants were instructed to abstain from alcohol for 24 hours prior to each session. Each session consisted of four basic components, including verification of alcohol abstinence, prebeverage measurement, beverage intake, and postbeverage measurement. Upon arrival at each session in the early afternoon, participants were taken to a resting room illuminated with a red light (needed for a separate mesopic visual function study). They were required to complete a questionnaire and had their breath alcohol concentration level measured by an Alco-Sensor III (Intoximeter Inc., St. Louis, MO) to confirm abstinence. They were provided with a light snack (non-caffeine, low-fat meal at 20% of daily kilocalorie needs, based on body weight [Schofield, 1985]) to help reduce the potential for alcohol-induced nausea. Then they were taken to a dark room for visual testing. Following a tutorial and practice period for the pedestal task (and the mesopic vision task), participants completed prebeverage measurements. Participants then drank the assigned beverage through a straw from a plastic, lidded cup (to help conceal the scent and identification of the alcohol content). Participants were again reminded that the drink could contain either alcohol or a placebo. They had 5 min to consume one half of the beverage, followed by a 5-min rest period, and then another 5 min to finish the second half. This beverage administration procedure has been used extensively in previous studies in Dr. King's laboratory and has shown reliable rising and declining breath alcohol concentration (BrAC) curves across participants (Brumback, Cao, & King, 2007; King, de Wit, McNamara, & Cao, 2011). Participants rested for 45 min before starting postbeverage measurements. The pedestal task started at approximately 60 min after beverage consumption, a time which corresponds to peak BrAC (Brumback et al., 2007). BrAC levels were measured when the pedestal task started as well as it finished. In the alcohol session, the BrAC levels were at 0.04% for four of the participants and ranged from 0.07% to 0.10% for the other 12 participants, while BrAC levels were all zero during the placebo session.
Visual display
The visual stimuli were presented on a ViewSonic 19” CRT color monitor (refresh rate 60 Hz) controlled by an iMac computer. CRT calibrations included the spectral outputs of the red, green, and blue guns of the CRT measured with a Photo Research PR-650 Spectrophotometer, and the linearity of the light outputs of each gun, measured at 1,024 light levels using an International Light Radiometer/Photometer (IL-1700).
Visual paradigms and staircase procedure
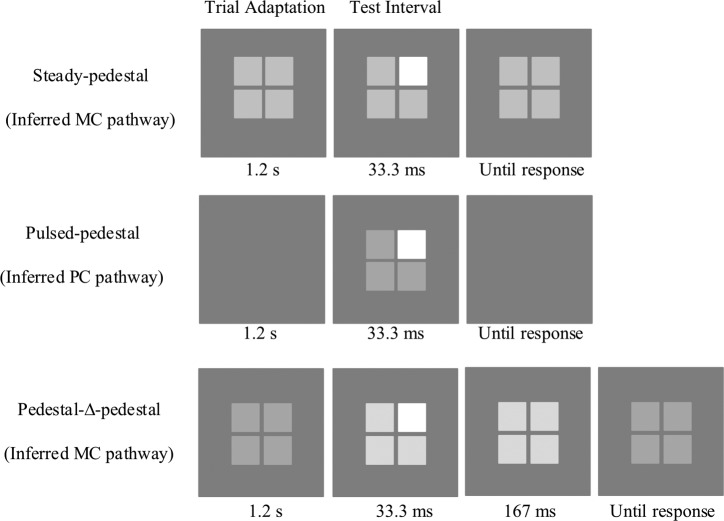
Figure 1 depicts the three pedestal paradigms, including steady-pedestal, pulsed-pedestal, and pedestal-Δ-pedestal paradigms, for measuring the contrast sensitivity and contrast gain of the inferred PC and MC pathways (Pokorny & Smith, 1997; for a review, see Pokorny, 2011). All of the paradigms used the same stimuli but differed only pre- and posttest adaptation. The stimuli consisted of a pedestal (2 × 2 array of four squares) surrounded by a uniform achromatic 32.8° × 22.3° rectangular field (15.0 cd/m2 luminance). Each square subtended 1° × 1° of visual angle with a 0.08° of separation between two squares at 57 cm viewing distance. A cross of 0.08° × 0.08° was presented at the center of the array as the fixation throughout the experiment.
Figure 1.
Diagrams of the stimulus displays for the steady pedestal (top panel), the pulsed pedestal (middle panel), and the pedestal-Δ-pedestal (bottom panel) paradigms. The pedestal consisted of a 2 × 2 array of four 1° squares (separated by 0.08°) surrounded by a uniform achromatic 32.8° × 22.3° rectangular field of 15.0 cd/m2. The experiment started with a 1-min adaptation to the background luminance and a 5-s additional adaptation to each pedestal luminance for the steady-pedestal or pedestal-Δ-pedestal paradigm. The three paradigms were similar in the test interval but differed in the adaptation phase. For the steady pedestal and pedestal-Δ-pedestal paradigms, there was 1.2 s adaptation to pedestal luminance for each trial; for the pulsed pedestal paradigm, however, the adaptation was to the background luminance. During the 33.3 ms test interval of each trial, one randomly selected square had a higher luminance from the other three squares that had the pedestal luminance for the steady pedestal and pulsed pedestal paradigms or the pedestal-Δ-pedestal luminance for the pedestal-Δ-pedestal paradigm. Participants were instructed to identify which square differed from the others during the test interval. For the pedestal-Δ-pedestal paradigm, after the test interval, the squares maintained the pedestal-Δ-pedestal luminance for an additional 167 ms before it returned to the pedestal luminance.
To assess an alcohol effect on visual processing, it is important that the blood alcohol concentration level does not change significantly during the testing period. We shortened the original paradigms (Pokorny & Smith, 1997) so that the total time to collect data for the three paradigms was less than 15 min. We made two modifications from the original paradigms. First, in the original paradigms (Pokorny & Smith, 1997), both incremental and decremental (i.e., the pedestal luminance was higher and lower luminance, respectively, than the surround,) contrast discrimination thresholds were measured. Here, we only measured incremental contrast discrimination thresholds. Second, we used a single staircase instead of two-interleaved staircases (as in the original paradigm) to estimate the threshold for each pedestal luminance. Staircase parameters were empirically established in pilot experiments (including the number of staircases, step size, the number of reversals and termination criterion) for efficient and reliable threshold measurement.
The steady pedestal paradigm started with a 1 min adaptation to the background luminance followed by a 5 s adaptation to a pedestal (the 2 × 2 array of squares). Then a single two-Down-one-Up staircase procedure was used to determine a contrast discrimination threshold at each pedestal luminance. During the staircase procedure, each trial started with 1.2 s adaptation to the pedestal luminance then followed by a 33.3 ms test interval (Figure 1, top panel), in which one square was randomly chosen to have luminance incremented and to serve as the test square while the other three served as the reference squares. Participants were required to make a 4-alternative forced choice to identify which square differed from the other three by pressing one of four buttons on a gamepad. The change in test square luminance relative to the pedestal luminance was determined by the step size (in log units) of the staircase. The staircase started with an easily discriminable test square luminance increment, a 0.2 log unit step size (test square luminance 58.5% higher than the pedestal luminance). Whenever a reversal occurred, that is, the moving direction of the staircase changed, the step size (in log units) was halved until a minimum step size of 0.03125 log units (equivalent to 1.07% higher than the pedestal luminance) was reached. The staircase procedure continued until five reversals at the minimum step size occurred. The average value of the last three reversals was taken as the discrimination threshold for that pedestal luminance. For the steady pedestal paradigm, five pedestal luminances were used: 15.0, 18.9, 23.8, 29.9, and 37.7 cd/m2, leading to Weber contrasts of 0%, 26%, 58%, 100%, and 151%, respectively. The order of the five pedestal luminances was randomized.
The staircase procedures to determine discrimination thresholds for the pulsed-pedestal paradigm and the pedestal-Δ-pedestal paradigm were the same as for the steady-pedestal paradigm. For the pulsed pedestal paradigm, there was no adaptation to the pedestal luminance. The four-squares, with three of the squares having the pedestal luminance and the test square having a higher luminance, was presented only during the test interval for 33.3 ms (Figure 1, middle panel). Discrimination thresholds were determined at four pedestal luminances: 18.9, 23.8, 29.9, and 37.7 cd/m2. For the pedestal-Δ-pedestal paradigm, the four-square array appeared at a fixed luminance at 23.8 cd/m2 (pedestal luminance) at the beginning of each staircase procedure. During the test interval (33.3 ms) of each trial, the three reference squares were presented at the pedestal-Δ-pedestal luminance; while the test square had higher luminance than the other three squares (Figure 1, bottom panel). To make the task easier, after the test interval all 4 squares remained at the pedestal-Δ-pedestal luminance for an additional 167 ms before changing back to the pedestal luminance of 23.8 cd/m2 (Sun, Swanson, Arvidson, & Dul, 2008). For the pedestal-Δ-pedestal paradigm, thresholds were measured at four pedestal-Δ-pedestal luminances: 24.3, 24.9, 25.5, and 26.1 cd/m2.
The three paradigms were tested in the same order starting with steady-pedestal, then pulsed-pedestal and finally pedestal-Δ-pedestal paradigm, such that each paradigm was tested at about the same time after beverage drinking for all participants. The adaptation (surround) luminance was the same for the three paradigms; thus the choice of a fixed or random order would not alter adaptation.
Models for contrast detection and discrimination threshold
Based on physiological findings in primates, the contrast response characteristics of a MC or PC cell can be described by a Michaelis-Menten saturation function (Kaplan & Shapley, 1986):
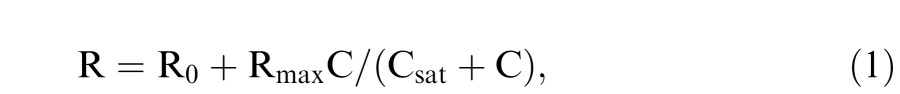 |
where R0 represents the resting response rate after adapting to a steady stimulus field, Rmax represents the maximum response rate of a cell, Csat is the contrast level at which the response reaches half of Rmax, and C is the contrast of the stimulus. Contrast gain is defined as the derivative of Equation 1 at zero contrast; that is, Rmax/Csat. In primates, MC cells have much higher contrast gain than PC cells do (Kaplan & Shapley, 1986) and show response saturation at relatively low achromatic contrasts whereas PC cell responses are relatively linear across a wide range of contrasts. This difference in contrast response characteristics is the theoretical foundation of the psychophysical assessment of contrast sensitivity and gain in the MC and PC pathways using the steady or pulsed pedestal paradigms (Pokorny, 2011; Pokorny & Smith, 1997).
For the pulsed pedestal paradigm, the background luminance is B and the pedestal luminance is P such that the pedestal has a contrast of C [= (P-B)/B] from background. The test square has a luminance of P*(1+ΔC). The contrast discrimination threshold is defined as the value of ΔC such that the differential responses with pedestal alone [luminance: P] and test [luminance: P*(1+ΔC)] to reach a criterion δ (Pokorny & Smith, 1997; Pokorny, 2011). Typically, the discrimination threshold is expressed as luminance; that is, ΔL = P*ΔC (“L” for luminance). Therefore, ΔL can be modeled using an alternative form derived from Equation 1 for the pulsed pedestal paradigm as follows:
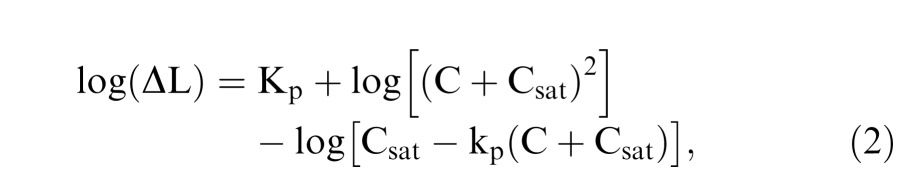 |
where Kp is the vertical scaling constant in logarithmic units, representing the absolute thresholds from the pulsed-pedestal tasks, which indicate the contrast sensitivities for the inferred PC pathways. Csat is related to contrast gains for the inferred PC pathways. kp represents the criterion parameter that is equivalent to δ/Rmax, which is typically very small. Therefore the term log[Csat − kp(C + Csat)] ≈ log[Csat] in Equation (2). Since there were only four data points for each participant, to reduce the number of free parameters, a simplified version of Equation (2) was used to fit the pulsed-pedestal data:
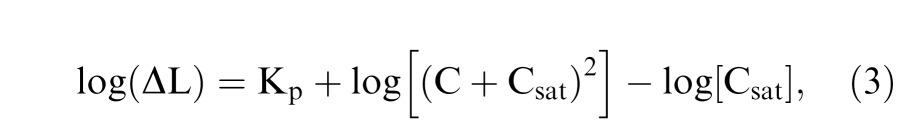 |
where Kp and Csat were free parameters.
For the pedestal-Δ-pedestal paradigm, suppose the pedestal-Δ-pedestal luminance is PdP. Then the contrast in the pedestal-Δ-pedestal paradigm C is computed as C = (PdP − P)/P. The model for the pedestal-Δ-pedestal paradigm has the same form as that for the pulsed pedestal paradigm:
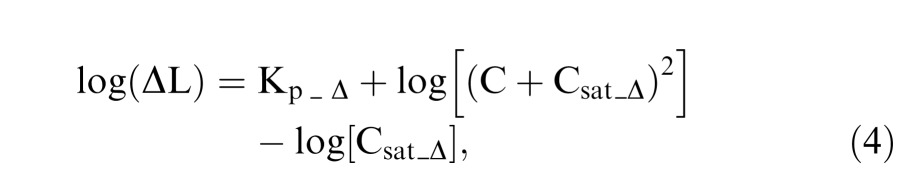 |
where Kp_Δ represents contrast sensitivity and Csat_Δ related to contrast gain for the inferred MC pathway.
For the steady-pedestal condition, the threshold data are described by Weber's law, that is, the slope of Threshold Versus Radiance (TVR) function is 1:
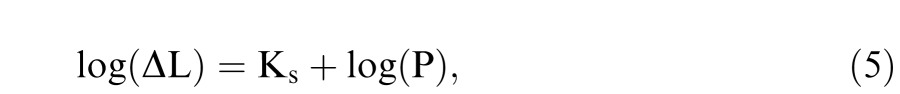 |
where Ks is the vertical scaling constant that represents the absolute contrast sensitivity mediated by the inferred MC pathway.
Data analysis
Each participant's pre- and postbeverage staircases during the alcohol and placebo sessions were inspected to assess the reliability of the threshold measurements. We checked the standard deviation of the last three reversals that were used for the threshold measurement and did not find significant differences in the standard deviations between pre- and postbeverage tests, and between the two sessions, suggesting the staircase measurement was equally reliable in all test conditions. Additionally, for any contrast discrimination function, the threshold at a contrast was ruled as an outlier if inclusion resulted in an unusually large standardized residual (the ratio of the residual for an observation over the average residual from the model fits using the remaining data points for the same contrast discrimination function ≥2.0) or an unusual large or small fitted parameter (≥2.0 standard deviations away from the group mean of the parameter without including the participant for the same testing condition). Further, it was expected that for the same session, the differences in post- and prebeverage thresholds as a function of contrast would form a monotonic pattern. Therefore, if a postbeverage threshold at one contrast was much lower than the prebeverage value at that contrast, while at other contrasts the postbeverage values were higher or at least similar to the prebeverage values, then the data point was ruled as an outlier. These outliers were excluded from final model fits.
Each participant's pre- and postbeverage threshold data from the alcohol and placebo sessions were independently fitted to the models described in Equations 3, 4, and 5, and five fitted parameters were obtained: Kp, Csat, Kp_Δ, Csat_Δ, and Ks for each testing condition. Among the five fitted parameters obtained, −Kp and −log(Csat) are linearly related to the PC contrast sensitivity and contrast gain, whereas, −Ks (and −Kp_Δ) and −log(Csat_Δ) are linearly related to the MC contrast sensitivity and contrast gain. Therefore, −Kp, −log(Csat), −Ks, −Kp_Δ, and −log(Csat_Δ) were independently analyzed. For each participant and each parameter; the difference between the post- and prebeverage values were computed separately for the alcohol [(Post − Pre)A] and placebo [(Post − Pre)P] sessions. To be more conservative, we used the Wilcoxon matched-pairs signed-rank test, a non-parametric counterpart of paired t tests to compare (Post − Pre)A and (Post − Pre)P. Non-parametric Spearman correlation was used to investigate the association between the net alcohol effect [(Post − Pre)P − (Post − Pre)A] on each parameter and BrAC level. A positive value of this net alcohol effect would indicate worse performance under alcohol than under placebo.
Results
We examined each participant's discrimination thresholds from the pre- and postbeverage testing conditions in both sessions. As an example of an individual participant's data, Figure 2 shows the contrast discrimination thresholds [expressed in log(ΔL)] as a function of log pedestal luminance, log(P), or pedestal-Δ-pedestal luminance, log(PdP), and the model fits from the pre- (open symbols) and postbeverage (filled symbols) measurements in the placebo session from one participant. The data for this participant show typical patterns as those of Pokorny and Smith (1997). That is, the discrimination thresholds from the steady-pedestal paradigm could be described by a straight line with a slope of 1, following Weber's law. The thresholds from the pulsed-pedestal paradigm increased with pedestal contrast but formed a curve that had a steeper slope than that from the steady-pedestal paradigm. The threshold function from the pedestal-Δ-pedestal paradigm had the steepest slope among the three paradigms, suggesting higher contrast gain in the inferred MC pathway than in the PC pathway.
Figure 2.
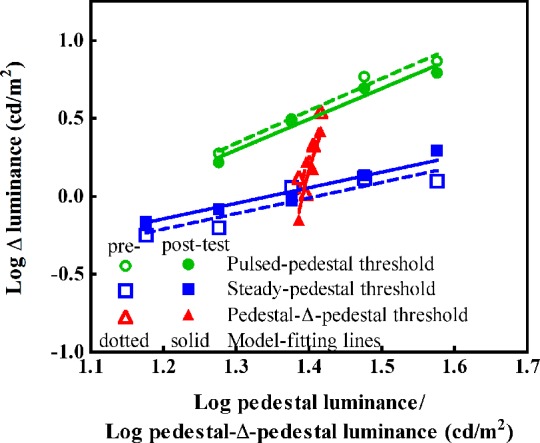
The discrimination thresholds (in log luminance) and the model fits from one participant's pre- and postbeverage testing condition during the placebo session. The abscissa is log pedestal luminance for steady (squares) or pulsed (circles) pedestal paradigm, or log pedestal-Δ-pedestal luminance for the pedestal-Δ-pedestal paradigm (triangles). Open symbols represent data from the prebeverage condition and filled symbols show data from the postbeverage condition. The dashes and lines show the model fits for the pre- and postbeverage data, respectively. The steady pedestal data could be described well by Weber's law with a slope of 1 in the log-log plot.
Contrast detection and discrimination threshold function
Figure 3 shows the individual threshold function during the placebo (Figure 3a) and alcohol (Figure 3b) sessions. For each paradigm, the pre- and postbeverage thresholds during the placebo session were very similar (Figure 3a) for all participants, suggesting a minimal or weak placebo effect. Further, the majority of the participants showed typical threshold patterns as those of Pokorny and Smith (1997), indicating that (a) our staircase parameters determined empirically to minimize the test time were reliable for threshold determination, and (b) using a fixed test order for the three paradigm did not disrupt the threshold pattern. For a few participants, the slopes of the pulsed-pedestal were not much steeper than that of the steady-pedestal but were still in the range of the slopes obtained from PC cell contrast response function (Kaplan & Shapley, 1986).
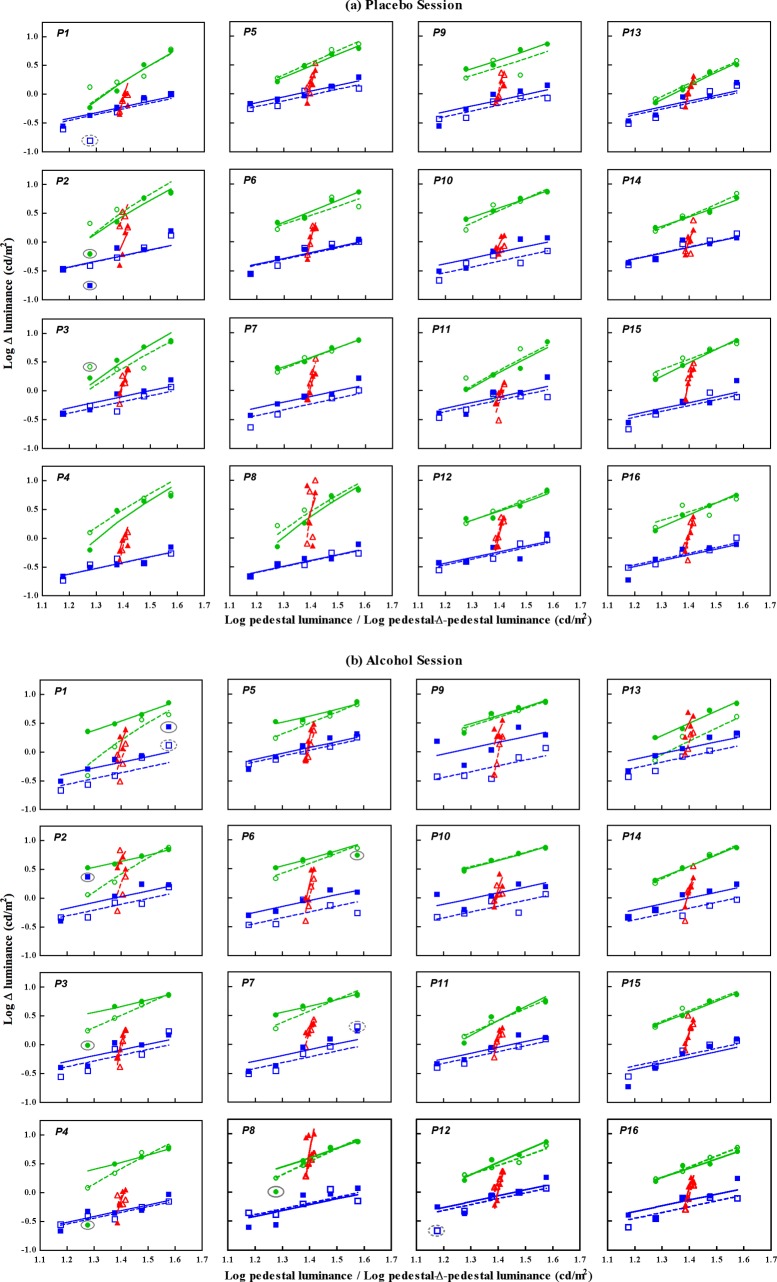
Figure 3.
Individual contrast detection and discrimination thresholds and model fits from the pre- and postbeverage testing conditions in the (a) placebo sessions and (b) alcohol sessions. Each panel has the same format as Figure 2. The individual data are presented in an order based on the pattern and magnitude of the slope change in the pulsed-pedestal function in the alcohol session. The first two columns show the individual data that are consistent with the account of increase in neural noise, and the panels are ordered by the change magnitude from large to small. The last two columns show the individual data with smaller or minimal change. The data points encircled in the solid (for the postbeverage session) and dashed (for the prebeverage session) ovals were excluded from model fits for the reason described in the Methods section.
The data during the alcohol session are shown in Figure 3b. There were individual differences when comparing the pre- and postbeverage results. Compared with prebeverage data, the postbeverage threshold for the steady pedestal paradigm was elevated for the majority of the participants. For the pulsed pedestal paradigm, eight participants (P1–P8) showed a much shallower postbeverage curve than prebeverage curve. The data for the pedestal-Δ-pedestal paradigm were elevated in those participants who showed elevated steady pedestal thresholds, but the slope of the curves were similar between the pre- and postbeverage measurements.
Contrast sensitivity and contrast gain parameters
The dashed and solid lines in Figure 3 are the models' fits based on Equations 3, 4, and 5. There were 13 data points (four in placebo sessions and nine in alcohol sessions, as shown in the solid or dashed ovals in Figure 3) considered as outliers, among which, seven measurements from the steady pedestal paradigm had high standardized residuals (P1, preplacebo, prealcohol, and postalcohol; P2, postplacebo and postalcohol; P7, postalcohol; and P12, prealcohol); three were due to the fitted parameters too high or too low for the pulsed pedestal paradigm (P2, postplacebo; P3, preplacebo; P6, postalcohol), and the remaining three had nonmonotonic pattern in the difference of the post- versus premeasurements for the pulsed pedestal paradigm (P3, postalcohol; P4, postalcohol; P8, postalcohol). Figure 4 plots the change in contrast sensitivity and gain parameters with outliers excluded between the post- and prebeverage values between the two sessions [(Post − Pre)A vs. (Post − Pre)P] for all of the participants.
Figure 4.
The within-subject difference [(Post − Pre)A vs. (Post − Pre)P] in the contrast sensitivity (left column: panel a for MC sensitivity and b for PC sensitivity) and gain parameters (right column: panel c for MC contrast gain and d for PC contrast gain), in the inferred MC (first row) and PC (second row) pathways. In each panel, open circles represent individual values. The square represents the mean values during the two sessions with the error bars for 95% confidence interval (95% CI). The diagonal line indicates equal change in the fitted parameters in the alcohol and placebo sessions. The shaded area represents the 95%CI of (Post − Pre)P. Data points below the shaded area as well as the diagonal line are considered to show an alcohol impairment effect; those above the shaded area and diagonal line are considered to have an improvement from alcohol; other data points are considered to be equal between placebo and alcohol session, that is, no alcohol effect. Based on the criteria, the numbers of participants who showed impairment (-), no effect ( = ) or improvement (+) from alcohol with different parameters are shown in each panel.
The MC and PC contrast sensitivity parameters are shown in Figure 4a and 4b. Compared to placebo beverage, alcohol significantly reduced MC sensitivity parameters [(Post − Pre)A vs. (Post − Pre)P for −Ks from the steady-pedestal paradigm: z = −2.38, p = 0.017, see Figure 4a; or −Kp_Δ from the pedestal-Δ-pedestal paradigm: z = −2.87, p = 0.004, fitted parameter not shown]. The 95% confidence interval for the mean beverage effect during both placebo and alcohol sessions did not cover 0 (Figure 4a), suggesting a significant placebo effect as well as alcohol effect, but the reduction in the MC sensitivity in the placebo was smaller than that in the alcohol session. The inspection of individual (Post − Pre)A relative to the 95% CI of the (Post − Pre)P as well as individual placebo effect indicates that eight participants showed alcohol impairment relative to placebo while two participants showed improvement in MC contrast sensitivity, and six had no alcohol effect. These results suggested that for the majority of participants there was a significant loss of sensitivity in the MC pathway after acute alcohol consumption. By contrast, the PC sensitivity parameters (−Kp) from the pulsed-pedestal paradigm were not significant [z = −0.879, p = 0.379; see Figure 4b], with four participants showing alcohol impairment while all remaining 12 participants were covered by the 95% CI of (Post − Pre)P. The net alcohol effect [(Post − Pre)P − (Post − Pre)A] on the MC and PC contrast sensitivity did not correlate with each other (ρ = −0.009, p = 0.974), suggesting alcohol modified MC and PC pathway function independently. BrAC level was not significantly correlated with net alcohol effect on MC contrast sensitivity or PC contrast sensitivity (MC: ρ = −0.409, p = 0.118; PC: ρ = −0.371, p = 0.157.
The MC and PC contrast gain parameters are shown in Figures 4c and 4d. Alcohol and placebo produced comparable effects on −log(Csat_Δ) measured from the pedestal-Δ-pedestal paradigm [z = 0.931, p = 0.352; see Figure 4c]. Inspection of individual data indicate that four participants showed an alcohol impairment effect but alcohol improved contrast gain for six participants, indicating large individual differences in the alcohol effect on MC contrast gain. Compared to placebo beverage, alcohol significantly reduced −log(Csat) measured from the pulsed-pedestal paradigm [z = −2.534, p = 0.011; see Figure 4d]. Eleven participants showed alcohol impairment on PC contrast gain, one participant showed improvement effect, and four had no effect. The net alcohol effect [(Post − Pre)P − (Post − Pre)A] on MC and PC contrast gain did not correlate (ρ = −0.088, p = 0.745), again indicating that alcohol affected the MC and PC pathway independently. BrAC level did not significantly correlate with net alcohol effect on MC or PC contrast gain (MC: ρ = −0.377, p = 0.150; PC: ρ = −0.363, p = 0.168).
Discussion
Using paradigms developed by Pokorny and Smith (1997) to investigate inferred MC and PC pathway function separately, we showed that, compared to the placebo session, alcohol produced differential effects on contrast processing mediated by the inferred MC and PC pathways. Specifically, for the majority of participants, alcohol reduced MC mediated contrast sensitivity and PC mediated contrast gain. On the other hand, for the majority of participants, alcohol did not reduce PC mediated contrast sensitivity. For MC mediated contrast gain, there were large individual differences in alcohol effects, with some participants showing contrast gain impaired, improved, or having no effect by alcohol intake. A potential concern might be that the fixed testing order for the three paradigms could alter results because of alcohol expectancy effects, i.e., changes in behavior based on the belief that one has consumed alcohol. While we cannot rule out such effects completely, they were at least minimized in that participants were unaware of the sequence of the alcohol and placebo sessions and the same testing order was used in both pre- and postbeverage phase within a session. Statistical analyses were repeated controlling for session order (receiving alcohol in the first or second session), and showed equivalent main results. Other work by our group has shown that subjective and psychomotor effects of alcohol did not differ in persons who correctly identified alcohol versus those who did not; nor did effects differ in those who believed the placebo contained an active substance versus those who guessed it was placebo (Conrad, McNamara, & King, in press). A manipulation check was not undertaken in the present study; and since the intoxicating dose of alcohol does produce mood and behavioral changes in some participants, whether or not different levels of expectancy may affect alcohol effect on behavior such as contrast processing, mediated by the early stage of the visual system, remains an empirical question. In particular, given that contrast gain is mainly mediated by retinal processing, it is unlikely that expectancy could change contrast gain, as was observed for the Pulsed-Pedestal data.
It has been proposed that alcohol may selectively impair the MC but not the PC pathway function (Hill & Toffolon, 1990; Khan & Timney, 2007b). Our results indicate that alcohol may selectively impair MC mediated contrast sensitivity, but not contrast gain. Other studies also reported the spatial contrast sensitivity for high spatial frequencies, which are inferred to be mediated by the PC pathway, may be reduced by alcohol intake (Andre et al., 1994; Nicholson et al., 1995; Pearson & Timney, 1998, 1999b; Roquelaure et al., 1995; Zulauf et al., 1988). These results and our current results suggest that the selective impairment on the MC pathway hypothesis is not complete.
Potential locus for alcohol effects
Contrast gain originates in the retina, likely at the bipolar cell layer (Beaudoin, Borghuis, & Demb, 2007; Burkhardt, 2011). If cells were to produce smaller changes in spike density to a stimulus contrast change, the measured contrast response function would be shallower and contrast sensitivity would be reduced. Our data shows alcohol does not initiate concomitant changes in contrast gain and sensitivity. Rather, two distinct patterns emerge. For the MC pathway, there was a sensitivity loss not accompanied by a loss in contrast gain. The pattern of a decrease in contrast sensitivity with unaltered contrast gain could be caused by a decrease of quantum efficiency, an increase in phototransduction noise in photoreceptors, a change of sensory information processing efficiency at postphotoreceptor sites, or alteration of the decision making criterion in the cortex (Pokorny, 2011). If the change of contrast sensitivity was due to alteration of the quantum efficiency or phototransduction noise in photoreceptors, one ought to expect a similar influence on both PC and MC pathway function. The dissimilar effects of alcohol ingestion on sensitivity in the two pathways points to a postreceptoral stage, likely at cortical level in visual processing, as the locus of the loss in MC mediated contrast sensitivity.
For the PC pathway, alcohol produced a shallowing effect of the contrast gain function with no loss of sensitivity. A reduction in contrast gain could result from an increase in postretinal neural noise or response compression (Pokorny, 2011). In principle, both noise and response compression subsequent to the generation of retinal contrast gain can alter measured contrast gain, but the sensitivity profiles would be different. Noise lowers sensitivity near the adapting luminance while leaving discrimination for large contrast steps unimpaired, whereas response compression may leave sensitivity near the adapting luminance unimpaired while decreasing threshold sensitivity for large contrast steps. The results from 8 out of the 16 participants in the current study (Figure 3b, columns 1 and 2) follow the pattern expected for an increase in neural noise, altering sensitivity at the adapting light level as well as contrast gain for the PC pathway. However, because the signal-to-noise ratio for low contrast pedestals is poorer than that for high contrast pedestals, an increase of neural noise would have a larger impact on discrimination for low contrast pedestals than for higher contrast pedestals, resulting in a shallower contrast gain slope. In cats, it is also reported that alcohol intake worsens the signal-to-noise ratio in visual cortex area 17 neurons (Chen, Xia, Li, & Zhou, 2010).
In the present study, we demonstrated that alcohol affected visual information processing in both the PC and MC pathways but in different ways. Interestingly, these results are very similar to what we obtained using similar paradigms in some patients with optic neuritis, an inflammation of the optic nerve (Cao et al., 2011). It has been suggested that the reduced contrast sensitivity for low spatial frequency grating observed among alcohol dependent participants may be attributed to a toxic effect of alcohol on the optic nerve (Roquelaure et al., 1995). Given this, it is possible that acute alcohol may produce impairment effects on the optic nerve similar to those of optic neuritis, limiting information transfer from retina to cortex. The findings of the current study may provide further insights into the mechanisms underlying other higher order visual functional losses after acute alcohol consumption and impairment of coherent motion perception and speed discrimination (Wegner, Günthner, & Fahle, 2001).
Acknowledgments
Supported by grants: ABMRF/Foundation for Alcohol Research (D. Cao), National Eye Institute R01-EY019651 (D. Cao), P30-EY01792 (UIC core grant for vision research), National Institute on Alcohol Abuse and Alcoholism R01-AA013746 (A. King).
Commercial relationships: none.
Corresponding author: Dingcai Cao.
Email: dcao98@uic.edu.
Address: Department of Ophthalmology & Visual Sciences, The University of Illinois at Chicago, Chicago, IL, USA.
Contributor Information
Xiaohua Zhuang, Email: xzhuang@uic.edu.
Andrea King, Email: aking@yoda.bsd.uchicago.edu.
Patrick McNamara, Email: pmcnamar@yoda.bsd.uchicago.edu.
Joel Pokorny, Email: j-pokorny@uchicago.edu.
Dingcai Cao, Email: dcao98@uic.edu.
References
- Andre J. T. (1996). Visual functioning in challenging conditions: Effects of alcohol consumption, luminance, stimulus motion, and glare on contrast sensitivity. Journal of Experimental Psychology: Applied , 2(3), 250–269 [Google Scholar]
- Andre J. T., Tyrrell R. A., Leibowitz H. W., Nicholson M. E., Wang M. (1994). Measuring and predicting the effects of alcohol consumption on contrast sensitivity for stationary and moving gratings. Attention, Perception, & Psychophysics , 56(3), 261–267 [DOI] [PubMed] [Google Scholar]
- Beaudoin D. L., Borghuis B. G., Demb J. B. (2007). Cellular basis for contrast gain control over the receptive field center of mammalian retinal ganglion cells. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience , 27(10), 2636–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumback T., Cao D. C., King A. (2007). Effects of alcohol on psychomotor performance and perceived impairment in heavy binge social drinkers. Drug and Alcohol Dependence , 91(1), 10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt D. A. (2011). Contrast processing by ON and OFF bipolar cells. Visual Neuroscience , 28(1), 69–75 [DOI] [PubMed] [Google Scholar]
- Campbell F., Kulikowski J. (1966). Orientational selectivity of the human visual system. The Journal of Physiology , 187(2), 437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D., Zele A. J., Pokorny J., Lee D. Y., Messner L. V., Diehl C., et al. (2011). Functional loss in the magnocellular and parvocellular pathway in patients with optic neuritis. Investigative Ophthalmology and Visual Science , 52: 8900–8907, http://www.iovs.org/content/52/12/8900. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., Xia J., Li G., Zhou Y. (2010). The effects of acute alcohol exposure on the response properties of neurons in visual cortex area 17 of cats. Toxicology and Applied Pharmacology , 243(3), 348–358 [DOI] [PubMed] [Google Scholar]
- Conrad M. C., McNamara P. J., King A. C. (in press). The alternative substance paradigm: Effectiveness of beverage blinding and effects on acute alcohol responses. Experimental and Clinical Psychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crego A., Holguín S. R., Parada M., Mota N., Corral M., Cadaveira F. (2009). Binge drinking affects attentional and visual working memory processing in young university students. Alcoholism: Clinical and Experimental Research , 33(11), 1870–1879 [DOI] [PubMed] [Google Scholar]
- Dacey D. M. (2000). Parallel pathways for spectral coding in primate retina. Annual Review of Neuroscience , 23, 743–775 [DOI] [PubMed] [Google Scholar]
- Donnelly M., Miller R. (1995). Ingested ethanol and binocular rivalry. Investigative Ophthalmology & Visual Science , 36(8): 1548–1554, http://www.iovs.org/content/36/8/1548. [PubMed] [Article] [PubMed] [Google Scholar]
- Foley J. M., Legge G. E. (1981). Contrast detection and near-threshold discrimination in human vision. Vision Research , 21, 1041–1053 [DOI] [PubMed] [Google Scholar]
- George S., Rogers R. D., Duka T. (2005). The acute effect of alcohol on decision making in social drinkers. Psychopharmacology , 182(1), 160–169 [DOI] [PubMed] [Google Scholar]
- Hill J. C., Toffolon G. (1990). Effect of alcohol on sensory and sensorimotor visual functions. Journal of Studies on Alcohol , 51(2), 108–113 [DOI] [PubMed] [Google Scholar]
- Kaplan E. (2004). The m, p, and k pathways of the primate visual system. In Chalupa L. M., Werner J. S. (Eds.), The visual neuroscience (Vol. 1, pp 481–493) Cambridge, MA: MIT Press [Google Scholar]
- Kaplan E., Lee B. B., Shapley R. M. (1990). New views of primate retinal function. In Osborne N., Chader J. (Eds.), Progress in retinal research (Vol. 9, pp 273–336) Oxford: Pergamon Press [Google Scholar]
- Kaplan E., Shapley R. M. (1986). The primate retina contains two types of ganglion cells, with high and low contrast sensitivity. Proceedings of the National Academy of Sciences of the United States of America , 83, 2755–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S. A., Timney B. (2007a). Alcohol does not affect dark adaptation or luminance increment thresholds. Journal of Studies on Alcohol and Drugs , 68(4), 493–502 [DOI] [PubMed] [Google Scholar]
- Khan S. A., Timney B. (2007b). Alcohol slows interhemispheric transmission, increases the flash-lag effect, and prolongs masking: Evidence for a slowing of neural processing and transmission. Vision Research , 47(13), 1821–1832 [DOI] [PubMed] [Google Scholar]
- King A. C., de Wit H., McNamara P. J., Cao D. (2011). Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Archives of General Psychiatry , 68(4), 389–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingdom F. A. A., Whittle P. (1996). Contrast discrimination at high contrasts reveals the influence of local light adaptation on contrast processing. Vision Research , 36, 817–829 [DOI] [PubMed] [Google Scholar]
- Kirchner T. R., Sayette M. A. (2003). Effects of alcohol on controlled and automatic memory processes. Experimental and Clinical Psychopharmacology , 11(2), 167–175 [DOI] [PubMed] [Google Scholar]
- Lee B. B., Martin P. R., Valberg A. (1989). Sensitivity of macaque retinal ganglion cells to chromatic and luminance flicker. Journal of Physiology (London) , 414, 223–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. B., Pokorny J., Smith V. C., Martin P. R., Valberg A. (1990). Luminance and chromatic modulation sensitivity of macaque ganglion-cells and human observers. Journal of the Optical Society of America a-Optics Image Science and Vision , 7(12), 2223–2236 [DOI] [PubMed] [Google Scholar]
- Livingstone M. S., Hubel D. H. (1987). Psychophysical evidence for separate channels for the perception of form, color, motion and depth. Journal of Neuroscience , 7, 3416–3468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M. S., Hubel D. H. (1988). Segregation of form, color, movement and depth: Anatomy, physiology and perception. Science , 240, 740–749 [DOI] [PubMed] [Google Scholar]
- Nicholson M. E., Andre J. T., Tyrrell R. A., Minqi W., Leibowitz H. W. (1995). Effects of moderate dose alcohol on visual contrast sensitivity for stationary and moving targets. Journal of Studies on Alcohol , 56(3), 261–266 [DOI] [PubMed] [Google Scholar]
- Pearson P., Timney B. (1998). Effects of moderate blood alcohol concentrations on spatial and temporal contrast sensitivity. Journal of Studies on Alcohol , 59(2), 163–173 [DOI] [PubMed] [Google Scholar]
- Pearson P., Timney B. (1999a). Alcohol does not affect visual contrast gain mechanisms. Visual Neuroscience , 16(4), 675–680 [DOI] [PubMed] [Google Scholar]
- Pearson P., Timney B. (1999b). Differential effects of alcohol on rod and cone temporal processing. Journal of Studies on Alcohol , 60(6), 879–883 [DOI] [PubMed] [Google Scholar]
- Pokorny J. (2011). Review: Steady and pulsed pedestals, the how and why of postreceptoral pathway separation. Journal of Vision, 11(5):7 , 1–23, http://journalofvision.org/11/5/7, doi:10.1167/11.5.7. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Pokorny J., Smith V. C. (1997). Psychophysical signatures associated with magnocellular and parvocellular pathway contrast gain. Journal of the Optical Society of America A , 14, 2477–2486 [DOI] [PubMed] [Google Scholar]
- Roquelaure Y., Le Gargasson J., Kupper S., Girre C., Hispard E., Dally S. (1995). Alcohol consumption and visual contrast sensitivity. Alcohol and Alcoholism , 30(5), 681–685 [PubMed] [Google Scholar]
- Saults J. S., Cowan N., Sher K. J., Moreno M. V. (2007). Differential effects of alcohol on working memory: Distinguishing multiple processes. Experimental and Clinical Psychopharmacology , 15(6), 576–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield W. N. (1985). Predicting basal metabolic rate, new standards and review of previous work. Human Nutrition . Clinical Nutrition , 39 (Supple. 1), 5–41 [PubMed] [Google Scholar]
- Shapley R. M., Victor J. D. (1979). The contrast gain control of the cat retina. Vision Research , 19, 431–434 [DOI] [PubMed] [Google Scholar]
- Smith V. C., Pokorny J. (2003). Psychophysical correlates of Parvo- and Magnocellular function. In Mollon J., Pokorny J., Knoblauch K. (Eds.), Normal and defective colour vision (pp 91–107) Oxford: Oxford University Press [Google Scholar]
- Sun H., Swanson W. H., Arvidson B., Dul M. W. (2008). Assessment of contrast gain signature in inferred magnocellular and parvocellular pathways in patients with glaucoma. Vision Research , 48(26), 2633–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson W. H., Wilson H. R., Giese S. C. (1984). Contrast matching data predicted from contrast thresholds. Vision Research , 24, 63–75 [DOI] [PubMed] [Google Scholar]
- Watson P., Watson I., Batt R. (1980). Total body water volumes for adult males and females estimated from simple anthropometric measurements. The American Journal of Clinical Nutrition , 33(1), 27–39 [DOI] [PubMed] [Google Scholar]
- Watten R. G., Lie I. (1996). Visual functions and acute ingestion of alcohol. Ophthalmic and Physiological Optics , 16(6), 460–466 [PubMed] [Google Scholar]
- Wegner A. J., Günthner A., Fahle M. (2001). Visual performance and recovery in recently detoxified alcoholics. Alcohol and Alcoholism , 36(2), 171–179 [DOI] [PubMed] [Google Scholar]
- Whittle P. (1992). Brightness, discriminability and the “crispening effect”. Vision Research , 32(8), 1493–1507 [DOI] [PubMed] [Google Scholar]
- Zele A. J., Wood J. M., Girgenti C. C. (2010). Magnocellular and parvocellular pathway mediated luminance contrast discrimination in amblyopia. Vision Research , 50(10), 969–976 [DOI] [PubMed] [Google Scholar]
- Zoethout R. W. M., Delgado W. L., Ippel A. E., Dahan A., van Gerven J. M. A. (2011). Functional biomarkers for the acute effects of alcohol on the central nervous system in healthy volunteers. British Journal of Clinical Pharmacology , 71(3), 331–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulauf M., Flammer J., Signer C. (1988). Short-term influence of alcohol on spatial brightness contrast sensitivity. Ophthalmologica , 197(3), 159–165 [DOI] [PubMed] [Google Scholar]