Abstract
Background
While the impact of active maternal smoking during pregnancy on child health has been well investigated, the association between maternal passive smoking, or environmental tobacco smoke (ETS), or second-hand smoke, and behavioral development of offspring is less clear. This study examines the association between maternal ETS exposure during pregnancy and child behavior problems.
Methods
Cross-sectional data of 646 mother–child pairs from the Jintan China Cohort Study were used in the analyses. Mother’s exposure to tobacco smoking at home, the workplace, and other places during pregnancy (for the determination of maternal ETS exposure) and children’s behaviors (via Child Behavior Checklist) were assessed when the children were 5–6 years old. Logistic regression models were constructed to examine associations between maternal exposure to ETS during pregnancy and internalizing and externalizing behavior problems, adjusting for potential cofounders including child sex and parental characteristics.
Results
37% of mothers reported ETS during pregnancy. Children of mothers exposed to ETS during pregnancy had higher scores for externalizing and total behavior problems, with 25% of children whose mothers were exposed to ETS compared to 16% of children of unexposed mothers. After adjusting for potential confounders, ETS exposure was associated with a higher risk of externalizing behavior problems in offspring of exposed mothers (OR = 2.08, 95% confidence interval [CI] 1.27–3.43). Analysis after multiple imputations and sensitivity analysis further verified the association, but no dose–response relationship was found. ETS exposure, however, was not associated with internalizing or total behavior problems.
Conclusion
This study suggests that maternal ETS exposure during pregnancy may impact child behavioral development, particularly externalizing behaviors.
Keywords: Environmental tobacco exposure, Second-hand smoke, Child behavior problem, Externalizing behavior, Pregnancy exposure
1. Introduction
According to the World Health Organization, the number of women who are smokers is growing rapidly, with a projected prevalence of 20% by 2025 (compared to only 12% in 2005) (The World Health Organization, 2007). Pregnancy is a critical period during which tobacco exposure may impact fetal development. Experimental studies have consistently shown histopathologic changes in the fetus from maternal smoking, particularly in the lungs and brain (Centers for Disease Control and Prevention, 2010). The changes in the brain are associated with adverse neurodevelopmental outcomes. Epidemiologic studies suggest maternal active smoking (i.e. direct intake of tobacco through cigarettes) during pregnancy is associated with child behavior problems, such as attention deficit and hyperactivity disorder (ADHD) (Button et al., 2005; Fergusson et al., 1993; Hutchinson et al., 2010; Kotimaa et al., 2003; Linnet et al., 2005; Milberger et al., 1996, 1998; Tiesler et al., 2011), conduct problems (Fergusson et al., 1993; Wakschlag et al., 1997, 2006), aggression, and other externalizing behaviors (Boutwell et al., 2011; Brook et al., 2006; Fergusson et al., 1998; Huijbregts et al., 2008; Orlebeke et al., 1997, 1999), as well as adult criminal behavior (Brennan et al., 1999). One possible mechanism for such an association involves the fetus potentially experiencing brain growth retardation, which may result in hypoxia or anoxia through pregnancy and birth complications. It is known that hypoxia selectively damages the hippocampus, which brain-imaging research indicates is one component involved in aggression regulation (Raine et al., 2004; Liu, 2011). Furthermore, neurological deficits may represent a potential link between maternal smoking and adverse behavioral outcomes, such as criminal behavior in adolescence and adulthood (Brennan et al., 1999; Paradis et al., 2011).
While the impact of maternal active smoking on offspring behavior is well established, the impact of maternal environmental tobacco smoking (ETS) exposure during pregnancy has been less studied despite the large number of women exposed to ETS during pregnancy (Centers for Disease Control and Prevention, 2006). ETS, also known as second hand smoking, is a form of passive smoking, which is defined as inhalation of smoke by persons other than the intended ‘active’ smoker (Centers for Disease Control and Prevention, 2009). Few studies have examined maternal ETS exposure during pregnancy and the adverse behavior outcomes in offspring, and the U.S. Centers for Disease Control and Prevention has concluded that the evidence supporting a causal relationship between is inadequate (Centers for Disease Control and Prevention, 2006). Three studies included less than 200 mother–child pairs and found that child prenatal exposure through maternal ETS exposure was related to a higher risk of aggression and externalizing behavior problems (Gatzke-Kopp and Beauchaine, 2007; Hsieh et al., 2010; Makin et al., 1991). The only other study identified, which included more than 4000 mother–child pairs, did not support the previous findings (Roza et al., 2009).
Less than 2% of women in China are smokers (Li et al., 2011), but more than 60% of nonsmoking women are exposed to ETS (Yang et al., 1999). In fact, a recent survey found that 42% of women in China were exposed to ETS during pregnancy (Fu et al., 2008). The low active smoking prevalence but high ETS exposure rate makes women in China a unique population for studying health outcomes of maternal ETS exposure. This study aims to use the data from the China Jintan Child Cohort Study (CJCCS) (Liu et al., 2010) to address the gap in the literature regarding the effects of maternal ETS exposure during pregnancy and child behavioral outcomes. Outcomes may provide much-needed clarity on this relationship and are particularly important, as tobacco exposure and child psychopathology each represent significant public health issues.
2. Methods
2.1. Study population
The current study was part of a larger population-based community cohort study of 1656 Chinese children (55.5% boys, 44.5% girls) initially recruited in the Spring of 2005 from four preschools in the city of Jintan, located in the southeastern coastal region of Mainland China. Detailed sampling and research procedures of this larger cohort study have been described elsewhere (Liu et al., 2011b, 2010). Briefly, the China Jintan Child Cohort Study is an on-going prospective longitudinal study with the main aim of assessing the early health risk factors for the development of child neurobehavioral outcomes. Institutional Review Board approval was obtained from both the University of Pennsylvania and the ethical committee for research at Jintan Hospital in China.
Children were recruited in 2005 (included children in their junior (3 years old), middle (4 years old), and senior (5 years old) levels of preschool) and had their blood tested for lead levels (Liu et al., 2012). Children were followed periodically to evaluate physical and behavioral development. Two hundred and seventy-one children dropped out of the study due to changing schools, leaving 1385 children in the later study waves. This analysis was based on cross-sectional data on mother’s ETS exposure and children’s neurobehavioral assessment during spring 2005 to spring 2007, when children were in their last year of preschool. Parents completed a questionnaire regarding child nutrition, behavior, prenatal characteristics, and family characteristics and Child Behavior Checklist for 1.5- to 5-year-olds (CBCL/1.5–5). CBCL assessments for 509 children were conducted after those children were older than 5 years, and these children were excluded from the primary analysis due to age cut-offs for CBCL/1.5–5 and for consistency with our recent publication (Liu et al., 2011a). Children of mothers who did not answer ETS exposure questions or provided incomplete ETS exposure information were not included (n = 230) in the primary analysis. Therefore, 646 mother–child pairs were included into the analysis.
2.2. Child behavior assessment
The CBCL is one of the most widely used scales for assessing behavioral and emotional problems in children and consists of 99 items which are scored on a 3-point scale ranging from “not true” (score = 0) to “often true” (score = 2) (Achenbach and Rescorla, 2000; Ivanova et al., 2010; Rescorla et al., 2011). The Chinese version of the CBCL has been validated (Leung et al., 2006; Liu et al., 2011a). The CBCL includes measures of seven syndromes: anxiety/depression, emotional reaction, withdrawn, somatic complaints, sleep problems, attention problems, and aggression. Standardized T scores were calculated from raw scores. Standardized T scores for the syndromes equal to or greater than 65 (93rd percentile of Chinese norm group) indicate the presence of behavior problems in these areas in the borderline/clinical range (Achenbach and Rescorla, 2000). The CBCL also allows the examination of two behavioral problems: internalizing behavior problems (the sum of emotional reaction, anxiety/depression, withdrawn, and somatic complaints) and externalizing behavior problems (the sum of attention problems and aggression). The total problems score is the sum score of all 99 problem items. Those children with standardized T scores greater than 60 (83rd percentile) are considered having internalizing, externalizing, or total problems in borderline and clinical range (Achenbach and Rescorla, 2000).
2.3. Maternal exposure to ETS during pregnancy
In the questionnaire, mothers were asked how many persons smoked in the home, workplace, and other places where exposure could occur, and for how many minutes she was exposed to smoke in the environment each day during pregnancy. Exposure to ETS was considered present when at least one person smoked in the environment for at least 30 min each day. Maternal prenatal and postnatal active smoking status could not be assessed due to social stigma against women’s smoking in China, especially in rural areas and small towns like Jintan. The dose of ETS exposure was categorized as no ETS exposure, <30 min, 30–60 min, and ≥60 min under any one of those three circumstances.
2.4. Covariates
The first set of covariates included parental characteristics (father’s education, father’s occupation, whether the father smoked after the child was born, mother’s age when the child was born, mother’s education, mother’s occupation, parental psychopathologic problems, and marital status). Parental education at the time the children entered the study was categorized as less than high school, high school, and college or university. Parents reported the occupation that they had held for the longest period in their lives (unemployed, general labor, technician/professional worker, and other). Fathers also reported current smoking status (no, yes but less than 10 cigarettes per day, 10–20 cigarettes per day, and over 20 cigarettes per day). Both mothers and fathers were asked to report histories of psychological problems, alcoholism, and police arrests; responses were combined to define parental psychopathological problems due to the small number of cases. We also asked maternal age at childbirth (<25, 25–30, 30+ years). Information for these covariates was collected at the same time we collected ETS exposure and children’s behavior rating. Child sex, school area, and child blood lead level were also included.
2.5. Statistical analysis
Characteristics of parents and children were compared between children of mothers with and without maternal exposure to ETS, using chi-square test for categorical variables, Wilcoxon rank sum test for blood lead levels, and t-test for standardized T scores for behavioral problems. Parent and child characteristics between children with and without externalizing behavior problems were compared. The association between ETS exposure (yes vs. no) and internalizing, externalizing, and total behavior problems (yes vs. no) was examined using logistic regression models. All potential confounders described above were included in the initial model, and the final model included child sex and school area; parental education, occupation, and psychopathologic problems; maternal age during pregnancy; and father’s current (postnatal) smoking status, using the change-in-estimate method (Rothman et al., 2008). Odds ratios (ORs) and 95% confidence intervals (95% CI) were estimated for ETS and covariates. The relationship between the risk of externalizing behavior problem and ETS dose (no exposure, <30 min, 30–60 min, and ≥60 min.) was also examined. Diagnosis of the logistic regression model did not show significant collinearity between variables. Effect modification by parental characteristics was also examined using likelihood ratio statistics (Rothman et al., 2008). Effect modification was considered potentially important if the p-value of the likelihood ratio test was <0.1.
Several sensitivity analyses were undertaken to test the robustness of the association. We included children whose CBCL assessment was completed when they were close to 6 years old (n = 509). We also tested the association by assigning mothers with missing ETS exposure status as non-exposed and exposed, respectively. Multiple imputation was also conducted and combined estimations for model parameters were calculated in SAS (MI and MIANLYZE procedures). In addition, we expanded the definition of ETS exposure to include exposure to smoke for at least 1 h daily. All analyses were conducted in SAS 9.2 (SAS Institute, Cary, NC).
3. Results
Two hundred and forty (37%) women were exposed to ETS during pregnancy (Table 1). Home and workplace are the main sources of pregnant women’s ETS exposure, with 62% and 46% of those exposed women reporting home and workplace as exposure sources, respectively. More importantly, 41% of exposed women reported home the only exposure source. Among children of mothers with ETS exposure during pregnancy, 27% attended rural schools. This proportion was higher than that of children of mothers without exposure. Eighty-four percent of husbands of ETS exposed women smoked after their children were born, compared with 72% of husbands of unexposed women. Children of mothers with ETS during pregnancy had higher (worse) scores for externalizing behavior (51.8 ± 11.1 vs. 50.5 ± 9.8, p = 0.03) and behavior problems (51.6 ± 10.9 vs. 50.2 ± 9.8, p = 0.06).
Table 1.
Comparison of behavioral problem prevalence and socio-demographic characteristics between children with and without maternal ETS exposure
| Variables | Maternal ETS exposure? |
p value | |
|---|---|---|---|
| No (n = 406) | Yes (n = 240) | ||
| Externalizing problem (mean ± SD) | 50.5 ± 9.8 | 51.8 ± 11.1 | 0.03 |
| Internalizing problem (mean ± SD) | 50.8 ± 10.1 | 51.8 ± 10.9 | 0.17 |
| Total problem (mean ± SD) | 50.2 ± 9.8 | 51.6 ± 10.9 | 0.06 |
| Blood lead level (μg/dL, median and range) | 5.9 (1.8–27.5) | 5.8 (1.9–32.0) | 0.26 |
| Sex | |||
| Boy | 217 (53.5) | 128 (53.3) | 0.97 |
| Girl | 189 (46.5) | 112 (46.7) | |
| Sibling | |||
| No | 305 (75.1) | 182 (75.8) | 0.84 |
| Yes | 101 (24.9) | 58 (24.2) | |
| School area | |||
| City | 168 (41.4) | 88 (36.7) | 0.04 |
| Suburban | 163 (40.1) | 87 (36.3) | |
| Rural | 75 (18.5) | 65 (27.1) | |
| Father’s education | |||
| Less than high school | 148 (36.5) | 93 (38.8) | 0.76 |
| High school | 124 (30.5) | 74 (30.8) | |
| College and University | 134 (33.0) | 73 (30.4) | |
| Father’s occupation | |||
| Unemployed | 11 (2.7) | 7 (2.9) | 0.61 |
| General labor | 208 (51.2) | 124 (51.7) | |
| Technician/professional | 162 (39.9) | 100 (41.7) | |
| Other | 25 (6.2) | 9 (3.8) | |
| Father smoked after the child was born | |||
| Missing | 6 (1.5) | 7 (2.9) | <0.0001 |
| No | 108 (26.6) | 32 (13.3) | |
| <10 cigarettes/day | 217 (53.4) | 132 (55.0) | |
| 10–20 cigarettes/day | 62 (15.3) | 49 (20.4) | |
| 20+ cigarettes/day | 13 (3.2) | 20 (8.3) | |
| Mother’s age when the child was born | |||
| <25 | 122 (30.0) | 76 (31.7) | 0.32 |
| 25–30 | 251 (61.8) | 152 (63.3) | |
| 30+ | 33 (8.1) | 12 (5.0) | |
| Mother’s education | |||
| Less than high school | 204 (50.3) | 100 (41.7) | 0.09 |
| High school | 109 (26.8) | 80 (33.3) | |
| College and University | 93 (22.9) | 60 (25.0) | |
| Mother’s occupation | |||
| Unemployed | 18 (4.4) | 13 (5.4) | 0.75 |
| General labor | 170 (41.9) | 97 (40.4) | |
| Technician/professional | 127 (31.3) | 82 (34.2) | |
| Other | 91 (22.4) | 48 (20.0) | |
| Parental psychopathologic problem? | |||
| No | 385 (94.8) | 226 (94.2) | 0.72 |
| Yes | 21 (5.2) | 14 (5.8) | |
| Parental divorce | |||
| Missing | 20 (4.9) | 13 (5.4) | 0.84 |
| No | 369 (90.9) | 219 (91.3) | |
| Yes | 17(4.2) | 8 (3.3) | |
SD, standard deviation; numbers in parenthesis are percentages for categorical variables and range for blood lead level.
Overall, 127 (20%) children had externalizing behavior problem scores in the borderline/clinical range, 18% had internalizing problem scores in the borderline/clinical range, and 19% had total problem scores in the borderline/clinical range. As shown in Fig. 1, children of mothers with ETS exposure had a higher prevalence (25%) of externalizing behavior problems than did children of unexposed mothers (16%, p = 0.005, chi-square test). Although the prevalence rates of both internalizing problems (20.4%) and total problems (21.2%) in children of ETS exposed mothers were higher than those of children of unexposed mothers, the differences were not statistically significant.
Fig. 1.
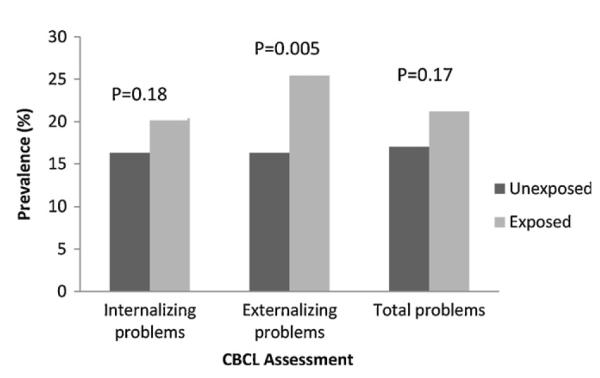
Prevalence of behavior problems among children of mothers with and without ETS exposure during pregnancy
Table 2 shows the comparison of characteristics of children and parents by presence and absence of externalizing behavior problems. The two groups were similar in most characteristics. Children with externalizing behavior problems were more likely than children without externalizing behavioral problems to be born to mothers with ETS exposure during pregnancy.
Table 2.
Comparison of mother’s exposure to ETS during pregnancy and socio-demographic characteristics between children with and without externalizing behavior problems
| Variables | Externalizing behavior problems? |
p value | |
|---|---|---|---|
| No (n = 519) | Yes (n = 127) | ||
| Mother exposed to ETS during pregnancy | |||
| No | 340 (65.5) | 66 (51.9) | 0.0046 |
| Yes | 179 (34.5) | 61 (48.0) | |
| Sex | |||
| Boy | 275 (52.9) | 70 (55.1) | 0.66 |
| Girl | 244 (47.1) | 57 (44.9) | |
| Sibling | |||
| No | 394 (75.9) | 93 (73.2) | 0.53 |
| Yes | 125 (24.1) | 34 (26.8) | |
| School area | |||
| City | 211 (40.7) | 45 (35.4) | 0.45 |
| Suburban | 195 (37.6) | 55 (43.3) | |
| Rural | 113 (21.7) | 27 (21.3) | |
| Blood lead level (μg/dL, median and range) | 5.9 (1.8–27.5) | 5.8 (2.2–32.0) | 0.12 |
| Father’s education | |||
| Less than high school | 193 (37.2) | 48 (37.8) | 0.93 |
| High school | 158 (30.4) | 40 (31.5) | |
| College and University | 168 (32.4) | 39 (30.7) | |
| Father’s occupation | |||
| Unemployed | 12 (2.3) | 6 (4.7) | 0.32 |
| General labor | 266 (51.2) | 66 (51.9) | |
| Technician/professional | 211 (40.7) | 51 (40.2) | |
| Other | 30 (5.8) | 4 (3.2) | |
| Father smoked after the child was born | |||
| Missing | 10 (1.9) | 3 (2.4) | 0.87 |
| No | 116 (22.3) | 24 (18.9) | |
| <10 cigarettes/day | 276 (53.2) | 73 (57.5) | |
| 10–20 cigarettes/day | 91 (17.5) | 20 (15.8) | |
| 20+ cigarettes/day | 26 (5.0) | 7 (5.5) | |
| Mother’s age when the child was born | |||
| <25 | 158 (30.4) | 40 (31.5) | 0.77 |
| 25–30 | 323 (62.2) | 80 (62.9) | |
| 30+ | 38 (7.3) | 7 (5.5) | |
| Mother’s education | |||
| Less than high school | 239 (46.1) | 65 (51.2) | 0.11 |
| High school | 148 (28.5) | 41 (32.3) | |
| College and University | 132 (25.4) | 21 (16.5) | |
| Mother’s occupation | |||
| Unemployed | 24 (4.6) | 7 (5.5) | 0.37 |
| General labor | 208 (40.1) | 59 (46.5) | |
| Technician/professional | 176 (33.9) | 33 (25.9) | |
| Other | 111 (21.4) | 28 (22.1) | |
| Parental psychopathologic problem? | |||
| No | 493 (95.0) | 118 (92.9) | 0.35 |
| Yes | 26 (5.0) | 9(7.1) | |
| Parental divorce | |||
| Missing | 26 (5.0) | 7 (5.5) | 0.84 |
| No | 474 (91.3) | 114 (89.8) | |
| Yes | 19 (3.7) | 6 (4.7) | |
Numbers in parenthesis are percentages expect for blood lead level.
Maternal ETS exposure was not associated with either internalizing problems or total problems (data not shown). It was, however, associated with a higher risk of externalizing behavior problems in offspring before and after adjusting for confounders (Table 3). The unadjusted OR was 1.75 (95% confidence interval [CI] 1.23–3.09). After controlling for potential confounders, the association was somewhat stronger (OR = 2.08, 95% CI 1.27–3.43). However, no dose-response relationship was observed between ETS (no exposure, <30 min, 30–60 min, >60 min) and externalizing behavior problems. After including children who completed CBCL assessment at approximately 6 years old, the association remained (OR = 1.50, 95% CI 1.00–2.27, p = 0.05). When the expanded definition of ETS exposure (i.e. 1-h cut-off) was used, ETS exposure was associated with an increased risk of behavioral problems, with an OR of 1.69 and 95% CI 1.02–2.81 (p = 0.04). When categorizing the 230 women with absent or incomplete ETS information as non-ETS exposed, we found a stronger association (OR = 2.36, 95% CI 1.49–3.75, p = 0.003). Conversely, even after assigning these women to the exposed group, we still found an association, albeit a weaker one, with an OR of 1.48 (95% CI 0.95–2.31, p = 0.09). After multiple imputations, the association between ETS and externalizing problem remained (OR = 1.53, 95% CI 1.05–2.22). No effect modification was found between maternal ETS exposure and parental characteristics (i.e. parental characteristics did not change the association between maternal ETS exposure and externalizing behavioral problem). Neither the attention syndrome nor the aggression syndrome alone was associated with any factors, including ETS (data not shown). None of the covariates were associated with either internalizing or externalizing problems (see Supplemental Table 1).
Table 3.
Logistic regression analysis for externalizing behavior of children from China Jintan Child Cohort Study
| Variables | Odd ratio | 95% confidence limits | Wald chi-square | p value |
|---|---|---|---|---|
| Primary analysis | ||||
| Unadjusted | 1.75 | 1.23–3.09 | 8.93 | 0.004 |
| Adjusteda | 2.08 | 1.27–3.43 | 8.80 | 0.004 |
| Sensitivity analysis (adjusted for confounders) | ||||
| Including children who completed CBCL assessment at 6 | 1.50 | 1.00–2.27 | 3.76 | 0.05 |
| 1-h cutoff for ETS | 1.69 | 1.02–2.81 | 4.21 | 0.04 |
| Women with missing ETS assigned as exposed | 2.36 | 1.49–3.75 | 13.21 | 0.003 |
| Women with missing ETS assigned as unexposed | 1.48 | 0.95–2.31 | 2.94 | 0.09 |
| After multiple imputations | 1.53 | 1.05–2.22 | 4.52 | 0.03 |
Adjusted for child sex and school area, parental education and occupation, father’s postnatal smoking status, and whether parents were separate or not.
Supplementary data related to this article found, in the online version, at http://dx.doi.org/10.1016/j.neuro.2012.11.005
4. Discussion
Our findings show that after adjusting for potential confounders, self-reported maternal ETS exposure during pregnancy was independently associated with a higher risk of having externalizing behavior problems in offspring at age 5 years. Earlier studies have consistently reported evidence of an association between maternal active smoking and antisocial behaviors in offspring (Wakschlag et al., 2002). While previous studies suggest that maternal active smoking during pregnancy is linked to behavior problems in offspring, including aggressive behavior and externalizing behavior in children (Brook et al., 2006; Fergusson et al., 1998; Huijbregts et al., 2008; Orlebeke et al., 1997, 1999) and criminal behavior in adults (Brennan et al., 1999; Paradis et al., 2011), our study extends these findings by being among the first to show that ETS specifically, rather than direct exposure, is associated with negative behavioral outcomes in children.
The findings from the present study are consistent with those from two previous studies, both of which also used the CBCL (Gatzke-Kopp and Beauchaine, 2007; Hsieh et al., 2010). In the U.S. study, children born to mothers exposed to ETS had higher mean scores in the two externalizing syndromes (attention and aggression) (Gatzke-Kopp and Beauchaine, 2007). The magnitude of the effects was similar to that seen in children of mothers exposed to active smoking. The second study, in Taiwan, evaluated approximately 200 children of mothers who did not smoke during pregnancy and found maternal ETS exposure during pregnancy was associated with externalizing behavior problems (Hsieh et al., 2010). Another study, from the Netherlands, used paternal indoor smoking as an indicator of maternal ETS exposure (i.e. workplace and other exposures were excluded) and found an association between ETS exposure and child externalizing behavior problems (assessed using the CBLC) after adjusting for children’s age and sex (Roza et al., 2009). The association, however, disappeared after additionally adjusting for parental education, family income, national origin, and parental psychopathology. An earlier small study compared neuropsychological performance of children of actively smoking mothers (i.e. mothers who smoked any quantity of cigarettes at any time during their pregnancy), children of passively smoking mothers, and children of non-smoking mothers (Makin et al., 1991). Compared with children of non-smoking mothers, children of passive smoking mothers demonstrated worse performance on tests of speech and language skills, intelligence, and behavioral outcomes (conduct disorders). This study also demonstrated that the long-term neurobehavioral effects of maternal passive smoking during pregnancy are similar to that of active smoking, although they may be milder. Maternal exposure to ETS during pregnancy has been previously associated with neurobiological and behavioral changes in offspring (Huijbregts et al., 2011), and adverse neurodevelopment outcomes in infants, including lower motor system cluster scores (Hernandez-Martinez et al., 2011), decreased mental developmental index scores (Lee et al., 2011), lower birth weight, greater congenital anomalies, and smaller head circumferences (Salmasi et al., 2010). Together with the present study, these results indicate that maternal exposure to ETS during pregnancy may increase the risk of externalizing behavioral problems in offspring. The lack of association between ETS and the two externalizing syndromes alone (aggression and attention) might be related to smaller numbers of cases and lower statistical power.
Tobacco smoke contains thousands of chemical compounds, and many of these compounds are known neurotoxicants (Liu and Wuerker, 2005; Liu, 2011). Nicotine itself is a neuroteratogen and binds to the cholinergic receptor during brain development, causing brain cell deaths or structural alteration in regional brain areas, which leads to neurobehavioral and functional impairment in offspring (Slotkin, 2004). The magnitude of effect seen in mothers exposed to ETS was comparable to that seen in active maternal smokers, suggesting that ETS should be regarded with equal seriousness in terms of its potential threat. Other components may also play important roles in these effects (Centers for Disease Control and Prevention, 2010). For instance, high prenatal exposure to airborne polycyclic aromatic hydrocarbons among non-smoking African-American and Dominican mothers was associated with lower mental development index and cognitive developmental delay of children at age 3 years (Perera et al., 2006). Metals including lead, cadmium, and mercury, which are neurotoxicants, have been detected in tobacco smokes. A recent study found that tobacco products in China had about three times higher levels of heavy metals (lead, cadmium, and arsenic) than those sold in Canada (O’Connor et al., 2010).
Although our findings are compelling, several limitations should be noted. First, our assessment of ETS was based on women’s self-report. The validity of self-reported ETS exposure is still a concern, although some studies, particularly recent studies in Asia, have found this methodology acceptable and useful (Chiu et al., 2008; Kaufman et al., 2002; Nondahl et al., 2005). The women were asked to recall exposures that happened 5–6 years ago, and thus recall bias could not be excluded. The proportion of women with ETS exposure during pregnancy in the present study was consistent with that observed in two previous studies in China (Fu et al., 2008; Hsieh et al., 2010). In our study, one-quarter of the women did not answer or provided incomplete information regarding ETS exposure during pregnancy. The association remained after including those subjects through multiple imputations and assigning exposure status. Second, there is no uniformed definition for ETS. In our study, we defined ETS by the number of smokers in the environment (home, workplace, and other place) and number of minutes exposed to smoke in that environment daily. Presence of ETS in our study was defined by at least one person having smoked for at least 30 min in the environment. When the more conservative 1-h cutoff was used, ETS was still associated with an increased risk of externalizing behavior in offspring, although the association was slightly attenuated. This difference was attributed to the higher prevalence of mothers exposed to ETS for 30 rather than 60 min. These sensitivity analyses indicate the association between maternal ETS exposure and child externalizing behavior problems is robust. In addition, information for potential factors (covariates) was collected at the time we collected ETS exposure. Status of some of these factors such as occupation might have changed between pregnancy and this time, but we did not have this information.
Very few women (less than 2%) in China are smokers (Li et al., 2011) and the prevalence is even lower in small city like Jintan. We were not able to assess women’s active smoking status due to the social stigma against women’s smoking behavior in this area. There might have been several (≤5) mothers who were active smokers during pregnancy in our study, which may cause confounding effects. However, we expect this number is negligible given the relatively strong association observed and the likely very small number of active smokers.
Parental postnatal smoking is consistently associated with children’s neurobehavioral problems (Herrmann et al., 2008). Paternal postnatal smoking was not associated with either externalizing or internalizing problems in the present study, but it is worth noting that more than three quarters of fathers who did not smoke at home during pregnancy resumed smoking after pregnancy. It is a Chinese phenomenon that a husband does not smoke at home when his wife is pregnant but continues to smoke outside the home and resumes smoking at home soon after the child is born (Wang et al., 2007).
Familial background factors and other environmental factors may account for the association between smoking during pregnancy and child externalizing and anti-social behaviors (D’Onofrio et al., 2010, 2008). Some potential confounders, such as family income, were not included in this study, while our study did adjust for parental and child characteristics. Children’s school area and neighborhood condition, which reflect social economic conditions of families, were also controlled in our analyses. However, we should not exclude a possibility of residual confounding by these socio-economic factors. Gestational age (preterm birth) and birth weight (low birth weight), which have been adjusted in similar studies (Gatzke-Kopp and Beauchaine, 2007; Roza et al., 2009), were not included in our analyses due to the lack of information. Birth complications have also been linked to externalizing behavior (Liu et al., 2009). Future study will take these covariates into account. The differences in parental demographic characteristics, such as education and occupation, between children included in the present analysis and the whole study population or child population in China also limits the generalizability of the finding. Further studies with larger sample size and more representative samples are warranted to validate the findings. In addition, our study is limited in that it lacks the biomarker serum cotinine to measure exposure to ETS. However, Kaufman et al. (2002) has noted that omission of this marker would actually lead to an underestimation of ETS exposure. Therefore, the results of our study may not necessarily be negatively affected. Nevertheless, future longitudinal prospective studies that include both biomarkers and questionnaires would be more informative.
Nevertheless, this study has several strengths. First, to our knowledge, this is the first study in China to examine the association between maternal ETS exposure and externalizing behaviors in offspring. Given the high prevalence of ETS exposure during pregnancy, more studies, in particular smoking reduction intervention studies, are warranted to clarify this relationship and determine the prognostic effects of ETS. Externalizing behavior problems were assessed using the CBCL, which has been validated in diverse samples (Ivanova et al., 2010). The longitudinal design of this cohort study makes it possible to examine whether the effect persists in future. Such findings could inform public health efforts to reduce public smoking and underscores the need for including ETS avoidance as a potential component of prenatal care among pregnant women. Finally, this study extends a small but important segment of the literature on tobacco use and child development. Although additional, more longitudinal, research is needed to continue parsing these effects, this study gives further clarity to the relationship between ETS and behavioral disturbance in children.
5. Conclusion
We found that maternal ETS exposure during pregnancy was associated with higher risk of externalizing behavior problems in offspring, after adjusting for potential confounders. Given the high prevalence of ETS exposure among pregnant women in China and far-reaching effects of child behavioral disturbance on public health outcomes, it is critical to reduce ETS exposure in order to improve the health of not only mothers and their children but that of society at large.
Supplementary Material
Acknowledgements
Thanks are extended to the participating children and their families from Jintan City, and to the Jintan Cohort Study Group.
Funding This study is supported, in part, by the National Institute of Environment Health Sciences (NIEHS, K01-ES015 877; R01-ES018858; K02-ES019878-01). Funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Abbreviations
- ETS
environmental tobacco smoke
- CBCL/1.5-5
Child Behavior Checklist
- CJCCS
China Jintan Child Cohort Study
Footnotes
Conflicts of interest The authors declare that there are no conflicts of interest. No conflict of interest or competing financial interest has been declared by the authors.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA preschool forms & profiles. University of Vermont, Research Center for Children, Youth & Families; Burlington, VT: 2000. [Google Scholar]
- Boutwell BB, Beaver KM, Gibson CL, Ward JT. Prenatal exposure to cigarette smoke and childhood externalizing behavioral problems: a propensity score matching approach. Int J Environ Health Res. 2011;21:248–59. doi: 10.1080/09603123.2010.544032. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Grekin ER, Mednick SA. Maternal smoking during pregnancy and adult male criminal outcomes. Arch Gen Psychiatry. 1999;56:215–9. doi: 10.1001/archpsyc.56.3.215. [DOI] [PubMed] [Google Scholar]
- Brook DW, Zhang C, Rosenberg G, Brook JS. Maternal cigarette smoking during pregnancy and child aggressive behavior. Am J Addict. 2006;15:450–6. doi: 10.1080/10550490600998559. [DOI] [PubMed] [Google Scholar]
- Button TM, Thapar A, McGuffin P. Relationship between antisocial behaviour, attention-deficit hyperactivity disorder and maternal prenatal smoking. Br J Psychiatry. 2005;187:155–60. doi: 10.1192/bjp.187.2.155. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and PreventionSurgeon general’s report—the health consequences of involuntary exposure to tobacco smoke. Centers for Disease Control and Prevention; Atlanta, GA, USA: 2006. [PubMed] [Google Scholar]
- Centers for Disease Control and PreventionNHIS adult tobacco use information—glossary. Centers for Disease Control and Prevention; Atlanta, GA, USA: 2009. http://www.cdc.gov/nchs/nhis/tobacco/tobacco_glossary.htm. [Google Scholar]
- Centers for Disease Control and PreventionSurgeon General’s report—how tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease. Centers for Disease Control and Prevention; Atlanta, GA, USA: 2010. [PubMed] [Google Scholar]
- Chiu HT, Wu HI, Kuo H. The relationship between self-reported tobacco exposure and cotinine in urine and blood for pregnant women. Sci Total Environ. 2008;406:331–6. doi: 10.1016/j.scitotenv.2008.07.005. [DOI] [PubMed] [Google Scholar]
- D’Onofrio BM, Singh AL, ILiadou A, Lambe M, Hultman CM, Grann M, et al. Familial confounding of the association between maternal smoking during pregnancy and offspring criminality. Arch Gen Psychiatry. 2010;67:529–38. doi: 10.1001/archgenpsychiatry.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Van Hulle CA, Waldman ID, Rodgers JL, Harden KP, Rathouz PJ, et al. Smoking during pregnancy and offspring externalizing problems: an exploration of genetic and environmental confounds. Dev Psychopathol. 2008;2013:9–164. doi: 10.1017/S0954579408000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Lynskey MT. Maternal smoking before and after pregnancy: effects on behavioral outcomes in middle childhood. Pediatrics. 1993;92:815–22. [PubMed] [Google Scholar]
- Fergusson DM, Woodward LJ, Horwood LJ. Maternal smoking during pregnancy and psychiatric adjustment in late adolescence. Arch Gen Psychiatry. 1998;55:721–7. doi: 10.1001/archpsyc.55.8.721. [DOI] [PubMed] [Google Scholar]
- Fu C, Chen Y, Wang T, Edwards N, Xu B. Exposure to environmental tobacco smoke in Chinese new mothers decreased during pregnancy. J Clin Epidemiol. 2008;61:1182–6. doi: 10.1016/j.jclinepi.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Gatzke-Kopp LM, Beauchaine TP. Direct and passive prenatal nicotine exposure and the development of externalizing psychopathology. Child Psychiatry Hum Dev. 2007;38:255–69. doi: 10.1007/s10578-007-0059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Martinez C, Arija Val V, Escribano Subias J, Canals Sans J. A longitudinal study on the effects of maternal smoking and secondhand smoke exposure during pregnancy on neonatal neurobehavior. Early Hum Dev. 2011;88:403–8. doi: 10.1016/j.earlhumdev.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Herrmann M, King K, Weitzman M. Prenatal tobacco smoke and postnatal secondhand smoke exposure and child neurodevelopment. Curr Opin Pediatr. 2008;20:184–90. doi: 10.1097/MOP.0b013e3282f56165. [DOI] [PubMed] [Google Scholar]
- Hsieh CJ, Jeng SF, Su YN, Liao HF, Hsieh WS, Wu KY, et al. CYP1A1 modifies the effect of maternal exposure to environmental tobacco smoke on child behavior. Nicotine Tob Res. 2010;12:1108–17. doi: 10.1093/ntr/ntq157. [DOI] [PubMed] [Google Scholar]
- Huijbregts SC, Seguin JR, Zoccolillo M, Boivin M, Tremblay RE. Maternal prenatal smoking, parental antisocial behavior, and early childhood physical aggression. Dev Psychopathol. 2008;20:437–53. doi: 10.1017/S0954579408000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbregts SCJ, van Berkel SR, Swaab-Barneveld H, van Goozen SHM. Neurobiological and behavioral stress reactivity in children prenatally exposed to tobacco. Psyneuroendocrinology. 2011;36:913–8. doi: 10.1016/j.psyneuen.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Hutchinson J, Pickett KE, Green J, Wakschlag LS. Smoking in pregnancy and disruptive behaviour in 3-year-old boys and girls: an analysis of the UK Millennium Cohort Study. J Epidemiol Community Health. 2010;64:82–8. doi: 10.1136/jech.2009.089334. http://dx.doi.org/10.1136/ jech.2009.089334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova MY, Achenbach TM, Rescorla LA, Harder VS, Ang RP, Bilenberg N, et al. Preschool psychopathology reported by parents in 23 societies: testing the seven-syndrome model of the child behavior checklist for ages 1.5–5. J Am Acad Child Adolesc Psychiatry. 2010;49:1215–24. doi: 10.1016/j.jaac.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman FL, Kharrazi M, Delorenze N, Eskenazi GN, B. Bernert JT. Estimation of environmental tobacco smoke exposure during pregnancy using a single question on household smokers versus serum cotinine. J Expo Anal Environ Epidemiol. 2002;12:286–95. doi: 10.1038/sj.jea.7500224. [DOI] [PubMed] [Google Scholar]
- Kotimaa AJ, Moilanen I, Taanila A, Ebeling H, Smalley SL, McGough JJ, et al. Maternal smoking and hyperactivity in 8-year-old children. J Am Acad Child Adolesc Psychiatry. 2003;42:826–33. doi: 10.1097/01.CHI.0000046866.56865.A2. [DOI] [PubMed] [Google Scholar]
- Lee BE, Hong YC, Park H, Ha M, Kim JH, Chang N, et al. Secondhand smoke exposure during pregnancy and infantile neurodevelopment. Environ Res. 2011;111:539–44. doi: 10.1016/j.envres.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Leung PW, Kwong SL, Tang CP, Ho TP, Hung SF, Lee CF, et al. Test–retest reliability and criterion validity of the Chinese version of CBCL, TRF, and YSR. J Child Psychol Psychiatry. 2006;47:970–3. doi: 10.1111/j.1469-7610.2005.01570.x. [DOI] [PubMed] [Google Scholar]
- Li Q, Hsia J, Yang G. Prevalence of smoking in China in 2010. N Engl J Med. 2011;364:2469–90. doi: 10.1056/NEJMc1102459. [DOI] [PubMed] [Google Scholar]
- Linnet KM, Wisborg K, Obel C, Secher NJ, Thomsen PH, Agerbo E, et al. Smoking during pregnancy and the risk for hyperkinetic disorder in offspring. Pediatrics. 2005;116:462–7. doi: 10.1542/peds.2004-2054. [DOI] [PubMed] [Google Scholar]
- Liu J. Early health risk factors for violence: conceptualization, review of the evidence, and implications. Aggress Violent Behav. 2011;16:63–73. doi: 10.1016/j.avb.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ai Y, McCauley L, Pinto-Martin J, Yan C, Shen X, et al. Blood lead levels and associated socio-demographic factors among preschool children in the south eastern region of China. Paediatr Perinat Epidemiol. 2012;26:61–9. doi: 10.1111/j.1365-3016.2011.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Cheng H, Leung PW. The application of the preschool child behavior checklist and the caregiver-teacher report form to mainland Chinese children: syndrome structure, gender differences, country effects, and inter-informant agreement. J Abnorm Child Psychol. 2011a;39:251–64. doi: 10.1007/s10802-010-9452-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, McCauley LA, Leung P, Wang B, Needleman H, Pinto-Martin J. Community-based participatory research (CBPR) approach to study children’s health in China: experiences and reflections. Int J Nurs Stud. 2011b;48:904–13. doi: 10.1016/j.ijnurstu.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, McCauley LA, Zhao Y, Zhang H, Pinto-Martin J. Cohort profile: the China Jintan Child Cohort Study. Int J Epidemiol. 2010;39:668–74. doi: 10.1093/ije/dyp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Raine A, Wuerker A, Venables P, Mednick SA. The association of birth complications and externalizing behavior in early adolescents. J Res Adolesc. 2009;19:93–111. doi: 10.1111/j.1532-7795.2009.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wuerker A. Biosocial bases of violence: implications for nursing research. Int J Nurs Stud. 2005;42:229–41. doi: 10.1016/j.ijnurstu.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Makin J, Fried PA, Watkinson B. A comparison of active and passive smoking during pregnancy: long-term effects. Neurotoxicol Teratol. 1991;13:5–12. doi: 10.1016/0892-0362(91)90021-n. [DOI] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Chen L, Jones J. Is maternal smoking during pregnancy a risk factor for attention deficit hyperactivity disorder in children. Am J Psychiatry. 1996;153:1138–42. doi: 10.1176/ajp.153.9.1138. [DOI] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Jones J. Further evidence of an association between maternal smoking during pregnancy and attention deficit hyperactivity disorder: findings from a high-risk sample of siblings. J Clin Child Psychol. 1998;27:352–8. doi: 10.1207/s15374424jccp2703_11. [DOI] [PubMed] [Google Scholar]
- Nondahl DM, Cruickshanks KJ, Schubert CR. A questionnaire for assessing environmental tobacco smoke exposure. Environ Res. 2005;97:76–82. doi: 10.1016/j.envres.2004.02.005. [DOI] [PubMed] [Google Scholar]
- O’Connor RJ, Li Q, Stephens WE, Hammond D, Elton-Marshall T, Cummings KM, et al. Cigarettes sold in China: design, emissions and metals. Tob Control. 2010;19(Suppl. 2):i47–53. doi: 10.1136/tc.2009.030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlebeke JF, Knol DL, Verhulst FC. Increase in child behavior problems resulting from maternal smoking during pregnancy. Arch Environ Health. 1997;52:317–21. doi: 10.1080/00039899709602205. [DOI] [PubMed] [Google Scholar]
- Orlebeke JF, Knol DL, Verhulst FC. Child behavior problems increased by maternal smoking during pregnancy. Arch Environ Health. 1999;54:15–9. doi: 10.1080/00039899909602231. [DOI] [PubMed] [Google Scholar]
- Paradis AD, Fitzmaurice GM, Koenen KC, Buka SL. Maternal smoking during pregnancy and criminal offending among adult offspring. J Epidemiol Community Health. 2011;65:1145–50. doi: 10.1136/jech.2009.095802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tsai WY, Tang D, Diaz D, et al. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ Health Perspect. 2006;114:1287–92. doi: 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A, Ishikawa SS, Arce E, Lencz T, Knuth KH, Birhle S, et al. Hippocampal structure asymettry in unsuccessful psychopaths. Biol Psychiatry. 2004;l55:185–91. doi: 10.1016/s0006-3223(03)00727-3. [DOI] [PubMed] [Google Scholar]
- Rescorla LA, Achenbach TM, Ivanova MY, Harder VS, Otten L, Bilenberg N, et al. International comparisons of behavioral and emotional problems in preschool children: Parents’ report from 24 societies. J Clin Child Adolesc Psychol. 2011;40:456–67. doi: 10.1080/15374416.2011.563472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman K, Greenland S, Lash T. Modern epidemiology. Lippincott, Williams & Wilkin; Philadelphia, PA: 2008. [Google Scholar]
- Roza SJ, Verhulst FC, Jaddoe VW, Steegers EA, Mackenbach JP, Hofman A, et al. Maternal smoking during pregnancy and child behaviour problems: the Generation R Study. Int J Epidemiol. 2009;38:680–9. doi: 10.1093/ije/dyn163. [DOI] [PubMed] [Google Scholar]
- Salmasi G, Grady R, Jones J, McDonald SD. Environmental tobacco smoke exposure and perinatal outcomes: a systematic review and meta-analyses. Acta Obstet Gynecol Scand. 2010;89:423–41. doi: 10.3109/00016340903505748. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198:132–51. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Tiesler CM, Chen CM, Sausenthaler S, Herbarth O, Lehmann I, Schaaf B, et al. Passive smoking and behavioural problems in children: results from the LISAplus prospective birth cohort study. Environ Res. 2011;111:1173–9. doi: 10.1016/j.envres.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Lahey BB, Loeber R, Green SM, Gordon RA, Leventhal BL. Maternal smoking during pregnancy and the risk of conduct disorder in boys. Arch Gen Psychiatry. 1997;54:670–6. doi: 10.1001/archpsyc.1997.01830190098010. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Pickett KE, Cook E, Jr, Benowitz NL, Leventhal BL. Maternal smoking during pregnancy and severe antisocial behavior in offspring: a review. Am J Public Health. 2002;92:966–74. doi: 10.2105/ajph.92.6.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Pickett KE, Kasza KE, Loeber R. Is prenatal smoking associated with a developmental pattern of conduct problems in young boys. J Am Acad Child Adolesc Psychiatry. 2006;45:61–467. doi: 10.1097/01.chi.0000198597.53572.3e. [DOI] [PubMed] [Google Scholar]
- Wang T, Fu C, Chen Y, Xu B. Infant exposure to environmental tobacco smoke: a prevalence study in Shanghai. Chin J Primary Care. 2007;21:1–5. [Google Scholar]
- The World Health OrganizationGender and tobacco control: a policy brief. The World Health Organization; Geneva, Switzerland: 2007. [Google Scholar]
- Yang G, Fan L, Tan J, Qi G, Zhang Y, Samet JM, et al. Smoking in China: findings of the 1996 national prevalence survey. JAMA. 1999;282:1247–53. doi: 10.1001/jama.282.13.1247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


