Abstract
Holding negative aging stereotypes can lead older adults to perform poorly on memory tests. We attempted to improve older adults’ memory performance by giving them task experience that would counter their negative performance expectations. Before participating in a memory experiment, younger and older adults were given a cognitive task that they could either successfully complete, not successfully complete, or they were given no prior task. For older adults, recall was significantly higher and self-reported anxiety was significantly lower for the prior task success group relative to the other groups. There was no effect of prior task experience on younger adults’ memory performance. Results suggest that older adults’ memory can be improved with a single successful prior task experience.
Keywords: memory, aging, memory improvement, task success, stereotypes
Many older adults believe that their memory abilities are declining and they expect to perform poorly on memory tests (e.g., Berry, 1999; Lineweaver & Hertzog, 1998). These worries may stem from personal experiences of memory failures, but they might also be influenced by societal and cultural expectations that memory and other abilities will decline with age (see Heckhausen, Dixon, & Baltes, 1989; Hertzog, Lineweaver, & McGuire, 1999; Levy & Langer, 1994; Ryan, 1992; Ryan & Kwong See, 1993).
Holding negative aging stereotypes can have detrimental consequences for older adults. The finding that knowledge of a stereotype influences behavior is called the stereotype threat effect (Steele & Aronson, 1995) and has been demonstrated for a variety of stereotypes in addition to those associated with aging. Research with older adults shows that activating negative stereotypes of aging can cause older adults to perform more poorly on subsequent memory tests, relative to when positive stereotypes or no stereotypes of aging are activated (Hess, Auman, Colcombe, & Rahhal, 2003; Hess, Hinson, Statham, 2004; Levy, 1996). Aging stereotypes can be activated in a variety of ways. In some cases simply telling older adults that the purpose of the upcoming experiment is to determine why younger and older adults perform differently on memory tests is sufficient to impair older adults’ memory performance (Hess, Hinson, & Hodges, 2009). In other studies, older adults may read a narrative containing negative information about aging (Hess & Hinson, 2006) or solve word puzzles containing negative stereotype-relevant words, such as “frailty” prior to participating in a standard memory experiment (Hess, et al., 2004, see also Levy, 1996; Stein, Blanchard-Fields, & Hertzog, 2002, for other priming paradigms). In the Hess et al. (2004) study, younger and older participants were primed with negative words, such as “forgetfulness” in one condition and positive words such as “wisdom” in another condition. After being primed, participants studied a list of words (unrelated to the priming task) and were then given a free recall test for the studied words. For older adults, recall performance was significantly higher in the positive prime condition compared to the negative prime condition (.53 vs. .40). In contrast, younger adults’ recall performance was not impaired by the negative primes.
The results just described demonstrate that when negative aging stereotypes are experimentally activated, older adults’ subsequent memory performance is reduced. However, it is not necessary for stereotype threat to be experimentally induced for older adults’ memory performance to be influenced by negative performance expectations. Indeed, under standard testing circumstances older adults may perform poorly simply because they are aware that their memory is being tested, which itself is sufficient to activate negative thoughts about one’s memory and create test anxiety (see Chasteen, Bhattacharyya, Horhota, Tam, & Hasher, 2005; Rahhal, Hasher, & Colcombe, 2001; Hess et al., 2009). Age differences on explicit and implicit memory tests provide one example of how older adults perform poorly under conditions in which they know their memory is being tested. When younger and older adults are not aware that their memory is being tested, as is the case with implicit memory tests, there are often no age differences in performance (see Fleischman & Gabrieli, 1998, Light, Prull, La Voie, & Healy, 2000 for reviews). There is good evidence that explicit tests differ from implicit ones because they require more controlled, often conceptual, and attention-demanding processes that are typically impaired with age, whereas implicit tests require automatic processes that are not impaired with age (e.g., Gabrieli et al., 1999; Geraci, 2006; Light et al., 2000). While explicit and implicit tests certainly do require different cognitive processes, they also differ in another fundamental way: by definition, participants know that their memory is being tested on explicit tests, whereas they do not know that their memory is being tested on implicit tests. Indeed, directly activating negative age-related stereotypes influences older adults’ explicit memory performance, but not their implicit memory performance (Eich, Knowlton, & Castel, 2008). Thus, it appears that having some awareness that memory is being tested may be sufficient to cause older adults to perform poorly.
Researchers have attempted to reduce the detrimental effects of older adults’ negative performance expectations by training older adults to think more positively about aging, but these attempts are not always effective. For example, in one study, participants read two newspaper articles that either contradicted or supported stereotypical views about aging and memory before taking an explicit memory test (Hess & Hinson, 2006). Results from age groups ranging from ages 30 to 80 showed that memory performance was not statistically improved for any age group after reading positive aging stereotypes. In other longer-term training studies, attempts have been made to improve memory performance by increasing memory self-efficacy, or one’s confidence in their memory abilities (see Rebok, Carlson, and Langbaum, 2007, for review). Yet, these attempts at improving memory self-efficacy do not always lead to improvements in memory performance.
One recent study did show significant improvements in both memory self efficacy and performance. This study combined self-efficacy and strategy training and showed improvements in older adults’ memory performance across various tasks (West, Bagwell, & Dark-Freudeman, 2008). In this 6-week memory training course, older adults (ages 54 to 92) took weekly memory training classes in which they were given various memory tests. Participants were given memory tests that included items that had been previously encountered to allow them a chance to apply their newly-learned strategies and also to provide an opportunity for them to believe that they could improve their memory performance. To further improve memory self-efficacy, participants read and discussed the potential for cognitive improvement at any age. Overall, results showed memory improvement and increased self-efficacy from pre-test to post-test for the training group, but not for the control group who did not receive training. The authors concluded that the program was successful because self-efficacy training was incorporated into each step of the program; they stated that “…trainees have to be convinced that they should believe in themselves and their ability to gain from practice and effort” (2008, p. 326).
We hypothesize that one powerful way to convince older adults of their abilities might be to give them direct experience successfully completing cognitive tasks. In the West et al. (2008) study and in other training studies, task success is often confounded with self-efficacy and strategy training—easier tasks may be introduced first and then increased in difficulty. It may be that successfully completing prior cognitive tasks, even unrelated ones, serves as a powerful way to convince older adults of their abilities and counter the detrimental effects of negative performance expectations. To our knowledge, this hypothesis has yet to be directly examined.
In the current study, we sought to determine if giving older adults direct experience successfully completing a prior cognitive task, even one unrelated to the memory experiment, could provide information to counter their negative performance expectations and lead to improved memory performance. To test this hypothesis, younger and older adults were given a cognitive task that they could perform successfully. Then, participants partook in a memory experiment. We compared memory performance for this group (referred to as the task success group) to memory performance for two other groups: a group that was given the same cognitive task that they could not complete successfully because of an unrealistic time limit (the task failure group) and a group that received no task experience prior to participating in the memory experiment (the control group). We predicted that older adults who successfully performed the prior sentence scramble task would have better subsequent memory performance than older adults who did not complete a prior task (the control condition) or who failed to successfully complete the prior sentence task (the failure condition). Further, we expected that prior task success would primarily benefit older adults’ subsequent memory performance and have little effect on younger adults’ performance. An age-specific effect of this sort would be consistent with the interpretation that prior task success improves memory performance by targeting stereotypical age-related performance expectations.
Method
Participants
Seventy-five older adults (M age = 73.29, SD = 6.70; 55% female) from the community participated in the experiment and were given an honorarium for their time. As well, seventy-five younger adults (M age = 18.52, SD = .66; 71% female) participated and were given course credit for their time. One third (25) of the older adults participated in the task success condition, one third participated in the control condition, and one third participated in the task failure condition. Similarly, one third of the younger adults participated in the task success condition, one third participated in the control condition, and one third participated in the task failure condition. All participants were tested individually or in groups of up to four at a time and the testing session lasted approximately 1 hour. Consent and demographic documentation were completed prior to participation. Education level was higher for older adults (M = 16.19 years; SD = 3.06) than younger adults (M = 13.05; SD = .44), F(1, 148) = 77.78, MSE = 4.77, η2p = .34. Vocabulary was significantly higher for older adults (M = 34.61, SD = 3.73) than it was for younger adults (M = 30.25, SD = 2.96), F(1, 148) = 44.95, MSE = 11.32, η2p = .30. There were no differences in age, F(2, 72) = .89, MSE = 45.03, η2p = .02, education, F(2, 72) = 1.41, MSE = 9.26, η2p = .04, or vocabulary, F(2, 72) = 1.69, MSE = 13.66, η2p = .05, between the three groups of older adults. There were also no differences in education (F(2, 72) = 1.62, MSE = .19, η2p = .04) or vocabulary (F(2, 72) = .06, MSE = 8.96, η2p < .01) between the three groups of younger adults. There was a slight difference in age among the younger adult group (F(2, 72) = 4.79, MSE = .40, p = .01, η2p = .12) with those in the control condition (M age = 18.84 years, SD = .62) being older than those in the success (M age = 18.36 years, SD = .64) and failure condition (M age = 18.36 years, SD = .64). All older adults were screened for major cognitive impairment using the Mini-Mental Status Exam (MMSE) (Folstein, Folstein, & McHugh, 1975). There were no differences in average MMSE scores across groups, F(2, 72) = 1.98, MSE = 1.34, η2p = .05, and all participants had MMSE scores of 26 or higher.
Materials and Procedure
During participant recruitment, potential participants were told that they would receive a series of cognitive tests, including a word scramble test and a memory test. Upon entering the lab, participants were again told that they would receive a series of cognitive tests. They first completed a consent form followed by a brief demographic questionnaire that asked about age, education, and ethnicity and the MMSE. Next, participants in the task success condition were first given a packet containing 30 sets of five scrambled words. For each set of five words, participants were instructed to rearrange the words to form a grammatically correct four-word sentence (e.g., lamp, the, fell, run, over). Note that this task is similar to sentence scramble tasks used by Bargh and colleagues (e.g., Bargh, Chen, & Burrows, 1996), except that in the current experiment words used were not designed to prime any stereotypes. We used neutral words without any clear relationship to aging stereotypes (e.g., shoe, car, apple, book), see also Hess, et al., 2004). This type of task was selected to encourage older adults to engage in a language task in which they would excel. Participants in the success condition had unlimited time to complete the sentence scramble task. By design, older adults in this condition performed the task nearly perfectly (they correctly unscrambled 97% of the sets). The task failure group was given the same sentence scramble task, but they were given an unrealistic amount of time to unscramble each set of words at which time a beeper sounded indicating that participants should move on to the next sentence. Pilot testing indicated that with no time restriction older adults took approximately 20 seconds per sentence, so those in the task failure condition were given half this amount of time to unscramble each sentence. Indeed, results confirmed that participants in the task failure condition had difficulty with the task (they correctly unscrambled only 43% of the sets). Participants in the control condition did not complete the scramble task. They were given the memory experiment immediately after completing the MMSE.
Participants in the success and failure conditions were not explicitly told the results of their performance on the scramble task. However, participants in the task success condition could see whether they had written a grammatically correct sentence and know that they did the task correctly. The participants in the task failure group also experienced immediate feedback in the sense that they could see whether they had written a grammatically correct sentence before the buzzer sounded telling them to advance to the next item.
Next, all participants completed the memory experiment. We examined free recall of categorized lists because this procedure has been used in the stereotype threat literature and because age effects are regularly obtained in free recall. Participants were given a list of 30 common words to study (none of these words were related to words used in the previous word scramble task). The list of 30 words included five words from six different semantic categories (e.g., kitchen utensils) that were presented in a fixed random order. Participants had 2 minutes to study the list of words. Participants were given a numbered sheet of paper and instructions to recall as many of the words as they could, in any order. Participants were allowed as much time as they liked to recall the words but everyone finished recalling the words in about two minutes. Immediately after the recall test, older adults were asked to rate their anxiety level on a scale from 1-7. They were asked to: “Please rate how anxious you were during the previous memory test”. Younger adults were not asked to report their level of anxiety. Finally, all participants completed a vocabulary test (Zachary, 1986) designed to measure general intelligence.
Results
Results of statistical tests were significant at p < .05, unless otherwise noted. Effect sizes (Cohen’s d and partial eta squared, η2p) were included for each analysis.
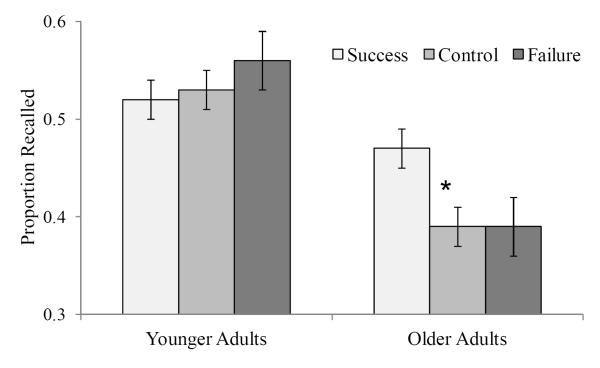
Results showed that prior task experience selectively affected older adults’ memory performance such that their performance was improved following task success. Results from the 2 (Age: older and younger adults) x 3 (Prior Task Experience: control, success, failure) ANOVA indicated a main effect of Age on recall (F(1, 144) = 39.19, MSE = .53, η2p = .21) and a significant interaction effect (F(2, 144) = 3.34, MSE = .05, η2p = .04) but no main effect of Prior Task on recall (F(2, 144) = 1.43, MSE = .02, η2p = .02). To follow-up the significant interaction effect, we completed separate one-way ANOVAs to determine the effect of condition on recall for older and younger adults. There was a significant effect of Prior Task Experience on recall in older adults (F(2, 72) = 3.83, MSE = .02, η2p = .10) but not in younger adults (F(2, 72) = .61, MSE = .01, η2p = .02) (see Figure 1). Planned comparisons indicated that participants in the prior task success condition had higher recall than participants in the control group, t(48) = 2.50, SE = .03, d = .71, and the prior task failure condition, t(48) = 2.41, SE = .03, d = .68. Experiencing prior task failure did not lead to worse memory performance compared to having no prior task experience, t(48) < 1.0, which could be consistent with the idea experiencing task failure may simply confirm older adults negative expectations, and not lead to a further decrease in subsequent memory performance, as compared to the control condition. Thus, the noteworthy finding is that having a single successful task experience in the lab significantly improved older adults’ subsequent memory performance. In fact, comparing younger and older adults’ recall performance within each condition shows that younger adults only had significantly better recall than older adults in the control (t(48) = 4.28, d = 1.23) and failure (t(48) = 4.52, d = 1.30) conditions. The standard age effect in recall did not reach significance in the success condition (t(48) = 1.78), d = .50, p = .08).
Figure 1.
The proportion of words correctly recalled for younger and older adults in each prior task condition. Error bars represent standard error. An * indicates a significant difference relative to control and failure conditions.
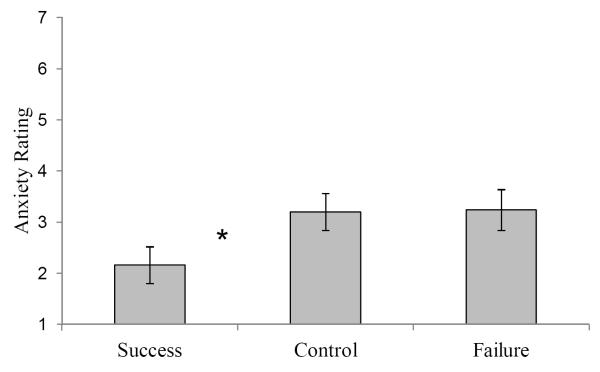
One reason that prior task success may lead to improvements in memory performance for older adults is that having a successful prior task experience could reduce older adults’ anxiety about taking the memory test. At the conclusion of the experiment, we asked participants to rate their current level of anxiety. Results showed that self-reported anxiety levels did differ across the three conditions, F (2, 72) = 3.13, MSE = 3.00, η2p = .08. Participants in the task success condition were significantly less anxious than participants in the task failure condition, t(48) = 2.25, SE = .48, d = .65, and participants in the control condition, t(48) = 2.31, SE = .45, d = .66 (see Figure 2). Further, we found that level of anxiety was significantly correlated with memory performance (r = -.34). We also found that anxiety mediates the relationship between prior task experience and memory performance. When we ran an ANCOVA with anxiety entered as a covariate, the results showed that prior task experience no longer significantly influenced memory performance (F(2, 71) = 2.12, MSE = .01, η2p = .06), suggesting that the benefits of task success on memory performance might be due to reductions in anxiety.
Figure 2.
Self-reported anxiety for older adults for each prior task condition. Error bars represent standard error. An * indicates a significant difference relative to control and failure conditions.
Discussion
Several studies have now shown that holding negative beliefs about memory can lead older adults to have poor memory performance (e.g., Hess, et al., 2003; Hess, et al., 2004; Hess, et al., 2009; Levy, 1996). Researchers have attempted to reduce the influence of these beliefs by training older adults to think less negatively about age-associated memory changes, but these attempts are only modestly effective and are often time-consuming to implement. Our results offer an efficient and effective method for improving older adults’ memory performance. We found that a single successful task experience significantly improved older adults’ subsequent memory performance relative to when they had no prior task experience or an unsuccessful task experience. We suggest that success on a prior cognitive task provides older adults with powerful direct evidence to counter their negative performance expectations, thereby allowing them to optimize their later memory performance.
We also have some preliminary evidence that prior task success may improve memory performance in older adults by reducing anxiety. In fact, older adults in the prior task success condition had lower self-reported anxiety than did participants in the control and task failure conditions. While the current study did not directly manipulate or measure stereotype threat, the role that anxiety plays in older adults memory performance has been examined in the threat literature and may be relevant here. One idea is that people under threat experience intrusive thoughts due to increased anxiety, which influences performance because the intrusive thoughts consume needed working memory resources (see Schmader & Johns, 2003; Schmader, Johns, & Forbes, 2008 for a review). But the existing work with older adults on stereotype threat does not clearly support this mechanism. Some studies show that stereotype threat increases anxiety in older adults under some conditions (Abrams, Eller, & Bryant, 2006; Hess, Hinson, & Hodges, 2009). However, in other studies, threat does not affect anxiety in older adults (see Hess, et al., 2003; Hess & Hinson, 2006). We note that in the Hess and Hinson study, the effects of threat on many of the dependent variables including anxiety were small or nonsignificant, so more work is needed to understand the relationship between threat and anxiety. Of course the current study did not directly examine threat and so the connections between the effects of task success and the mechanisms involved with threat effects are only speculative at this point. In addition, older adults in our study were asked to report on their level of anxiety at the conclusion of the experiment, so one might wonder whether reductions in anxiety related to prior task success on the scramble task or prior task success on the memory test. Future research will have to thoroughly investigate the mechanisms behind the prior task success effect on memory using more refined measures of anxiety directly following the task success manipulation. Future work will also need to investigate other potential mediators. For example, it’s been shown that stereotype threat is associated with poor performance expectations, which leads to poorer subsequent memory performance (Desrichard & Kopetz, 2005; Hess et al., 2009; see also Barber & Mather, in press, for review). It will be important to examine how performance expectations (either specific task expectations or general memory expectations and self-efficacy) might mediate the relationship between task success and later memory performance.
The lack of a difference in recall and self-reported anxiety between the control and failure conditions is also potentially interesting. It may be that recall would have been lower (and anxiety higher) in the task failure condition relative to the control condition if a stronger manipulation of task failure was used. In the current study, task success was certainly lower in the failure condition by design (participants correctly unscrambled fewer than half of the sentences), but success was not completely eliminated. Thus, it is possible that memory performance would be lower relative to control following complete failure on a prior task. We can examine this issue with the current data given that there is variability in performance on the scramble task for participants in the failure condition. Indeed, it appears that the number of sentences completed correctly in the failure condition is significantly correlated with subsequent recall performance (r =.586,). So, those with worse performance on the task did show worse subsequent memory performance. Of course this correlation has to be interpreted with caution as it may simply reflect individual differences in ability on both tasks. An alternate hypothesis is that the task failure condition led to the same performance as the control condition because older adults expect to have some difficulty with cognitive tasks given in a lab situation so that experiencing failure on the scramble task is consistent with their expectations.
Other interesting questions arise. For example, one might wonder if any type of prior task success (even success on a non cognitive task) could improve subsequent memory performance or cognitive performance in general. One might also want to examine other boundary conditions to this effect, including the time course and if additional task success experiences could further improve memory performance in older adults. Future work might examine this issue but for now, the noteworthy finding from the current study is that providing older adults with a single successful cognitive experience led to significant improvements in memory performance.
The current results may be of practical importance. For example, it could be beneficial to create various opportunities for success in late life to help improve or maintain cognitive abilities. The current findings also have clinical assessment implications, particularly for neuropsychological assessment, where oftentimes little attention is paid to how previously performed (easy or difficult) tasks might influence an individual’s performance on subsequent assessment tasks. It may also be critical to consider basic design issues in cognitive aging research regarding the placement of routinely administered tests, such as vocabulary tests, for example, that older adults complete successfully, versus working memory tests or verbal fluency tests that older adults perform less successfully. Future research might examine the influence of the placement of these common tasks on older adults’ subsequent task performance. Finally, the current results are of theoretical importance because they support the emerging notion that age-associated memory changes are influenced not only by cognitive and neurological mechanisms, but also by potentially controllable contextual factors.
Table 1.
Proportion correctly recalled and standard error for younger and older adults in each condition.
| Prior Task Experience | |||
|---|---|---|---|
|
| |||
| Success | Control | Failure | |
| Age | |||
| Younger adults | .52 (.02) | .53 (.02) | .56 (.03) |
| Older adults | .47 (.02) | .39 (.02) | .39 (.03) |
Acknowledgments
This research was supported by National Institute on Aging Grant RO1 AG039502 awarded to Lisa Geraci.
References
- Abrams D, Eller A, Bryan J. An age apart: The effects of intergenerational contact and stereotype threat on performance and intergroup bias. Psychology and Aging. 2006;21:691–702. doi: 10.1037/0882-7974.21.4.691. [DOI] [PubMed] [Google Scholar]
- Barber SJ, Mather M. Stereotype threat in older adults: When and why does it occur, and who is most affected? In: Verhaeghen P, Hertzog C, editors. The Oxford Handbook of Emotion, Social Cognition, and Everyday Problem Solving during Adulthood. (in press) [Google Scholar]
- Bargh JA, Chen M, Burrows L. Automaticity of social behavior: Direct effects of trait construct and stereotype activation on action. Journal of Personality and Social Psychology. 1996;71:230–244. doi: 10.1037//0022-3514.71.2.230. [DOI] [PubMed] [Google Scholar]
- Berry J. Memory self-efficacy in its social cognitive context. In: Hess TM, Blanchard-Fields F, editors. Social cognition and aging. Academic Press; San Diego, CA: 1999. pp. 69–96. [Google Scholar]
- Chasteen AL, Bhattacharyya S, Horhota M, Tam R, Hasher L. How feelings of stereotype threat influence older adults’ memory performance. Experimental Aging Research. 2005;31:235–260. doi: 10.1080/03610730590948177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrichard O, Kopetz C. A threat in the elder: The impact of task-instructions, self-efficacy and performance expectations on memory performance in the elderly. European Journal of Social Psychology. 2005;35:537–552. [Google Scholar]
- Eich TS, Knowlton B, Castel AD. Memory performance and stereotype threat across normal aging. Poster presented at the 16th Annual Cognitive Neuroscience Society Meeting; San Francisco, CA. Apr, 2008. [Google Scholar]
- Fleischman DA, Gabrieli JDE. Repetition priming in normal aging and Alzheimer’s disease: A review of findings and theories. Psychology and Aging. 1998;13:88–119. doi: 10.1037//0882-7974.13.1.88. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE, Vaidya CJ, Stone M, Francis WS, Thompson-Schill SL, Fleischman DA, Wilson RS. Convergent behavioral and neuropsychological evidence for a distinction between identification and production forms of repetition priming. Journal of Experimental Psychology: General. 1999;128:479–498. doi: 10.1037//0096-3445.128.4.479. [DOI] [PubMed] [Google Scholar]
- Geraci L. A test of the frontal lobe functioning hypothesis of age deficits in production priming. Neuropsychology. 2006;20:530–548. doi: 10.1037/0894-4105.20.5.539. [DOI] [PubMed] [Google Scholar]
- Heckhausen J, Dixon RA, Baltes PB. Gains and losses in development throughout adulthood as perceived by different age groups. Developmental Psychology. 1989;25:109–121. [Google Scholar]
- Hertzog C, Lineweaver TT, McGuire CL. Beliefs about memory and aging. In: Blanchard-Fields F, Hess TM, editors. Social Cognition and Aging. Academic Press; New York: 1999. pp. 43–68. [Google Scholar]
- Hess TM, Auman C, Colcombe SJ, Rahhal TA. The impact of stereotype threat on age differences in memory performance. Journal of Gerontology: Psychological Sciences. 2003;58B:3–11. doi: 10.1093/geronb/58.1.p3. [DOI] [PubMed] [Google Scholar]
- Hess TM, Hinson JT. Age-related variation in the influences of aging stereotypes on memory in adulthood. Psychology and Aging. 2006;21:621–625. doi: 10.1037/0882-7974.21.3.621. [DOI] [PubMed] [Google Scholar]
- Hess TM, Hinson JT, Hodges EA. Moderators of and mechanisms underlying stereotype threat effects on older adults’ memory performance. Experimental Aging Research. 2009;35:153–177. doi: 10.1080/03610730802716413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess TM, Hinson JT, Stratham JA. Explicit and implicit stereotype activation effects on memory: Do age and awareness moderate the impact of priming? Psychology and Aging. 2004;19:495–505. doi: 10.1037/0882-7974.19.3.495. [DOI] [PubMed] [Google Scholar]
- Levy B. Improving memory in old age through implicit self-stereotyping. Journal of Personality and Social Psychology. 1996;71:1092–1107. doi: 10.1037//0022-3514.71.6.1092. [DOI] [PubMed] [Google Scholar]
- Levy B, Langer E. Aging free from negative stereotypes: Successful memory in China among the American deaf. Journal of Personality and Social Psychology. 1994;66:989–997. doi: 10.1037//0022-3514.66.6.989. [DOI] [PubMed] [Google Scholar]
- Light LL, Prull MW, La Voie D, Healy MR. Dual-process theories of memory in older age. In: Perfect TJ, Maylor EA, editors. Models of cognitive aging. Oxford University Press; Oxford, England: 2000. pp. 238–300. [Google Scholar]
- Lineweaver TT, Hertzog C. Adults’ efficacy and control beliefs regarding memory and aging: Separating general from personal beliefs. Aging, Neuropsychology, and Cognition. 1998;5:264–296. [Google Scholar]
- Rahhal TA, Hasher L, Colcombe SJ. Instructional manipulations and age differences in memory: Now you see them, now you don’t. Psychology and Aging. 2001;16:697–706. doi: 10.1037//0882-7974.16.4.697. [DOI] [PubMed] [Google Scholar]
- Rebok GW, Carlson MC, Langbaum JBS. Training and maintaining memory abilities in healthy older adults: Traditional and novel approaches. The Journals of Gerontology: Psychological Sciences. 2007;1:53–61. doi: 10.1093/geronb/62.special_issue_1.53. [DOI] [PubMed] [Google Scholar]
- Roenker DL, Thompson CP, Brown SC. Comparison of measures for the estimation of clustering in free recall. Psychological Bulletin. 1971;76:45–48. [Google Scholar]
- Ryan EB. Beliefs about memory changes across the adult life span. Journal of Gerontology. 1992;47:41–46. doi: 10.1093/geronj/47.1.p41. [DOI] [PubMed] [Google Scholar]
- Ryan EB, Kwong See S. Age-based beliefs about memory changes for self and others across adulthood. The Journal of Gerontology. 1993;48:199–201. doi: 10.1093/geronj/48.4.p199. [DOI] [PubMed] [Google Scholar]
- Schmader T, Johns M. Converging evidence that stereotype threat reduces working memory capacity. Journal of Personality and Social Psychology. 2003;85:440–452. doi: 10.1037/0022-3514.85.3.440. [DOI] [PubMed] [Google Scholar]
- Schmader T, Johns M, Forbes C. An integrated process model of stereotype threat effects on performance. Psychological Review. 2008;115:336–356. doi: 10.1037/0033-295X.115.2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele CM, Aronson J. Stereotype threat and the intellectual test performance of African Americans. Journal of Personality and Social Psychology. 1995;69:797–811. doi: 10.1037//0022-3514.69.5.797. [DOI] [PubMed] [Google Scholar]
- Stein R, Blanchard-Fields F, Hertzog C. The effects of age stereotype priming on the memory performance of older adults. Experimental Aging Research. 2002;28:169–181. doi: 10.1080/03610730252800184. [DOI] [PubMed] [Google Scholar]
- West RL, Bagwell DK, Dark-Freudeman A. Self-efficacy and memory aging: The impact of a memory intervention based on self-efficacy. Aging, Neuropsychology, and Cognition. 2008;15:302–329. doi: 10.1080/13825580701440510. [DOI] [PubMed] [Google Scholar]
- Zachary RA. Shipley Institute of Living Scale, Revised Manual. Western Psychological Services; Los Angeles, CA: 1986. [Google Scholar]