Abstract
Purpose
To test the feasibility of using proposed quality indicators to assess radiotherapy quality in prostate cancer management based on a 2007 stratified random survey of treating academic and non-academic US institutions.
Methods and Materials
414 patients with clinically localized prostate cancer treated with external beam radiotherapy (EBRT) or brachytherapy were selected from 45 institutions. Indicators used as specific measurable clinical performance measures to represent surrogates for quality of radiotherapy delivery included established measures, such as the use of prescription doses ≥75 Gy for intermediate- and high-risk EBRT patients and androgen-deprivation therapy (ADT) in conjunction with EBRT for patients with high-risk disease, and emerging measures, including daily target localization (image-guidance) to correct for organ motion for EBRT patients.
Results
167 patients (47%) were treated with 6 MV photons, 31 (9%) were treated with 10 MV, 65 (18%) received 15 MV, and the remaining 90 (26%) 16–23 MV. For intermediate- plus high-risk patients (n=181), 78% were treated to ≥75 Gy. Among favorable-risk patients, 72% were treated to ≥75 Gy. Among high-risk EBRT patients, 60 (87%) were treated with ADT in conjunction with EBRT and 13% (n=9) with radiotherapy alone. Among low- and intermediate-risk patients, 10% and 42%, respectively, were treated with ADT plus EBRT. For 24% of EBRT patients (85/354), weekly electronic portal imaging was obtained as verification films without daily target localization and the remaining 76% were treated with daily localization of the target using various methods.
Conclusions
Adherence to defined quality indicators was observed in a majority of patients. ≈90% of high-risk patients are treated with ADT plus EBRT and ≈80% of intermediate- and high-risk patients receive prescription doses >=75 Gy, consistent with the published results of randomized trials.
Keywords: external beam radiotherapy, quality indicators, androgen deprivation therapy, dose escalation, prostate cancer
Introduction
With societal demands for improving the quality of cancer care in the United States, the importance of establishing quality indicators (QI) by which individual care can be assessed and compared with national practice is critical. (1–3) Such QIs can be derived from established clinical guidelines, results of clinical trials, expert consensus, and evolving QIs based upon rapidly emerging technologies. These QIs form the basis for assessing the quality of therapy and practice as well as the identification of deficiencies that could potentially benefit from practice improvement.
Since 1973, the Patterns of Care Study conducted detailed retrospective surveys of national radiation oncology practice. This unique quality-improvement initiative has had a major positive impact on contemporaneous practice and has kept pace with dramatic alterations in the radiation oncology structural base and clinical processes. (4–7) With an interest in placing greater emphasis on quality of care, the Patterns of Care Study evolved into the Quality Research in Radiation Oncology (QRRO) under the auspices of the American College of Radiology. As part of this effort, QRRO investigators established QIs for various disease subsites based upon published guidelines such as those from the National Comprehensive Cancer Network (NCCN) as well as emerging QIs for processes involving emerging technologies. QRRO then initiated a national process survey for various disease sites to obtain the necessary benchmark data to facilitate the evaluation of quality of care in radiation oncology as practiced in the United States.
The present study summarizes the results of a stratified random survey conducted by QRRO of prostate cancer patients treated in 2007 with external beam radiotherapy or brachytherapy collected from 45 academic and non-academic institutions in the United States. The purpose of this survey was to test the feasibility of using proposed clinical performance measures to estimate established and emerging quality indicators of radiotherapy treatment delivery in the treatment of prostate cancer among institutions surveyed.
Methods and Materials
The survey design utilized a two-stage stratified random sampling of radiation oncology facilities in the United States (first stage) and further random selection of treated patients for localized prostate cancer (second stage) within that facility. Eligibility criteria for inclusion in the survey were as follows: 1) patients with biopsy-proven adenocarcinoma of the prostate; 2) treatment consisted of brachytherapy, external beam radiotherapy (EBRT), or combination thereof; 3) patients received their treatment during one year (2007); 4) the use of androgen-deprivation therapy in conjunction with radiotherapy was acceptable if started no more than six months prior to initiation of radiation therapy; 5) patients who had a prior radical prostatectomy or were treated for recurrent/metastatic disease were excluded. Using this design and criteria, 414 patients with clinically localized prostate cancer treated with EBRT or brachytherapy were randomly selected from 45 institutions that participated out of 106 invited facilities.
Data were extracted on site at each of the facilities by highly trained QRRO research associates. All medical records, and radiotherapy charts and records, were carefully reviewed. Data collected included patient demographics and characteristics, clinical and pathological factors, and treatment details including dosimetric information. For the purposes of data collection and analysis, prognostic risk groups were defined according to the criteria of the NCCN Guidelines. (8)
Prior to the survey the members of the Genitourinary Committee of QRRO identified six QIs as specific measurable clinical performance measures (CPM) that would be surrogates for quality of radiotherapy delivery for the treatment of prostate cancer. The methods used for developing these CPMs have been previously reported. (9) At the time this study design was conceived in 2006, we identified three CPMs that were considered established measures of quality (based upon Level I evidence) and three emerging measures that also reflected quality of treatment delivery based on data from peer-reviewed published references (see Appendix 1: 2007 QRRO Clinical Performance Measures).
The CPMs (10) included the following:
-
1)
Use of high-energy linear accelerators (≥6MV) in men with non-metastatic prostate cancer treated with external beam radiotherapy (photons or protons)
-
2)
Use of prescription doses of ≥75 Gy for intermediate- and high-risk patients treated with EBRT (for this analysis patients treated with hypofractionation regimens were excluded)
-
3)
Use of androgen-deprivation therapy (ADT) in conjunction with EBRT for patients with high-risk disease
-
4)
Use of dose-volume histogram (DVH) evaluations of the target and normal tissue structures in the planning for EBRT (Emerging Measure 1)
-
5)
Dosimetric assessment of target coverage after brachytherapy using post-implantation computed tomography (CT) scanning. (Emerging Measure 2)
-
6)
Use of daily target localization (image-guidance) for correction of organ motion for patients treated with EBRT (Emerging Measure 3)
The above CPMs were analyzed based upon the prognostic risk-group classification, academic/non-academic strata and regional location in the USA (i.e., Northeast, South, Central, and West). Results for CPMs are reported by unweighted case counts in each category. Since the CPMs are computed for numerous small sub-sets of patients, they are reported by un-weighted case counts in each category. Other descriptive results are also reported by un-weighted case counts.
Although national averages are computed using weights reflecting the relative contribution of each institution and each patient in the sample, results comparing small subsets of patients are reported for the surveyed sample. Patients in academic facilities are more heavily represented in the sample compared to those in non-academic facilities. Statistical analyses were conducted using SAS software, version 9.2 of the SAS System for Windows (Copyright 2002–2008 by SAS Institute Inc., Cary, NC, USA). Statistical comparisons were made using Pearson Chi-Square test of significance.
Results
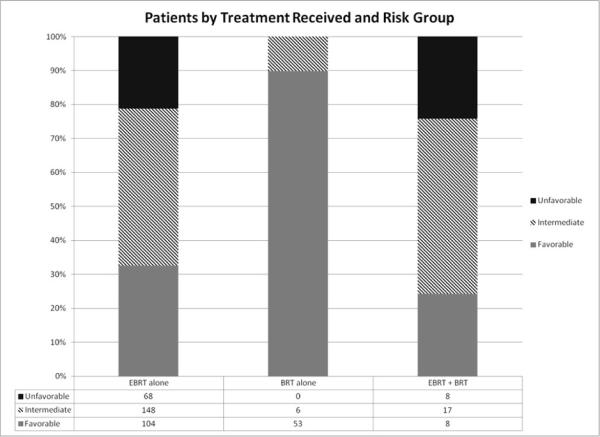
Table 1 demonstrates the patient characteristics of the surveyed population. Thirty-one (69%) of the institutions surveyed were non-academic settings and the remaining 14 (31%) were academic centers. The median age of the surveyed population at the time of treatment was 68 years (range, 46–89 years). The prognostic risk-group classification breakdown (according to the NCCN classification) in these patients were favorable risk, 165 (40%); intermediate risk, 171 (41%); and unfavorable risk, 76 (19%). The use of EBRT or brachytherapy according to the prognostic risk groupings is shown in Figure 1. Characteristics of patients receiving brachytherapy are shown in Table 2 and comparisons of patients receiving EBRT and brachytherapy are shown in Table 3.
Table 1.
Patient characteristics
| Patient Characteristics | Weighted Patients Number (%) | Unweighted Patients (n=414) Number (%) |
|---|---|---|
| Age at start of RT | ||
| ≤60 | 11,417 (17.8) | 93 (22.4) |
| 61–70 | 25,223 (39.2) | 170 (41.1) |
| >70 | 27,693 (43.0) | 151(36.5) |
| Stratum | ||
| Academic | 5,785 (9.0) | 140 (33.8) |
| Non-academic | 58,548 (91.0) | 274 (66.2) |
| Pretreatment PSAa | ||
| <10 | 46,259 (71.9) | 309 (74.8) |
| 10–20 | 14,194(22.1) | 75 (18.2) |
| >20 | 3,839 (6.0) | 29 (7.0) |
| Biopsy Gleasonb | ||
| 6 | 32,015 (49.8) | 206 (49.9) |
| 7 | 23,548 (36.6) | 155 (37.5) |
| 8–10 | 8,690 (13.5) | 52 (12.6) |
| Risk groupc | ||
| Favorable | 25,011 (38.9) | 165 (40.0) |
| Intermediate | 27,088 (42.1) | 171 (41.5) |
| Unfavorable | 12,074 (18.8) | 76 (18.5) |
| Use of ADT during or before treatment | ||
| No | 39,812 (61.9) | 263 (63.5) |
| Yes | 24,521 (38.1) | 151 (36.5) |
Excluding 1 patient (weighted n=41) with missing Pretreatment PSA.
Excluding 1 patient (weighted n=80) with missing Gleason score.
Excluding 2 patients (weighted n=160) for whom risk group could not be determined.
ADT, androgen deprivation therapy; PSA, prostate-specific antigen.
Figure 1.
Breakdown of patients according to treatment received.
Table 2.
Patients treated with brachytherapy
| Characteristic (n=93) | Patients Number (%) |
|---|---|
| Age at start of RT | |
| Median (66 years) | |
| Range (49–83 years) | |
| Stratum | |
| Academic | 23 (24.7) |
| Non-academic | 70 (75.3) |
| Risk groupa | |
| Low (favorable) | 61 (66.3) |
| Intermediate | 23 (25.0) |
| High (unfavorable) | 8 (8.7) |
| Type of implant | |
| Iodine-125 | 72 (77.4) |
| Palladium-103 | 14 (15.1) |
| High dose rate | 7 (7.5) |
| Endocrine manipulation | |
| No | 76 (81.7) |
| Yes | 17 (18.3) |
Excluding 1 patient for whom risk group could not be determined.
Table 3.
Comparison of patients treated with external beam radiotherapy and brachytherapy
| Characteristic | EBRT (n=321) | Brachytherapya (n = 93) | Total (n=414) | Chi-square p Value |
|---|---|---|---|---|
| Stratum | 0.035 | |||
| Academic | 117 (36%) | 23 (25%) | 140 (34%) | |
| Non-academic | 204 (64%) | 70 (75%) | 274 (66%) | |
| Region | NS | |||
| Northeast | 41 (13%) | 16 (17%) | 57 (14%) | |
| Midwest | 92 (29%) | 24 (26%) | 116 (28%) | |
| South | 112 (35%) | 39 (42%) | 151 (36%) | |
| West | 76 (24%) | 14 (15%) | 90 (22%) | |
| Age at start of RT | ||||
| ≤60 years | 71 (22%) | 22 (24%) | 93 (22%) | 0.009 |
| 61–70 years | 121 (38%) | 49 (53%) | 170 (41%) | |
| >70 years | 129 (40%) | 22 (24%) | 151 (36%) |
EBRT: External Beam Radiation Therapy.
NS= not significant
Includes 33 patients who were treated with both EBRT and Brachytherapy.
Clinical performance measure: use of high-energy linear accelerators for EBRT
Among the 354 patients treated with EBRT, the beam energy was recorded in 353 patients and the distribution of energies used was as follows: 167 patients (47%) were treated with 6 MV photons, 31 patients (9%) were treated with 10 MV photons, 65 patients (18%) received 15 MV photons, and the remaining 90 patients (26%) were treated with energies ranging from 16 to 23 MV, which included 7 patients treated with 250 MeV proton therapy. No patient surveyed was treated with Cobalt-60 therapy. There were significant differences observed in beam energy used based upon academic/non-academic stratification and by regional differences in the US. Of the 353 patients for whom energy was recorded, 121 (34%) were treated at academic facilities and 232 (66%) at non-academic facilities. Among patients treated in academic facilities 42% were treated with energy of 6 MV, 5% with energy of 10 MV, 20% with energy with energy of 15 MV, and 33% with energy of 16–23 MV. On the other hand among patients treated in non-academic facilities the corresponding percentages were 50%, 11%, 18%, and 22% respectively (p=0.038).
In the Northeast, 69% (29/42) of the patients received 6 MV whereas only 7% (3/42) were treated with energies ranging from 16 to 23 MV. Proportions of patients treated with the higher energies in the Midwest, South, and West were 31%, 24%, and 30%, respectively (p < 0.0001).
Clinical performance measure: use of higher radiation doses for intermediate- and high-risk patients
Prescription doses ranged from 2500 to 8100 cGy. In the cohort of intermediate- and high-risk patients (n=181), the majority (n=141, 78%) were treated to dose levels of ≥75 Gy. Within this high dose range, 138 patients (98%) were treated to 75–<80 Gy and 3 (2%) were treated to ≥80 Gy. Only 11 (6%) and 29 (16%) patients were treated to dose levels of <72 Gy and 72–74 Gy, respectively.
An additional 14 patients with intermediate- and high-risk disease who were treated with hypofractionated treatment schedules and received a dose per fraction of >200 cGy, were excluded from this measure and analyzed separately. In this latter group, 2/14 (14%) were treated to cumulative dose levels of 75 Gy; 7 (50%) and 5 (36%) received dose levels of <72 Gy and 72–74 Gy, respectively.
Although not included in this CPM, we observed among favorable-risk patients (n = 87) a similar breakdown of prescription doses. Sixty-three patients (72%) were treated to dose levels of ≥75 Gy, and 22 (25%) and 2 (2%) were treated to dose levels of 72–74 Gy and <72 Gy, respectively. There were no differences observed in the use of higher dose levels (≥75 Gy) based upon academic/non-academic stratification or regional variations in the United States (p = 0.1).
Clinical performance measure: use of ADT for high-risk patients
Among high-risk patients treated with EBRT, 87% (n = 60) were also treated with ADT and 13% (n = 9) were treated with radiotherapy alone. Among low- and intermediate-risk patients, 10% and 42%, respectively, were treated with ADT in conjunction with EBRT. Information regarding the planned duration of ADT was only available in 87 of 153 patients who were treated with ADT (57%). Sixty-three percent (55/87) of patients were treated with ADT regimens described as short duration or ≤6 months and the remaining 32 (37%) patients were treated with longer regiments up to 36 months. Among these 32 patients treated with longer ADT regimens, 23 (72%) of the patients were in the high-risk group, 9 (28%) were in the intermediate-risk group, and 1 was in the low-risk group (p < 0.0001). No differences were found by academic/non-academic classification or by region of the United States.
Emerging performance measure: use of DVH evaluations of the target and normal tissue structures in the planning for EBRT
Among patients treated with EBRT, 95% (337/354) underwent treatment planning that integrated DVH evaluations for assessment of rectum, bladder, and target dosing consistent with three-dimensional conformal or intensity-modulated radiation therapy (IMRT)-based treatment. Twelve percent of patients (39/337) were treated with three-dimensional conformal treatment, 82% (276/337) were treated with IMRT-based treatment planning, and the remaining 4% (13/337) were treated with regimens that combined three-dimensional conformal radiation therapy and IMRT. Academic facilities were significantly more likely to use DVH evaluation of the target structures compared to non-academic (p=0.11). Use of DVH evaluation differed significantly by census region with the highest rate in the Northeast (100%) and the lowest rate in the West (87%) (p=0.001). .
Emerging performance measure: use of CT-based post-implant dosimetric evaluation
Ninety-three patients were treated with brachytherapy and within this group 92% (86/93) received low-dose rate brachytherapy. The characteristics of all the brachytherapy-treated patients are shown in Table 2. The median dose for the patients treated with I-125 permanent implantation was 145 Gy and the median dose for the Pd-103 patients was 100 Gy, as this latter isotope was more often used as a boost during combined brachytherapy-EBRT treatments.
Ninety-nine percent (85/86) of patients underwent post-implantation dosimetric evaluation, which was obtained in 45% of treated cases 30 days after the implantation procedure. Because almost all patients had this evaluation, we did not test for differences by type of facility or region. The timing of post-implantation CT based evaluation ranged from day 0 to beyond 40 days after implantation.
Emerging performance measure: use of daily target localization (image-guidance) for correction of organ motion for patients treated with EBRT
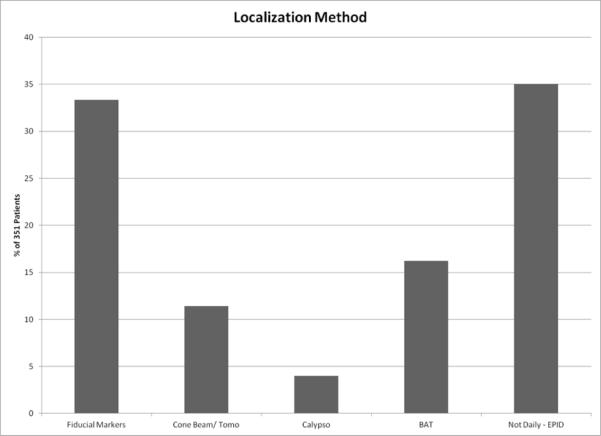
For 35% of patients treated with EBRT (123/354), weekly electronic portal imaging was obtained as verification films without daily target localization and for 3 patients this information was not reported. The remaining 64% were treated with daily localization of the target using the various methods indicated, as shown in Figure 2. Of patients treated in academic facilities 73% had daily localization compared to 60% in non-academic facilities, a significant difference (p=0.015). This measure was not found to be significantly different by region.
Figure 2.
Breakdown of external beam radiotherapy patients according to use of image-guided daily localization.
Discussion
This report is the first analysis of the QRRO survey for prostate cancer patients, and has revealed important practice patterns across the United States in the management of the disease. Dose escalation appears to be routinely utilized across all risk groups, with approximately 75% of patients treated to dose levels of >=75 Gy. These findings suggest a high penetration during the survey period of the results of published randomized trials demonstrating improved tumor control outcomes with higher radiation dose levels, which has been an observed benefit for all prognostic risk groups (11–14). Nevertheless it is interesting to note that a small but significant percentage of patients (~25%) were treated to dose levels that would be considered suboptimal for the treatment of intermediate- and high-risk disease. It is possible that in some of these patients, lower dose levels may have been used by the treating physician due to baseline medical comorbidities of the patient. Nevertheless, we could not demonstrate that the use of lower doses by some practitioners was associated with a particular region of the United States or based upon other variables such as race, age, or socioeconomic status of the patient.
The other important observation noted in this survey is that nearly 90% of practitioners utilized ADT in conjunction with EBRT for patients with high-risk disease, consistent with the results of published randomized trials (15–19). An equally interesting observation in our report was that there were significantly fewer patients (42%) with intermediate-risk disease treated with ADT in conjunction with EBRT. This latter observation is also consistent with the absence of a preponderance of Level 1 evidence at the time of this survey that ADT improved tumor control outcomes for intermediate-risk patients. One randomized trial demonstrating evidence for benefit for intermediate-risk patients was initially published in 2004 (20), and only recently further evidence has become available that supports a role for ADT with EBRT in this cohort of patients (21). These trials did use low dose irradiation in conjunction with ADT and may not demonstrate the necessity of this combined modality program in the setting of escalated radiation dose levels. Nevertheless, we anticipate that, as a result of this more recent trial, there will be a further increase in the use of ADT in conjunction with EBRT for intermediate-risk disease. We also note, consistent with appropriate standards of care, that only 10% of favorable-risk patients were treated with ADT in conjunction with EBRT, and we speculate that these patients were likely selectively treated with ADT for downsizing the prostate prior to initiation of EBRT. The low percentage of patients treated with ADT for favorable-risk patients appears to be significantly lower than we previously reported based on an earlier survey in 1998, when approximately 31% of favorable-risk patients were treated with ADT (22). Unfortunately, in the current report specific information regarding the duration of ADT given to these patients was not routinely documented in the medical charts.
Finally, nearly all patients were treated with conformal treatment techniques and a high percentage (82%) of the patients was treated with IMRT. These findings are consistent with recently reported observations (23). It is interesting to note that approximately 75% of the patients were treated with some form of daily image guidance as well. While the latter practice is not based on Level I evidence even at the time of this report, it nevertheless appears to be a significantly widespread practice for delivering image-guided therapy for patients with clinically localized prostate cancer. In retrospect, however, the CPM of DVH assessment as a surrogate for conformal based treatment planning (CPM 4) and the use of dosimetric assessment of implantation quality based on the performance of a post-implantation CT scan (CPM 5) were not likely robust measures of treatment quality. We recognize that the simple performance of these studies or assessments without linking these to a dosimetric measurement (such a target dose or normal tissue constraint) could be inadequate. It may be more appropriate in the future to consider for EBRT the D95 for the target volume (dose delivered to 95% of the target volume) and the volume of the rectum exposed to greater than 70 Gy as measures of quality of treatment delivery (24). For brachytherapy quality assessment, appropriate quality indicators would include D90 (dose to 90% of the prostate) that should receive ≥140 Gy and the volume of the rectum exposed to prescription doses ≤1 cm3 (25). In fact, these more specific evaluations of implant quality have been performed on the brachytherapy patients in this current cohort based on an external QRRO review of the post-implantation scans and these findings will be reported in a separate publication.
In conclusion, adherence to proposed QRRO quality indicators was observed in a high percentage of patients treated. Almost 90% of high-risk patients were treated with ADT plus EBRT and approximately 80% of intermediate and high-risk patients were treated with escalated dose levels of radiotherapy. A trend was noted for academic facilities to utilize higher radiation energies for EBRT as well as a greater likelihood to utilize DVH evaluation of target structures compared to non-academic centers. In addition and of interest was that approximately two thirds of EBRT patients were treated with image-guided radiotherapy using daily localization of the target. We also noted in this survey that of patients treated in academic facilities, a higher percentage utilized daily target localization compared to non-academic facilities.
Acknowledgements
The authors would like to thank all the radiation oncologists, physicists, and staff at participating facilities for their support and cooperation, which is essential to the QRRO Process Survey. They also thank Lisa Morabito for administrative support and Joanne Sorich, R.N. and Alex Ho, M.S., M.A. for data design, data management, and quality management.
Supported by NCI Grant CA065435. This project was funded, in part, under a grant with the Pennsylvania Department of Health. The Department specifically declaims responsibility for any analyses, interpretations or conclusions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Notification: Dr. Zelefsky has received honoraria from Mick Radionuclear. Dr. Lee is paid a stipend by ASTRO for his role as Editor-in-Chief of Practical Radiation Oncology has collected royalties for his role as Section Editor for UpToDate and fees related to medical record review. Dr. Zietman is a board member of the American Society for Radiation Oncology. Ms. Khalid's, Dr. Lee's, Dr. Owen's, and Dr. Wilson's institutions received grants from NCI.
References
- 1.Institute of Medicine, Crossing the quality chasm: a new health system for the 21st century. National Academy Press; Washington DC: 2001. [Google Scholar]
- 2.Hewitt M, Simone JL. Ensuring quality cancer care. Institute of Medicine, National Academy Press; Washington, D.C.: 1999. [PubMed] [Google Scholar]
- 3.McGlynn EA, Malin J. Selecting National Goals and Core Measures of Cancer Care Quality, report to Steering Committee on Cancer National Quality Forum. 2002. [Google Scholar]
- 4.Hanks GE, Teshima T, Pajak TF. 20 years of progress in radiation oncology: Prostate cancer – Seminars in Radiation Oncology. 1997;7(2):114–120. doi: 10.1053/SRAO00700114. [DOI] [PubMed] [Google Scholar]
- 5.Thomas GM. Over 20 years of progress in radiation oncology: Seminoma - Seminars in Radiation Oncology. 1997;7(2):135–145. doi: 10.1053/SRAO00700135. [DOI] [PubMed] [Google Scholar]
- 6.Hewitt M, Simone JV. Enhancing Data Systems to Improve the Quality of Cancer Care. Institute of Medicine and Commission on Life Sciences. National Research Council, National Academy Press; Washington, DC: 2000. pp. 18–37. [PubMed] [Google Scholar]
- 7.Earle CC, Emmanuel EJ. Patterns of Care Studies: Creating “An Environment of Watchful Concern”. J Clin Oncol. 2003;21(24):4479–4480. doi: 10.1200/JCO.2003.09.979. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Prostate Cancer. Version 2. 2007. [DOI] [PubMed] [Google Scholar]
- 9.Crozier C, Wittman-Erickson B, Movsas B, et al. Shifting the Focus to Practice Quality Improvement in Radiation Oncology. J for Healthcare Quality. 2011;33(5):49–57. doi: 10.1111/j.1945-1474.2011.00119.x. [DOI] [PubMed] [Google Scholar]
- 10. [Accessed 1/4/2012]; http://www.qrro.org/Prostate_CPM.pdf .
- 11.Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53:1097–1105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- 12.Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from Proton Radiation Oncology Group/American College of Radiology 95-09. J Clin Oncol. 2010;28:1106–1111. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peeters ST, Heemsbergen WD, Koper PC, et al. Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006;24:1990–1996. doi: 10.1200/JCO.2005.05.2530. [DOI] [PubMed] [Google Scholar]
- 14.Dearnaley DP, Sydes MR, Graham JD, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2007;8(6):475–487. doi: 10.1016/S1470-2045(07)70143-2. [DOI] [PubMed] [Google Scholar]
- 15.Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma-long-term results of phase III RTOG 85–31. Int J Radiat Oncol Biol Phys. 2005;61:1285–1290. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 16.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomized trial. Lancet. 2002;360:103–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 17.Pilepich MV, Winter K, John MJ, et al. Phase III radiation therapy oncology group (RTOG) trial 86–10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2001;50:1243–1252. doi: 10.1016/s0360-3016(01)01579-6. [DOI] [PubMed] [Google Scholar]
- 18.Hanks GE, Pajak TF, Porter A, et al. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate. The Radiation Therapy Oncology Group Protocol 92–02. J Clin Oncol. 2003;21:3972–3978. doi: 10.1200/JCO.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 19.Roach M, 3rd, Desilvio M, Lawton C, et al. Phase III trial comparing whole pelvic versus prostate only radiotherapy and neoadjuvant versus adjuvant combined androgen suppression: Radiation Therapy Oncology Group 9413. J Clin Oncol. 2003;21:1904–1911. doi: 10.1200/JCO.2003.05.004. [DOI] [PubMed] [Google Scholar]
- 20.D'Amico AV, Manola J, Loffredo M, et al. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer. JAMA. 2004;292:821–827. doi: 10.1001/jama.292.7.821. [DOI] [PubMed] [Google Scholar]
- 21.Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365:107–18. doi: 10.1056/NEJMoa1012348. [DOI] [PubMed] [Google Scholar]
- 22.Zelefsky MJ, Moughan J, Owen J, et al. Changing trends in national practice for external beam radiotherapy for clinically localized prostate cancer: 1999 patterns of care survey for prostate cancer. Int J Radiat Oncol Biol Phys. 2004;59:1053–1061. doi: 10.1016/j.ijrobp.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen PL, Gu X, Lipsitz SR, et al. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol. 2011;29:1517–1524. doi: 10.1200/JCO.2010.31.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michalski JM, Jackson A, Tucker SL. QUANTEC-Quantitative analyses of normal tissue effects in the clinic. Int J Radiat Oncol Biol Phys. 2010;76(suppl):123–129. doi: 10.1016/j.ijrobp.2009.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crook JM, Potter L, Stock RG, Zelefsky MJ. Critical organ dosimetry in permanent seed prostate brachytherapy: defining the organs at risk. Brachytherapy. 2005;4:186–194. doi: 10.1016/j.brachy.2005.01.002. [DOI] [PubMed] [Google Scholar]