Abstract
Objective
The purpose of this study was to assess the outcome of cardiac MRI (CMRI) with late gadolinium enhancement (LGE) at outpatient follow-up in a consecutive series of patients with troponin-positive chest pain but unobstructed coronary arteries at the index admission.
Methods
The study group comprised 91 consecutive patients who presented to our institution with cardiac chest pain, elevated troponin I and unobstructed coronary arteries on coronary angiography. All patients underwent an outpatient CMRI with LGE imaging in order to establish a definitive diagnosis.
Results
The average time from coronary angiography to LGE-CMRI was 2 months. 73% of patients had no abnormality on their LGE-CMRI, 16% of patients had patchy late enhancement consistent with myocarditis and 11% had focal subendocardial or full thickness late enhancement consistent with myocardial infarction. There were no deaths in this cohort during a mean follow-up of 21 months.
Conclusion
LGE-CMRI is a useful tool for establishing whether such patients have definitive evidence of non-ST-segment elevation myocardial infarction (NSTEMI), and can make an important contribution to the long-term management strategy of these patients as an inappropriate diagnosis of NSTEMI carries important medical, social and financial implications.
Chest pain is one of the commonest indications for acute hospital admission in the UK [1]. A history of cardiac-sounding chest pain stimulates a series of investigations including electrocardiography (ECG) recordings and cardiac biomarkers as part of risk stratification [2]. A large body of evidence has demonstrated that, in patients with troponin-positive non-ST-segment elevation myocardial infarction (NSTEMI), early revascularisation is associated with a significant reduction in the rates of major adverse cardiac events (MACE) [3,4]. Current American and European guidelines recommend early angiography and revascularisation in this population. For this reason the vast majority of patients presenting with troponin-positive chest pain undergo coronary angiography with a view to revascularisation on the index admission.
However, while the commonest reason for this presentation is NSTEMI due to atherosclerotic plaque rupture, there are other potential aetiologies, which include myocarditis or arrhythmia (particularly in the context of impaired left ventricular systolic function). Up to 10% of patients referred for angiography with troponin-positive chest pain have unobstructed coronary arteries [5,6]. This cohort of patients presents a diagnostic dilemma and as a result their subsequent management is heterogeneous. National and international guidelines lead us to an apparent diagnosis of NSTEMI [2,7] for patients presenting in this fashion. Given that it is possible for plaque rupture to occur at the site of a “non-obstructive” stenosis, which is generally defined as <50% by visual assessment on angiography, the finding of non-obstructed coronary arteries does not fully exclude the diagnosis of NSTEMI. By contrast, in many such cases, the true reason for admission may not be NSTEMI, with a differential diagnosis of myopericarditis most often considered. The management strategy adopted for this cohort of patients is variable. Many such patients are committed to post-myocantial infarction (MI) secondary prevention treatment for presumed NSTEMI and retain this diagnostic label. This in turn carries important potential implications for lifestyle outcomes, such as driving as well as insurance and job applications.
The development of late gadolinium enhancement (LGE) cardiac MRI (CMRI) provides a sensitive and specific tool for the detection of even small amounts of myocardial damage [8]. This group and others have previously described the application of CMRI for differentiation of myocardial damage due to coronary occlusion or inflammation [9-11]. Furthermore, the clinical implications of detecting no LGE in such patients is uncertain as the underlying cause for the troponin release remains unexplained.
The aim of this study was to assess the outcome of LGE-CMRI at outpatient follow-up in a consecutive series of patients who had presented to this regional cardiac centre with troponin-positive chest pain but who had no obstructive coronary artery disease at angiography on the index admission. We also sought to identify the relative proportions of patients with (a) no LGE, (b) LGE typical of MI and (c) patchy LGE typical of myocarditis in the largest cohort of such patients reported so far.
Methods and materials
Patient population
The study received full approval from the Hampshire and Isle of Wight Research Ethics Committee and from the Trust's Research and Development Department. 91 consecutive patients who had been referred for clinical reasons for outpatient CMRI with LGE to our unit were prospectively recruited into our data set. All patients had presented to our unit with (a) cardiac-sounding chest pain, (b) an elevated troponin I (as defined by local laboratory upper limit of normal range) and (c) unobstructed coronary arteries on angiography, defined as no atheromatous lesions causing stenosis of diameter >50% as assessed by visual assessment of experienced consultant interventional cardiologists. Patients with ST elevation on their admission ECG series were excluded from this study but other ECG abnormalities were not a reason for exclusion. Study follow-up was carried out by review of notes and by telephone interview with the patient's general practitioner, together with a postal questionnaire sent to the patient. Clinical follow-up was at the discretion of the referring cardiologist.
Cardiac MRI protocol
Patients were studied using a 1.5 T clinical CMRI scanner (Avanto®; Siemens Medical Solutions, Forchheim, Germany), with steady-state free-precession cine images acquired in three long axis planes and a stack of short axis images that encompassed the entire left ventricle (LV). Gadolinium dimeglumine (Multihance; Bracco spa, Milan, Italy) was then administered intravenously at a dose of 0.2 mmol kg–1 of body weight. Long and short axis LGE-CMRI images were acquired after a 10-min delay using an inversion recovery segmented gradient echo sequence. Typical voxel size was 1.9×1.4×7 mm. Two specialist consultant cardiac radiologists reported all the images on dedicated workstations and both were blinded to the troponin level and the appearance of the ECGs. LV end-diastolic and end-systolic volumes were generated using dedicated post-processing software and these were used to calculate the LV ejection fraction.
Statistical analysis
Data were analysed by using SPSS® software (v. 11; SPSS, Chicago, IL). Continuous variables are expressed as mean ± standard deviation (SD) and qualitative variables are expressed as percentages.
Results
The baseline characteristics of the 91 patients are summarised in Table 1. The mean age was 53.1±2.3 years (mean±SD). The risk factor profile was as follows: current smokers 28.6%, ex-smokers 37.4%, hypertension 40.7%, diabetes mellitus 3.3%, positive family history for premature coronary artery disease 42.9% and hyperlipidaemia 37.4%. At presentation all patients were given standard secondary prevention medication for NSTEMI. By the time of discharge 91.2% of patients were on aspirin, 64.8% on clopidogrel, 82.4% on a statin, 69.2% on an angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, 53.8% on a beta-blocker and 9.9% on a calcium channel antagonist. None of the patients received thrombolysis or abciximab (the glycoprotein IIb/IIIa inhibitor of choice in our unit). Normal presentation ECGs were documented in 30.8% of patients. 69.2% of patients had an abnormal presentation ECG, with ST depression/T wave changes being the most common abnormality (68.1%).
Table 1. Baseline characteristics.
| Characteristics | Valuea | n |
| Mean age (SD), years | 53.1 (12.3) | 91 |
| Female sex | 54 (59.3) | 91 |
| Family history of CAD | 39 (42.9) | 91 |
| Diabetes mellitus | 3 (3.3) | 91 |
| Hypertension | 37 (40.7) | 91 |
| Smokers | 26 (28.6) | 91 |
| Ex-smokers | 34 (37.4) | 91 |
| Hyperlipidaemia | 34 (37.4) | 91 |
| Mean creatinine, µmol l–1 (SD) | 84.9 (21.2) | 91 |
| Median interval from angiography to CMRI, months (range) | 2.00 (0–12) | 91 |
| Primary ECG abnormality | 91 | |
| Nil | 28 (30.8) | 91 |
| Q waves | 1 (1.1) | 91 |
| ST depression/T wave changes | 62 (68.1) | 91 |
| Medications on discharge | ||
| Aspirin | 83 (91.2) | 91 |
| Clopidogrel | 59 (64.8) | 91 |
| Statins | 75 (82.4) | 91 |
| ACE-I/AT receptor blocker | 63 (69.2) | 91 |
| Beta-blocker | 49 (53.8) | 91 |
| Ca-antagonist | 9 (9.9) | 91 |
ACE-I, angiotensin-converting enzyme inhibitors; AT, angiotensin; CAD, coronary artery disease; CMRI, cardiac MRI; ECG, electrocardiogram; SD, standard deviation.
aValues are given as number (percentage) unless otherwise indicated.
The mean concentration of haemoglobin was 14.2±1.4 g l–1, of serum creatinine was 84.9±21.2 μmol –1 and of troponin I was 3.4±5.8 ng ml–1.
The median interval from coronary angiography to LGE-CMRI was 2 months. The LGE-CMRI findings are summarised in Figure 1. 73% of patients had no abnormality on their LGE-CMRI (Figure 2). 16% of patients had patchy late enhancement consistent with myocarditis (Figures 3 and 4) and 11% had focal subendocardial or full thickness late enhancement consistent with myocardial infarction (Figure 5).
Figure 1.
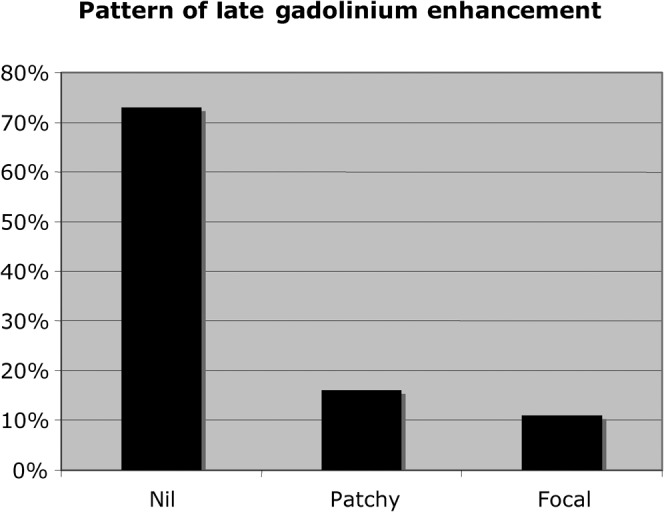
Pattern of late gadolinium enhancement in the patient cohort.
Figure 2.
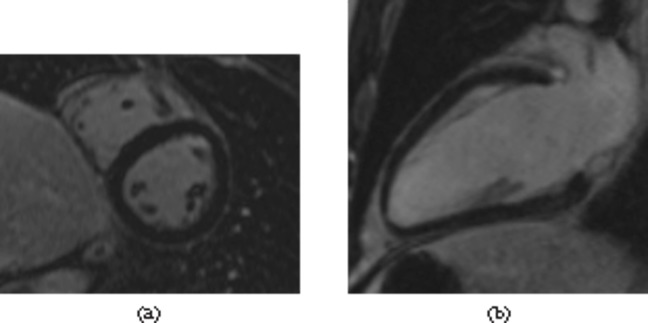
Normal late gadolinium enhancement images. The myocardium is uniformly of low signal. (a) Mid-ventricular short axis image using a three-dimensional multislice sequence; (b) a two-chamber view using a late gadolinium enhancement single slice sequence.
Figure 3.
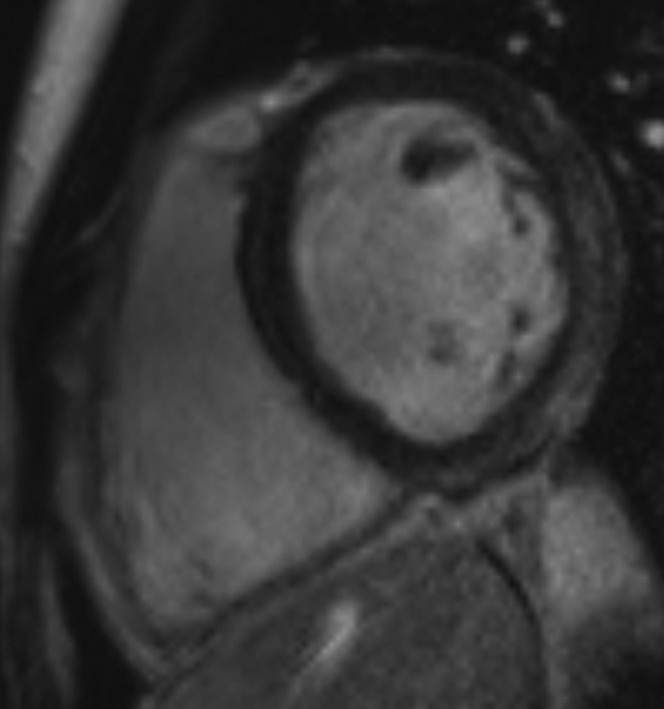
Patchy late gadolinium enhancement in the basal lateral and inferior walls with sparing of the subendocardial layer (which retains its normal low signal). This does not have the typical distribution of infarct and is consistent with myocarditis.
Figure 4.
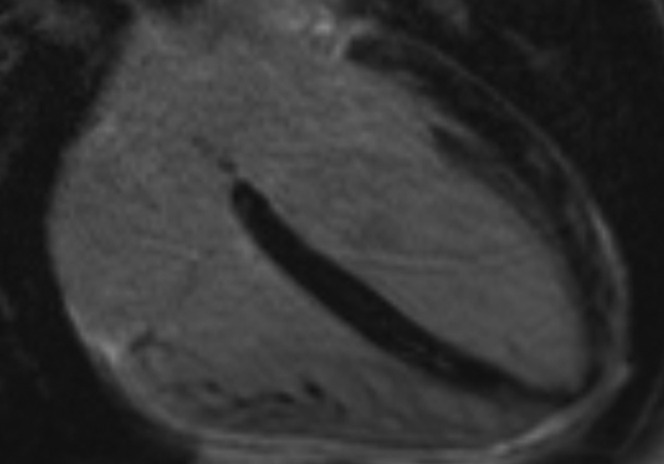
Patchy late gadolinium enhancement (LGE) in the distal septum and the mid and distal lateral wall on this four-chamber LGE image. Note the sparing of the subendocarial regions. The findings are not typical of myocardial infarction and are consistent with myocarditis.
Figure 5.
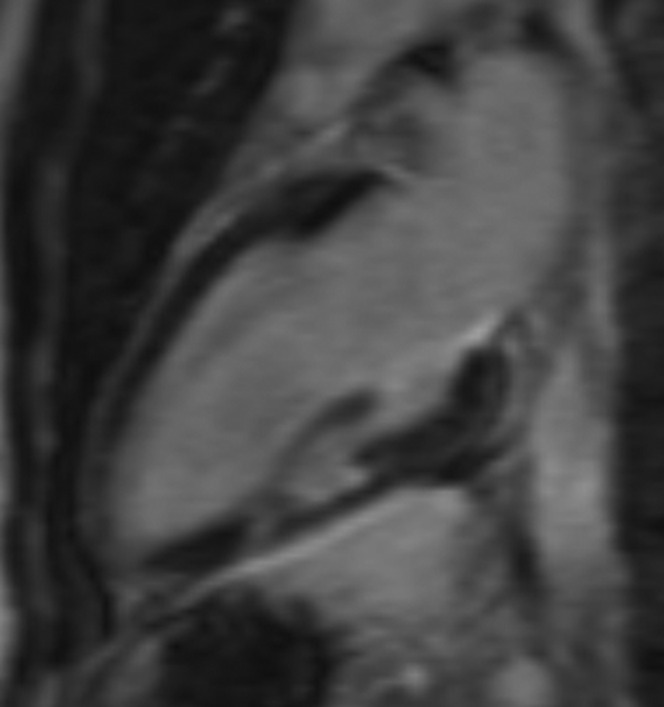
Focal late gadolinium enhancement (LGE) in the mid-inferior wall on this two-chamber three-dimensional image. The LGE includes the subendocardial region and this is therefore suggestive of infarct.
In the whole cohort, the left ventricular end-diastolic volume (LVEDV) was 132.7±24.3 ml, the LV end-systolic volume (LVESV) was 45±15.5 ml and the LV ejection fraction (LVEF) was 66.5%±8.1%. In the group in which the there was no late enhancement, the LVEDV, LVESV and LVEF were 129.4±24.3 ml, 42.2±14.4 ml and 67.9%±7.5%, respectively. In the group with patchy LGE, the values were 150±28 ml, 55.9±16.8 ml and 62.6±8.5%, respectively. These parameters are all significantly different to the no late enhancement group (p<0.05). In the group with focal LGE consistent with myocardial infarction, the values for the LV parameters were 129±19.1 ml, 47.8±14.9 ml and 62.7%±9.2%, respectively. CMRI parameters were not statistically different between the normal group and the focal group (p>0.05). These findings are summarised in Figure 6.
Figure 6.
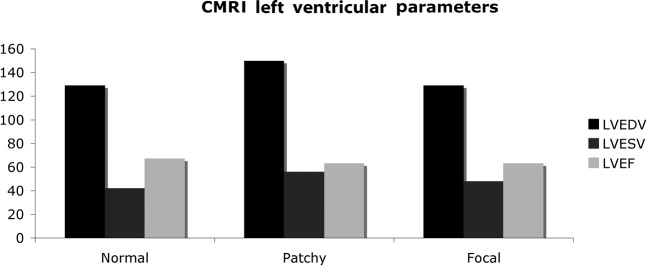
Cardiac MRI (CMRI) left ventricular end-diastolic volume (LVEDV), end-systolic volume (LVESV) and ejection fraction (LVEF) in the three groups in the patient cohort. The difference between the normal late gadolinium enhancement (LGE)-CMRI group and the patchy LGE-CMRI are statistically significant.
At a mean follow-up period of 21 months (range 2–45 months), there were no deaths in our cohort. Owing to restrictions set by the ethics committee in relation to approaching patients, we were unable to determine other components of major adverse cardiovascular and cerebrovascular events.
Discussion
In this study we report the largest consecutive series of patients investigated using LGE-CMRI after presentation with troponin-positive cardiac-sounding chest pain and unobstructed coronary arteries. In routine clinical practice, the vast majority of patients presenting with cardiac chest pain, no evidence of ST elevation on their ECG and an elevated troponin concentration are diagnosed as having sustained a NSTEMI. Following initial medical therapy, these patients then undergo coronary angiography and revascularisation as appropriate. However, in an important proportion of these patients, historically documented at about 10% [5,6], no flow-limiting lesions are identified at the time of coronary angiography and the coronary anatomy is commonly described as “unobstructed”. Further evaluation is therefore necessary to identify the mechanism of presentation in this cohort, particularly because many of them retain the diagnostic label of NSTEMI together with its psychological and social implications. The uncertainty as to how to manage these patients is illustrated in this population by the discharge medication as the rate of use of post-MI secondary prevention drugs is higher than expected for a group without coronary artery disease, but lower than the rate in revascularised patients after NSTEMI.
LGE-CMRI has emerged as a useful imaging modality for the assessment of this group of patients. LGE-CMRI is non-invasive, radiation free and has been extensively validated for its ability to identify even small areas of myocardial necrosis [12-14], with a good correlation with histopathological findings. It can also identify regions of myocardial damage that may be due not only to myocardial infarction but also to a variety of other conditions such as myocarditis, sarcoidosis and acute rejection post-cardiac transplantation [15-17].
In our study, 73% of patients had no abnormality on LGE-CMRI. The diagnosis of NSTEMI is therefore insecure, given the sensitivity of the technique, and such patients do not therefore warrant either the diagnostic label of MI or the secondary prevention drugs that accompany it. In this cohort, the elevation in troponin concentration may have been due to a variety of causes, and indeed it is possible that at least a proportion of these patients may have had a false-positive troponin result. We do not have serial troponin concentrations to exclude this possibility. The elevation in troponin concentration may have also arisen as a result of a transient tachyarrhythmia that was not captured on the ECG at the time of presentation or subsequent monitoring during their admission. However, none of the patients in this group had complained of palpitations at any time during their presentation. Although pulmonary embolism can also present with chest pain, troponin rise and non-specific ECG changes, the probability of this diagnosis was considered very low in our cohort and as a result neither a ventilation/perfusion scan nor a CT pulmonary angiogram were undertaken. Finally, as the median interval from angiography to LGE-CMRI was 2 months, it is possible that any low-grade inflammation that did not lead to scarring may have resolved by the time LGE-CMRI was undertaken and therefore have led to a normal study.
Focal late enhancement in a subendocardial distribution of a coronary artery territory characteristic of myocardial infarction was detected in 11% of this cohort. This is consistent with other such studies [8,10], and indicates that in patients with troponin-positive chest pain and unobstructed coronary anatomy, only a relatively small percentage of patients have truly sustained an MI and hence would benefit from conventional secondary prevention therapy. In fact, the majority of these patients were discharged on these drugs with no definitive plan as to long-term treatment. Potential mechanisms for infarction in this group include arterial occlusion and recanalisation, coronary spasm or embolism. There are important psychological and social consequences of labelling patients as having had a NSTEMI when this diagnosis is insecure. As well as the psychological impact of a “heart attack” label, the patients are obliged to take medications long term, with the associated potential for side effects. Furthermore, they are obliged to declare a history of “heart attack” on insurance, job application and driving authority forms.
Patchy late enhancement consistent with myocarditis was detected in 16% of patients. This is a smaller percentage compared with other studies such as those of Assomull et al (50%) [10], Bellenger et al (32%) [9], and Codreanu et al (44%) [11]. This difference is likely to be multifactorial. There is evidence that there are virus-related differences in the pattern of myocardial injury, as well as differences in the delay between disease onset and the timing of MRI (1–88 days in the Assomull et al study [10] and 9±7 days in the Codreanu et al study [11]). In this regard, it is particularly interesting that the LV volume parameters in this subgroup were significantly different to the groups with focal (MI-type) or no late enhancement. Furthermore, as our study represents the largest cohort of such patients studied to date it may be a more accurate reflection of the true prevalence of myocarditis in this group. Finally, although our clinical follow-up for the study is limited, the 100% survival at 21 months in this group highlights that this presentation has a benign short-term prognosis.
There are several limitations of this study. Firstly, as T2 weighted imaging was not performed routinely we are unable to provide evidence of inflammation without late enhancement. This will not affect the number of our patients found to have had an MI, however. Secondly, our clinical follow-up is limited to mortality and lacks other hard clinical end points. Thirdly, the lack of focal or patchy late enhancement does not make a definitive diagnosis to explain the admission, but it does help to exclude important components of the differential diagnosis. Fourth, given the median interval from angiography to LGE-CMRI of 2 months, it is possible that some patients with transient abnormalities or very small patchy foci of myocarditis may have been missed and labelled as having a normal scan. In a recent series by Monney et al [18] it was reported that myocarditis-related oedema and/or LGE were very commonly detected in patients undergoing CMRI within 2 weeks of presentation, so that 81% of the 79 patients were diagnosed with myocarditis. Importantly, in 20 patients who had both an early scan and a convalescent one, there was a time-dependent decrease in both (a) oedema (84% to 39%; p<0.01) and (b) LGE (5.6 to 3.0 segments; p=0.005). These data suggest that it is very likely that we have failed to detect myocarditis in some of our cohort because they did not have an early CMRI with oedema imaging. However, the principal aim of our study was to avoid inaccurate labelling as “heart attack”, and the exclusion of this diagnosis is much less likely to be influenced by the later timing of the CMRI. The practicalities of submitting all of these patients to early CMRI are likely to defeat most cardiac services for logistical and financial reasons. The final limitation of our study is that a retrospective analysis of the coronary angiograms in patients with an abnormal LGE-CMRI may have been useful in an attempt to exclude the possibility of missed lesions, particularly in small side braches of the major epicardial coronary arteries.
Although our study supports the clinical value of routine LGE-CMRI in patients presenting with troponin-positive chest pain and unobstructed coronary arteries, additional research will be required to determine the feasibility and cost-effectiveness of adopting this approach as “standard of care” in such patients. More specifically, such research should focus on earlier scanning, ideally on the index admission, incorporation of oedema imaging, adoption of international standards of CMRI imaging and reporting, as well as the utility of a more aggressive strategy to identify the mechanism(s) of myocarditis (for instance viral serology) and the troponin I release in patients who are found to have a normal LGE-CMRI.
In conclusion, our study provides support for the routine adoption of LGE-CMRI in the investigation of patients presenting with troponin-positive chest pain and unobstructed coronary arteries. Specifically, it demonstrates the relatively small proportion of patients who have experienced a true MI. This conveys clinically important information that could contribute to tailoring post-MI medical therapy accurately to those patients who qualify for it and, by contrast, remove an inappropriate label of “heart attack” from those in whom this diagnosis is not substantiated. It is likely that to ensure a robust differential diagnosis in the acute setting a CMRI would be needed earlier than performed in this study, but as a method for excluding the “heart attack” diagnosis, this methodology proves clinically useful.
References
- 1.Capewell S, McMurray J. “Chest pain—please admit”: is there an alternative? A rapid cardiological assessment service may prevent unnecessary admissions. BMJ 2000;321:452–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassand JP, Hamm CW, Ardissino D, Boersma E, Budaj A, Fernández-Avilés F, et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart Journal 2007;28:1598–660 [DOI] [PubMed] [Google Scholar]
- 3.Cannon CP, Weintraub WS, Demopoulos LA, Vicari R, Frey MJ, Lakkis N, et al. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med 2001;344:1879–87 [DOI] [PubMed] [Google Scholar]
- 4.Fox KA, Poole-Wilson PA, Henderson RA, Clayton TC, Chamberlain DA, Shaw TR, et al. Interventional versus conservative treatment for patients with unstable angina or non-ST-elevation myocardial infarction: the British Heart Foundation RITA 3 randomised trial. Lancet 2002;360:743–51 [DOI] [PubMed] [Google Scholar]
- 5.Curzen NP, Patel DJ, Kemp M, Hooper J, Knight CJ, Clarke D, et al. Can C reactive protein or troponin T and I predict outcome in patients with intractable unstable angina? Heart 1998;80:23–7 [PMC free article] [PubMed] [Google Scholar]
- 6.Germing A, Lindstaedt M, Ulrich S, Grewe P, Bojara W, Lawo T, et al. Normal angiogram in acute coronary syndrome-preangiographic risk stratification, angiographic findings and follow-up. Int J Cardiol 2005;99:19–23 [DOI] [PubMed] [Google Scholar]
- 7.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: executive summary. Circulation 2007;116:e148–304 [DOI] [PubMed] [Google Scholar]
- 8.Bellenger N, Peebles C, Curzen N. Cardiovascular magnetic resonance for the interventionist. EuroIntervention 2006;1:445–53 [PubMed] [Google Scholar]
- 9.Bellenger NG, Peebles C, Harden S, Dawkins K, Curzen N. Troponin-positive chest pain with unobstructed coronary arteries: a role for delayed enhanced cardiovascular magnetic resonance in the diagnosis of non-ST elevation myocardial infarction? J Invasive Cardiol 2006;18:594–8 [PubMed] [Google Scholar]
- 10.Assomull RG, Lyne JC, Keenan N, Gulati A, Bunce NH, Davies SW, et al. The role of cardiovascular magnetic resonance in patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Eur Heart 2007;28:1242–9 [DOI] [PubMed] [Google Scholar]
- 11.Codreanu A, Djaballah W, Angioi M, Ethevenot G, Moulin F, Felblinger J, et al. Detection of myocarditis by contrast-enhanced MRI in patients presenting with acute coronary syndrome but no coronary stenosis. J Magn Reson Imaging 2007;25:957–65 [DOI] [PubMed] [Google Scholar]
- 12.Kim RJ, Judd RM, Chen EL, Fieno DS, Parrish TB, Lima JA. Relationship of elevated 23Na magnetic resonance image intensity to infarct size after acute reperfused myocardial infarction. Circulation 1999;100:185–92 [DOI] [PubMed] [Google Scholar]
- 13.Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 2000;343:1445–53 [DOI] [PubMed] [Google Scholar]
- 14.Selvanayagam JB, Porto I, Channon K, Petersen SE, Francis JM, Neubauer S, et al. Troponin elevation after percutaneous coronary intervention directly represents the extent of irreversible myocardial injury: insights from cardiovascular magnetic resonance imaging. Circulation 2005;111:1027–32 [DOI] [PubMed] [Google Scholar]
- 15.Aletras AH, Tilak GS, Natanzon A, Hsu LY, Gonzalez FM, Hoyt RF, Jr, et al. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation 2006;113:1865–70 [DOI] [PubMed] [Google Scholar]
- 16.Marie PY, Angioï M, Carteaux JP, Escanye JM, Mattei S, Tzvetanov K, et al. Detection and prediction of acute heart transplant rejection with the myocardial T2 determination provided by a black-blood magnetic resonance imaging sequence. J Am Coll Cardiol 2001;37:825–31 [DOI] [PubMed] [Google Scholar]
- 17.Vignaux O, Dhote R, Duboc D, Blanche P, Dusser D, Weber S, et al. Clinical significance of myocardial magnetic resonance abnormalities in patients with sarcoidosis: a 1-year follow-up study. Chest 2002;122:1895–901 [DOI] [PubMed] [Google Scholar]
- 18.Monney PA, Sekhri N, Burchell T, Knight C, Davies C, Deaner A, et al. Acute myocarditis presenting as acute coronary syndrome: role of early cardiac magnetic resonance in its diagnosis. Heart 2011;97:1312–18 [DOI] [PubMed] [Google Scholar]


