Abstract
Objectives
Standard MRI of the cervical spine is performed in a different anatomical position to that utilised for traditional contrast myelography. Those well practised in myelography are familiar with the considerable changes in configuration of the bony and soft tissues of the cervical spine that may occur with changes in the degree of neck flexion and extension. We set out to compare the findings in a select group of patients with myeloradiculopathy who had undergone myelography and MRI in both standard and neck-extended positions. These findings were correlated with the clinical status.
Methods
29 patients underwent myelography with CT (CTM) and MRI in neutral and neck-extended positions. The imaging was assessed for the degree of cord compression and neural foraminal narrowing, quantified using a simple grading scheme suitable for routine clinical practice. The degree of neck extension was assessed using an angular measurement.
Results
For both CTM and MRI, scanning with the neck extended significantly increases the severity of cord compression compared with the standard supine position, to a degree similar to that shown during conventional prone myelography. The degree of perceived cord compression is related to the degree of neck extension achieved. Correlation of standard MRI findings and the clinical level of radiculopathy is poor. This correlation improves when the neck is extended.
Conclusions
The most appropriate position for routine MRI of the cervical spine in degenerative disease remains unknown, but in selected patients imaging with the neck extended may provide important additional information.
With the introduction of spinal surface coil technology and gradient echo sequences in the late 1980s, MRI rapidly replaced intrathecal contrast myelography as the standard imaging method for assessment of the cervical spinal cord and nerve roots. The two investigations are performed in very different anatomical positions. Standard cervical spine MRI is performed in a coil that is designed to make the patient comfortable, to minimise movement-related artefacts. This generally results in a position of mild extension of the neck. On the other hand, the majority of images for plain cervical myelography are obtained with the patient prone and the neck hyperextended so as to retain myelographic contrast within the cervical lordosis. CT myelography (CTM) is typically performed with the patient supine and with the neck straight or mildly flexed; however, CT myelography can also be performed in the prone position with the neck extended [1].
For a number of years we continued to use plain myelography, with CTM as a second-line investigation in patients with cervical myelopathy and/or radiculopathy, when the results of standard MRI were inconsistent with symptoms and signs. More recently we have also performed MRI with neck extension, predominantly in patients with myelopathic features but inconclusive supine MRI. We have been unsure how extended MRI compares with myelography and whether it is able to produce the same degree of extension as myelography. We have performed CT in the prone extended position as well, but have also been unsure how that relates to standard supine CT imaging or to extended MRI. We therefore wished to compare these various modalities in a heterogeneous group of clinically problematic patients.
Methods and materials
We retrospectively identified patients who had had cervical spine MRI and myelography between 2003 and 2009 for the same episode of cervical degenerative disease. 57 patients were studied. Myelography was always performed subsequent to an inconclusive MRI study. A maximum interval of 6 months between the investigations was accepted. At the beginning of this study period waiting times for imaging investigations in the English National Health Service were long, and 38 additional patients were excluded as the interval between the tests exceeded the standard.
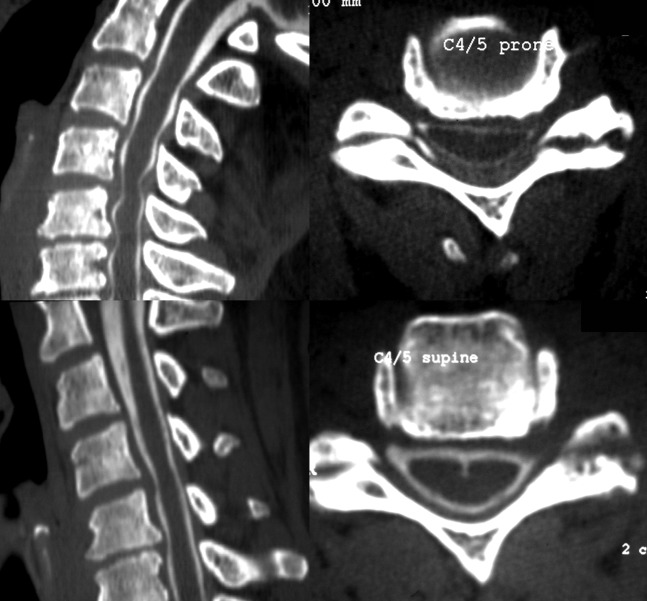
All myelograms were performed by two consultant neuroradiologists with extensive experience of the technique. Intrathecal contrast was introduced by the lumbar route in all cases. A standard set of fluoroscopic images was obtained, including frontal, oblique and lateral projections. CT was performed typically within 30 mins, acquiring from the foramen magnum to vertebra T1, but occasionally a more limited volume. All studies were performed on a 16- or 64-slice helical CT scanner. When CT was performed prone, the chin rested upon the headrest. Reconstructed sagittal and axial images parallel to individual discs (3 mm) were generated by one of the neuroradiologists in all cases. An example is shown in Figure 1. The dose–length product for a single acquisition on our equipment is about 800 mGy cm–1.
Figure 1.
Comparison of neutral and extended CT myelography studies. Top row: prone post-myelography CT sagittal reconstruction; and reconstructed axial at C4/5. Bottom row: supine study. Obvious cord compression only shown on extended imaging.
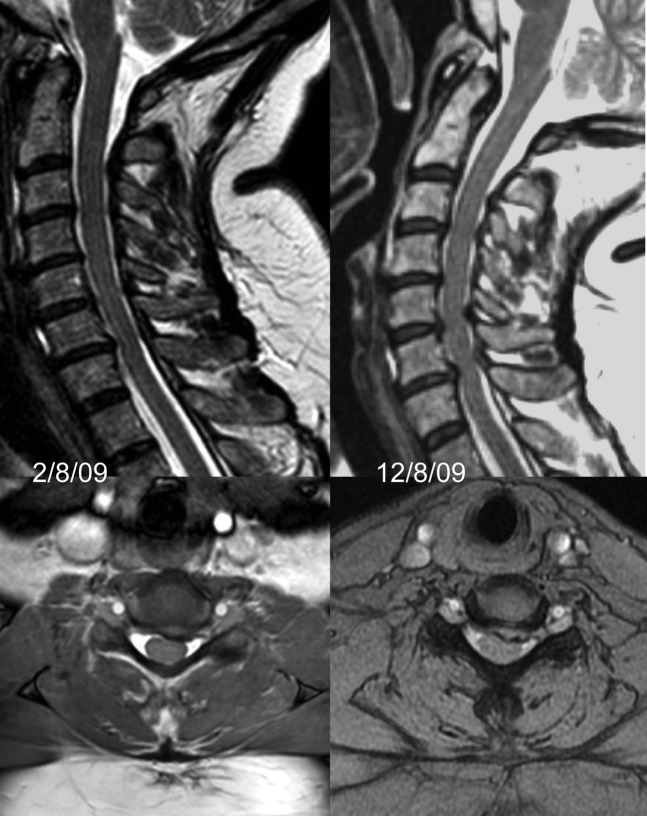
All MRI was performed at 1.5 T. Sequences included 3 mm sagittal T2 weighted fast spin-echo (FSE) and axial two-dimensional and/or three-dimensional T2* weighted gradient echo sequences with a maximum slice thickness of 3 mm. T1 weighted images were not used in this analysis. Standard images were obtained in a position of patient comfort using a standard cervical spine coil with or without an anterior element. For the extended neck studies the shoulders were elevated with robust foam padding so that the patient could look down the bore of the magnet with as much extension as they could comfortably manage (Figure 2). An example of such an image is shown in Figure 3. Some patients reported a transient exacerbation of their neck pain after this procedure, but no significant complications occurred in this patient group. The image quality for extended position sequences tended to be inferior to the standard sequences, and axial sequences were not always performed. With large patients it was sometimes not possible to use the anterior element of a spine coil when the neck was extended.
Figure 2.
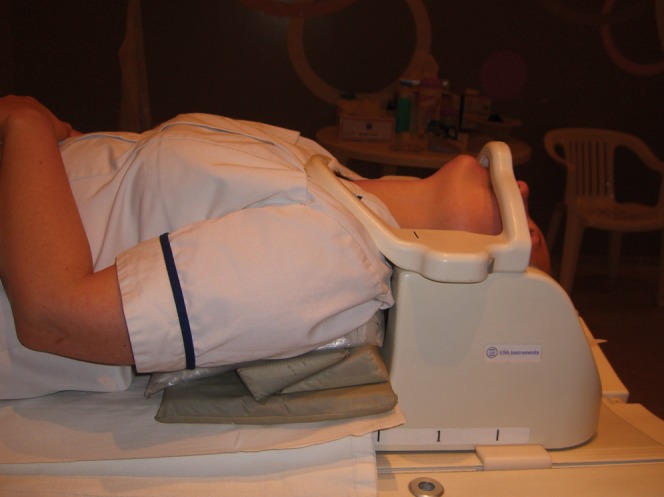
Patient position for extended MRI. Shoulders are elevated with robust foam padding and patient attempts to look down the bore of the scanner.
Figure 3.
Left: standard (neutral) MRI; mid-sagittal and axial at C6/7. Right: images at same locations obtained 10 days later, with 17° greater extension. There is increased disc bulging and buckling of the ligamentum flavum at two levels.
The five image sets (plain myelography, supine CTM, prone extended CTM, standard MRI and neck-extended MRI) for each patient were anonymised, separated and prepared for review in random order, without clinical information available. All studies were viewed blindly by a neuroradiologist (RJVB); 11 cases were reread 2 months later and were also viewed by a second neuroradiologist (CARH).
Image quality was recorded and graded as good, adequate or poor (non-diagnostic). Image artefacts (e.g. related to previous surgery) were recorded. If a particular spinal level was graded poor by one modality, all the results for that level were excluded from analysis. Intrinsic T2 weighted signal change in the cord was graded at each level as absent, moderate or severe.
The degree of extension of the spine was measured as the acute angle between a line drawn parallel to the posterior cortex of the C2 vertebral body and a line parallel to the posterior cortex of the C7 vertebral body (Figure 4). Occasionally this could not be ascertained (e.g. on myelography in patients with very large shoulders). If the spine was flexed a negative angle was recorded.
Figure 4.
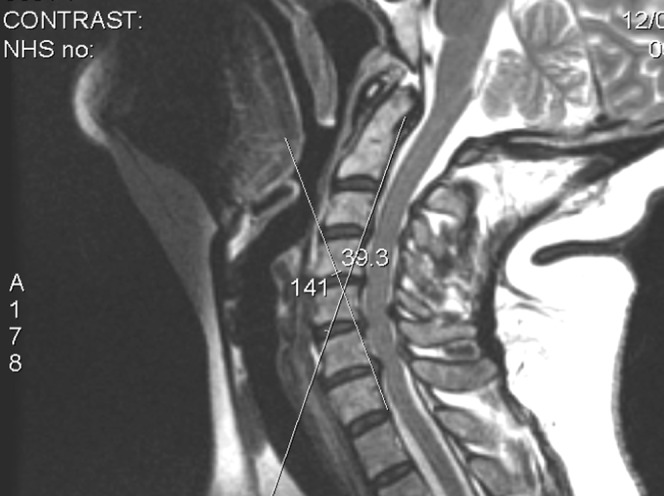
In this case angle of extension is 39°.
The mid-sagittal diameter of the bony spinal canal from mid-C7 vertebral body to the spinous process was measured on each investigation.
Cord compromise and neural foraminal narrowing at each level from C2/3 to C7/T1 were assessed using sagittal and axial images according to the following criteria.
Central canal
Normal (0): cerebrospinal fluid (CSF) visible dorsal or ventral, not indented on sagittal, and normal shape on axial.
Equivocal (1): no CSF visible dorsal and ventral on sagittal and/or axial, but not indented or displaced on sagittal (i.e. “nipped”); or atrophic or flattened but CSF visible dorsal and/or ventral.
Compressed (2): no CSF dorsal and ventral plus indented or displaced on sagittal, and/or flattened on axial.
Foramina
Normal (0): same size as adjacent or contralateral “normal” foramina.
Equivocal (1).
Compressed (2): >50% narrowing, displacement or impingement of root complex, disc material seen within foramen, root sheath underfilled or truncated, obvious swelling of rootlets or obliteration of individual rootlets.
Clinical analysis of the cases was a retrospective review of the case notes undertaken by a career grade neurosurgeon. The Ranwat classification of myelopathy was utilised (i.e. 1=no deficit; 2=subjective weakness with hyper-reflexia and dysaesthesiae; 3A=objective weakness with long tract signs, but able to walk; 3B=quadriparetic and non-ambulatory).
For patients with radiculopathy the presumed clinical level or levels were recorded and graded possible or definite.
During analysis, cord compromise at each of the six levels in the cervical spine, and compromise of the foramina on the left and right side, were considered to be independent variables.
Results
Demographics
This study focuses on 29 patients who had MRI in both standard and extended positions and post-myelography CT in the supine neutral position. 21 of these also had post-myelography CT performed in both standard and extended positions. 28 additional patients had post-myelography CT performed in extension, but only had MRI performed in the standard position.
Of the total 57 patients, 32 were male and 25 female; mean age was 52 (range 30–80 years). MRI and CTM were performed within 6 months (mean 3.1 months) for the same clinical complaint.
41 patients were myelopathic (13 Ranwat Grade 3A or 3B), 40 patients were radiculopathic and 24 were myeloradiculopathic.
The majority had a narrow, or relatively narrow, cervical canal. Canal diameters at C7 were 11 mm or less in 11 patients, 12 or 13 mm in 31 patients and more than 13 mm in 15 patients. 15 patients showed T2 signal change in the cord at one or more levels.
17 patients had had previous surgery. In one case this was a Ransford loop; the others were all anterior cervical discectomy and fusion, with or without anterior plates, and in 8 cases 2 levels had been operated upon.
Observer errors
Inter- and intra-observer errors for our technique of grading cord and foraminal compromise were determined after a pilot study in a subgroup of 11 cases (66 spinal levels, 132 foramina). This group contained only 2 patients with extended MRI studies. Results are shown in Table 1.
Table 1a. Cord compression intra/interobserver agreement kappa (95% confidence interval).
| Technique | Obs A1 vs Obs A2 | Obs A vs Obs B |
| CT extension | 0.79 (0.53–1.00) | 0.67 (0.44–0.91) |
| MRI supine | 0.85 (0.66–1.00) | 0.73 (0.55–0.92) |
| Myelography prone | 0.74 (0.57–0.93) | 0.61 (0.44–0.80) |
Table 1b. Foraminal compromise intra/interobserver agreement kappa (95% confidence interval).
| Technique | Obs A1 vs Obs A2 | Obs A vs Obs B |
| CT extension | 0.50 (0.31–0.70) | 0.37 (0.21–0.55) |
| MRI supine | 0.72 (0.59–0.86) | 0.53 (0.40–0.68) |
| Myelography prone | 0.58 (0.43–0.75) | 0.60 (0.47–0.74) |
Obs, observer.
Correlation was generally better for the assessment of cord compression than it was for the assessment of foraminal compromise. The assessment of foraminal compromise on CTM is difficult. Even when the nerve root sheath is opacified with subarachnoid contrast, CTM provides very little information about soft tissue changes in the foramen and primarily indicates the degree of bony narrowing. Otherwise the grading schemes utilised for both cord and foraminal compromise are reasonably reproducible. As anticipated, intra-observer agreement is slightly better than interobserver agreement. Subsequent analysis is based on the values determined by Observer A.
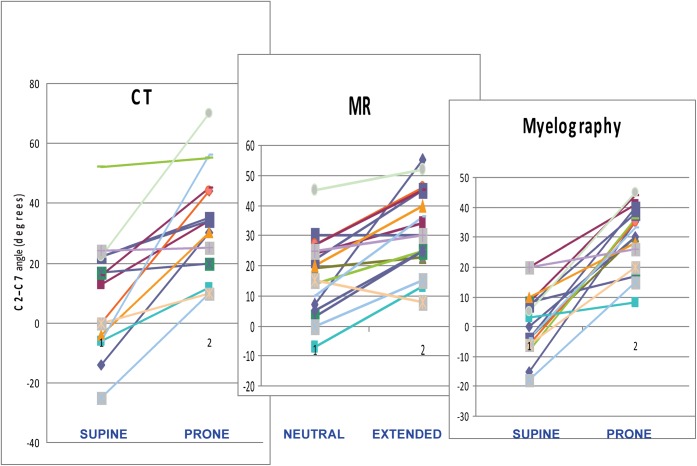
Angular changes and cord compression
Figure 5 shows graphically the ranges of movement of the neck for 17 patients with a complete data set. A number of patients were excluded because of radiographic factors that prevented measurement of the C2/C7 angle. Usually this was due to the dens being coned off on myelographic images, or C7 being poorly visualised through the shoulders.
Figure 5.
Angle between C2 and C7, neutral and extended studies. 17 patients, each with a different colour coding.
During standard MRI studies, the neck is usually slightly extended (mean 17.1° in the complete patient group), whereas with supine CTM it is only minimally extended, or even slightly flexed (mean 9.4°), so these studies are not strictly comparable. Extended MRI (mean 32.9°) and prone CTM (mean 32.6°) are both performed in a very similar position to prone myelographic images (mean 29.2°). Faulty radiographic technique means that it is occasionally possible to render the neck less extended after attempting to elevate the shoulders (Figure 5).
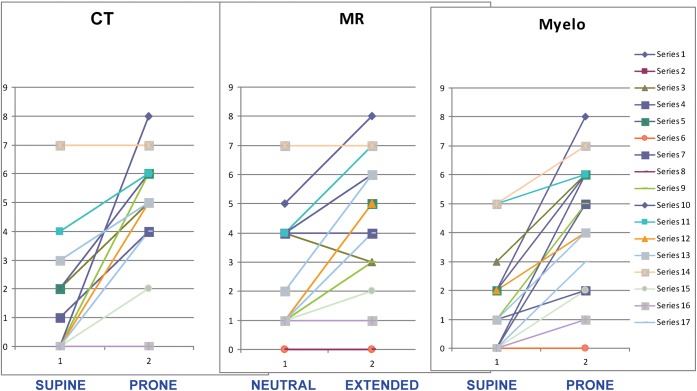
For each study the cord compression grade at each of the six cervical levels was summed to produce a “total compression score” for that study. The results obtained for the same 17 patients as in Figure 5 are shown in Figure 6. For most patients the extended position produces a clear increase in cord compression, and the extended studies all produce very similar findings. The difference between standard and extended MRI is less than that between supine and prone CT.
Figure 6.
Perceived cord compression on neutral and extended studies. Same patients and colour coding as in Figure 5.
To some extent the increase in cord compression is directly linked to the degree of neck extension achieved. In Figure 7 the change in angle between neutral and extended MRI is plotted against the change in the degree of perceived cord compression. Pearson's correlation coefficient is 0.51, but confidence intervals are wide.
Figure 7.
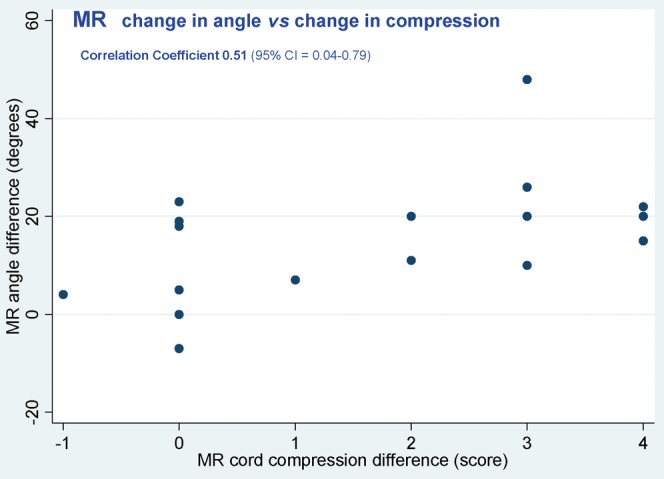
Difference in perceived cord compression versus change in angle between neutral and extended MRI.
Of the 29 patients who had an MRI study in extension, 12 demonstrated Grade 2 cord compression that was not demonstrated on the standard study.
For the whole group of 57 patients, using a logistic regression model and odds ratio, the clinical correlation of myelopathy (Ranwat Grades 2 and 3) with the total cord compression value determined from the various imaging techniques was poor. However, there was a weak correlation with the presence of T2 change in the cord (odds ratio 12.0; p=0.06).
Because of susceptibility artefacts, MRI is likely to overgrade the degree of cord compression compared with CTM. On a level-by-level analysis, standard MRI overscored in relation to supine CTM by one grade at 58 levels and by two grades at 8 levels. At 11 levels CTM was graded higher than MRI, but never by 2 grades.
Foraminal compromise and radiculopathy
The presence of foraminal compromise (Grade 1 or 2) was compared with the presence of clinical radiculopathy at exactly the same side and level using Spearman's correlation coefficient for categorical data. Myelographic images (without information from CTM) showed a strong correlation (0.70), whereas standard MRI was no better than chance (−0.02). Extended MRI was somewhat better (0.32). Looked at another way, the correlation of MRI images with the myelographic images was 0.02 for neutral MRI and 0.61 for extended MRI.
In practice, standard MRI is clinically more useful than the above would suggest, especially as the “surgical level” may be one level removed from the clinical level 2. Table 2 shows the clinical and radiological findings in 13 of the extended MRI group of patients who had Grade 2 (definite) foraminal compromise. Eight patients showed a direct correlation at at least one level. Three had an abnormality at the appropriate level, but on the opposite side; one had an abnormality at an adjacent level and one appeared to have no relevant abnormality. In this group the summated score for definite foraminal compromise was: prone myelography 78, supine CT 54, prone CT 64, standard MRI 68, extended MRI 80; this shows that foramina are more likely to appear abnormal if the spine is extended and the foramina become narrowed, as has long been known from plain film studies.
Table 2. Patients with definite foraminal abnormality on extension imaging: correlation with clinical levels of radiculopathy.
| Right |
Patient number | Left |
||
| MRI finding | Clinical level | Clinical level | MRI finding | |
| 4 | 6 | 104 | 6 | |
| 4 5 | 5 7 | 105 | 5 7 | |
| 7 | 106 | 5 6 7 | 5 6 | |
| 107 | 7 8 | 6 | ||
| 6 7 | 5 6 | 108 | ||
| 4 6 7 | 112 | 5 6 7 | ||
| 4 7 | 6 7 | 115 | 6 7 | |
| 6 7 | 117 | 6 7 | ||
| 6 7 | 5 6 | 118 | 5 6 | 4 6 7 |
| 4 | 7 8 | 121 | ||
| 5 6 | 5 | 123 | 5 | 5 6 7 |
| 4 | 125 | 5 | 5 7 | |
| 6 | 6 | 126 | 6 | |
There was no correlation between the presence of an abnormal foramen and cord compression at the same level (correlation coefficient 0.04).
Discussion
This study shows that in our group of patients the posture of the neck has a significant effect on the degree of cord compression demonstrated on both CTM and MRI. With the neck extended, the degree of compression correlates with that shown by conventional myelography. Neck extension comparable to that achieved in the prone position for plain myelography and CTM can be achieved with the patient supine in a standard 1.5 T MRI scanner.
Until 20 or so years ago, CTM was the primary modality for investigating cervical cord compression and therefore the imaging on which clinical management was largely based. Most studies comparing MRI and CTM [3,4] utilised surgical findings as the gold standard, which precludes identifying false negative observations. The lack of a satisfactory reference standard has significantly limited evaluation of the imaging of degenerative cervical pathology.
The greatest advantage of MRI over CTM is the ability to identify intrinsic abnormality of the cord. The relationship of the presence, absence or extent of intramedullary T2 weighted signal change to clinical myelopathy, and to the likely outcome after surgery, remains complex and controversial [5-8]. More recent publications tend to indicate that T2 weighted signal change, particularly if multisegmental, is a poor prognostic feature for response to surgery [9,10].
Despite the widespread acceptance of MRI, cautionary observations have been made. In 1999 Shafaie et al [11] compared CTM and MRI in the evaluation of myelopathy and radiculopathy. Agreement of the two tests in the assessment of canal compromise, foraminal encroachment and cord size was not very good (κ=0.42−0.46). CTM tended to upgrade spinal canal narrowing and foraminal encroachment even though the CT component was performed supine. Shafaie et al considered that the two investigations were complementary. Although they reviewed many of the reasons for discrepancies between the two tests, the issue of different degrees of neck extension was not included. Tsuruda [12] and others have shown that there is a tendency for gradient echo sequences to overestimate the degree of foraminal narrowing because of susceptibility blooming of bony structures, and that these effects are worse if there is also patient movement. Similar effects can also overestimate the degree of central canal stenosis, and for these reasons minimum practical TEs should be utilised. Reul et al [13] found that cervical stenosis was often overestimated by MRI in comparison with CTM, and that these effects were most marked in patients with a narrow canal. They attributed the problem to truncation artefacts and considered that CTM was still indicated, especially in patients with a narrow cervical canal. However, Dorenbeck et al [14] did not find any susceptibility-weighted discrepancies using the “medic” spoiled gradient echo MT sequence.
In 2003 an editorial in American Journal of Neuroradiology [15] bemoaned the demise of myelography and pointed out that it may still be appropriate “for cases in which nerve root compression is strongly clinically suspected, but for which MRI imaging has failed to confirm the suspicion”. In most UK centres myelography is reserved for patients who have an absolute contra-indication to MRI [16], although there are still a few enthusiastic advocates for myelography [17].
The potential importance of flexion/extension MRI in the elucidation of cervical cord compression has long been recognised [18]. Using a purpose-built positioning device, Muhle's group [19] showed a significant increase in cord impingement in 27% of patients when the neck was extended, but in only 5% of patients with the neck flexed. This study did not concentrate on patients with congenitally narrow canals, and there was no direct correlation with patient symptomatology. They did not undertake a formal comparative study with myelography, but did consider that in a small number of patients who underwent both studies, myelographic analysis was probably equivalent to the dynamic MRI assessment. A subsequent study [20] suggested that kinematic MRI might alter therapeutic management in up to 87% of patients with cervical degenerative disease. Chen et al [21] questioned whether dynamic MRI needs to be performed in every patient. Using standard equipment with positioning sponges, they found similar results to Muhle et al [19], with 31% of patients showing functional cord impingement in extension and only 3% during flexion. They considered that a sagittal canal diameter of ≤10 mm at C7 reflected severe canal stenosis, and showed that the chance of demonstrating dynamic cord impingement in extension rose to 79% in this patient group. They did not report whether dynamic MRI correlates with patient symptomatology any better than standard imaging. At least one commercial positioning device has been developed to facilitate these investigations, allowing an average of 42° of flexion and 47° of extension to be obtained [22]. Yoo et al [23] suggested that physiological narrowing of the exit foramina in extension may also be demonstrated.
The effect of axial loading of the cervical spine in the erect position by the weight of the head was reported by Jinkins et al [24] using the Fonar 0.6 T open system. In a number of cases he showed increased disc bulging in both cervical and lumbar regions, but the frequency of this occurrence, the correlation with symptomatology and the relationship to the degree of neck extension have not been established. The equipment is heavy, and has not become widely available.
Many techniques have been described to quantify the degree of narrowing of the subarachnoid space, or compromise of the spinal cord or exiting roots [25-30]. It is clear that no simple reliable technique exists and radiologists seem to be very poor at reliably assessing the degree of cord compression. Stafira et al [31] showed an interobserver agreement of κ=0.26 with CTM and κ=0.31 with MRI. Thus, we chose to utilise a simple grading scheme that can be used in routine clinical practice. We have demonstrated satisfactory inter- and intra-observer errors.
The role of electrophysiology in diagnosis of cervical myelopathy and radiculopathy is complex. Nardin et al [32] compared electromyography (EMG) and MRI in a mixed group of cervical and lumbar radiculopathies, although the majority were cervical. EMG was significantly abnormal at the clinically suspect or the adjacent level and side in 72% of clinically definite, 40% of clinically probable and 29% of clinically possible cases of radiculopathy. Surprisingly, the proportion of abnormal MRI findings was similar in each group, and abnormality was just as likely on the asymptomatic side as the symptomatic side. This casts doubt on the specificity of MRI. In an editorial in the same journal, Robinson [33] reworked the data, and noted a very low correlation between symptoms and MRI abnormalities. Many of these issues have been reviewed recently [34].
The patients in this study are highly selected on the basis that first-line imaging (i.e. standard cervical spine MRI) had not provided a clear answer or explanation for their symptoms and signs, and these were felt sufficiently troublesome to warrant further investigation. A high proportion had had previous cervical spine surgery, and several had associated myelomalacia and focal cord atrophy. The atypical nature of this group may explain, at least in part, the poor correlation between the clinical picture and the findings on standard MRI.
Conclusion
The use of CTM has decreased dramatically in recent years. Despite some methodological challenges, this study has provided a unique opportunity to compare the dynamic capabilities of MRI with a previously well-understood technique. It is unlikely that such a comparison will ever be performed again.
In this small group of patients, a retrospective (and subjective) clinical review indicated that in the majority of cases the information from extended neck imaging was useful in clinical management, either by revealing additional findings or by the absence thereof. Nevertheless, the role of extended-neck MRI in routine management remains unclear, and further prospective studies, with well-defined patient characteristics, are indicated. Until the significance of “dynamic” worsening of cord compression is better understood there is potential for demonstration of (perhaps incidental) increased cord compression in extension to lead to unnecessary surgical intervention. Findings on extension MRI should be interpreted with caution by multidisciplinary teams taking careful account of clinical and electrophysiological data.
CTM is still practised on occasion in some centres and still has a definite role where MRI is contra-indicated. Given the high dose of radiation associated with CTM, particularly to the thyroid, we suggest that CTM is best carried out in the prone extended position only, for maximum diagnostic information.
The poor specificity of standard MRI for mechanical radiculopathy is well recognised [32]. Our limited data suggest that extended-neck MRI may improve diagnostic accuracy, but further study is needed.
References
- 1.Graham CB, Wippold FJ, Bae KT, Pilgram TK, Shaibani A, Kido DK. Comparison of CT myelography performed in the prone and supine positions in the detection of cervical spinal stenosis. Clin Rad 2001;56:35–9 [DOI] [PubMed] [Google Scholar]
- 2.Benini A. Clinical features of cervical root compression C5–C8 and their variations. Nearal Orthop 1987;4:74–88 [Google Scholar]
- 3.Modic MT, Masaryk TJ, Mulopulos GP, Bundschuh C, Han JS, Bohlman H. Cervical radiculopathy: prospective evaluation with surface coil MR imaging, CT with metrizamide and metrizamide myelography. Radiology 1986;161:753–9 [DOI] [PubMed] [Google Scholar]
- 4.Statham PF, Hadley DM, Macpherson P, Johnston RA, Bone I, Teasdale GM. MRI in the management of suspected cervical spondylotic myelopathy. JNNP 1991;54:484–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi M, Yamashita Y, Sakamoto Y, Kojima R. Chronic cervical cord compression: clinical significance of increased signal intensity on MR images. Radiology 1989;173:219–24 [DOI] [PubMed] [Google Scholar]
- 6.Singh A, Crockard AH, Platts A, Stevens J. Clinical and radiological correlates of severity and surgery-related outcome in cervical spondylosis. J Neurosurgery 2001;94:189–98 [DOI] [PubMed] [Google Scholar]
- 7.Wada E, Kazuo Y, Suzuki S, Atsunori K, Takahiro O. Can intramedullary signal change on magnetic resonance imaging predict surgical outcome in cervical spondylotic myelopathy? Spine 1999;24:455–61 [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto M, Toyama Y, Ishikawa M, Kazuhiro C, Nobumasa S, Yoshikazu F. Increased signal intensity of the spinal cord on magnetic resonance images in cervical compressive myelopathy: does it predict the outcome of conservative treatment? Spine 2000;25:677–82 [DOI] [PubMed] [Google Scholar]
- 9.Chatley A, Kumar R, Jain V, Behari S, Sahu RN. Effect of spinal cord signal intensity changes on clinical outcome after surgery for cervical spondylotic myelopathy. J Neurosurg Spine 2009;11:562–7 [DOI] [PubMed] [Google Scholar]
- 10.Yukawa Y, Kato F, Yoshihara H, Yanase M, Ito K. MR T2 image classification in cervical compression myelopathy: predictor of surgical outcomes. Spine 2007;32:1675–8 [DOI] [PubMed] [Google Scholar]
- 11.Shafaie F, Wippold F, Gado M, Pilgram TK, Riew KD. Comparison of computed tomography myelography and magnetic resonance imaging in the evaluation of cervical spondylotic myelopathy and radiculopathy. Spine 1999;24:1781–5 [DOI] [PubMed] [Google Scholar]
- 12.Tsuruda JS, Remley K. Effects of magnetic susceptibility artifacts and motion in evaluating the cervical neural foramina on 3DFT gradient-echo MR imaging. AJNR 1991;12:237–41 [PMC free article] [PubMed] [Google Scholar]
- 13.Reul JF, Gievers BF, Weis JF, Thron A. Assessment of the narrow cervical spinal canal: a prospective comparison of MRI, myelography and CT–myelography. Neuroradiology 1995;37:187–91 [DOI] [PubMed] [Google Scholar]
- 14.Dorenbeck U, Schreyer AG, Schlaier J, Held P, Feuerbach S, Seitz J. Degenerative diseases of the cervical spine: comparison of a multiecho data image combination sequence with a magnetisation transfer saturation pulse and cervical myelography and CT. Neuroradiology 2004;46:306–9 [DOI] [PubMed] [Google Scholar]
- 15.Miller GM, Krauss WE. Myelography: still the gold standard. AJNR 2003;24:298. [PMC free article] [PubMed] [Google Scholar]
- 16.Puthuran M, Bartlett R. Myelography: a survey of current useage, and indications in the UK: Poster presented at the BSNR Annual Meeting 2007. London, UK: British Society of Neuroradiologists; 2007 [Google Scholar]
- 17.Goldstein M, Saklatvala J. The present position of myelography. Rad Magazine 2009;35:20–1 [Google Scholar]
- 18.Epstein NE, Hyman RA, Epstein JA, Rosenthal AD. “Dynamic” MRI scanning of the cervical spine. Spine 1988;13:937–8 [DOI] [PubMed] [Google Scholar]
- 19.Muhle C, Metzner J, Weinert D, Falliner A, Brinkman G, Mehdorn MH, et al. Classification system based on kinematic MR imaging in cervical spondylitic myelopathy. AJNR Am J Neuroradiol 1998;19:1763–71 [PMC free article] [PubMed] [Google Scholar]
- 20.Muhle C, Metzner J, Weinert D, Schon R, Rautenberg E, Falliner A, et al. Kinematic MR imaging in surgical management of cervical disc disease, spondylosis and spondylotic myelopathy. Acta Radiologica 1999;40:146–53 [DOI] [PubMed] [Google Scholar]
- 21.Chen C, Hsu H, Niu C, Chen T, Chen M, Tseng Y, et al. Cervical degenerative disease at flexion-extension MR imaging: prediction criteria. Radiology 2003;227:136–42 [DOI] [PubMed] [Google Scholar]
- 22.Schlamann M, Reischke L, Klassen D, Maderwald S, Bohner V, Kollia K, et al. Dynamic magnetic resonance imaging of the cervical spine using the neuroswing system. Spine 2007;32:2398–401 [DOI] [PubMed] [Google Scholar]
- 23.Yoo JU, Zou D, Edwards WT. Effects of cervical spine motion on the neuroforaminal dimensions of the human cervical spine. Spine 1992;17:1131–6 [DOI] [PubMed] [Google Scholar]
- 24.Jinkins JR, Dworkin JS, Green CA, Greenhaigh JF, Gianni M, Gelbien M, et al. Upright, weight-bearing, dynamic – kinetic magnetic resonance imaging of the spine – review of the first clinical results. J HK Coll Radiol 2003;6:55–74 [Google Scholar]
- 25.Penning L, Wilmink JT, van Woerden HH, Knol E. CT myelographic findings in degenerative disorders of the cervical spine: clinical significance. AJNR Am J Neuroradiol 1986;7:119–27 [DOI] [PubMed] [Google Scholar]
- 26.Yu YL, Du Boulay GH, Stevens JM, Kendall BE. Computer-assisted myelography in cervical spondylotic myelopathy and radiculopathy. Brain 1986;109 259–75 [DOI] [PubMed] [Google Scholar]
- 27.Muhle C, Weinert D, Falliner A, Wiskirchen J, Metzner J, Baumer M, et al. Dynamic changes of the spinal canal in patients with cervical spondylosis at flexion and extension using magnetic resonance imaging. Invest Radiol 1998;33:444–9 [DOI] [PubMed] [Google Scholar]
- 28.Golash A, Birchall D, Laitt RD, Jackson A. Significance of CSF area measurements in cervical spondylitic myelopathy. Br J of Neurosurg 2001;15:17–21 [DOI] [PubMed] [Google Scholar]
- 29.Kadanka Z, Kerkovsky M, Bednarik J, Jarkovsky J. Cross-sectional transverse area and hyperintensities on magnetic resonance imaging in relation to the clinical picture in cervical spondylotic myelopathy. Spine 2007;32:2573–77 [DOI] [PubMed] [Google Scholar]
- 30.Kanchiku T, Taguchi T, Kaneko K. A Correlation between magnetic resonance imaging and electrophysiological findings in cervical spondylotic myelopathy. Spine 2001;26:e294–9 [DOI] [PubMed] [Google Scholar]
- 31.Stafira JS, Sonnad JR, Yuh WTC, Huard DR, Acker RE, Nguyen DL, et al. Qualitative assessment of cervical spinal stenosis: observer variability on CT and MR images. AJNR Am J Neuroradiol 2003;24:766–9 [PMC free article] [PubMed] [Google Scholar]
- 32.Nardin RA, Patel MR, Gudas TF, Rutkove SB, Raynor EM. Electromyography and magnetic resonance imaging in the evaluation of radiculopathy. Muscle Nerve 1999;22:151–5 [DOI] [PubMed] [Google Scholar]
- 33.Robinson LR. Electromyography, magnetic resonance imaging, and radiculopathy: it's time to focus on specificity. Muscle Nerve 1999;22:149–50 [DOI] [PubMed] [Google Scholar]
- 34.Bartlett R. Imaging of degenerative cervical myeloradiculopathy in radiology update. CPD Journal of the RCR 2008;7:63–83 [Google Scholar]