Abstract
Objectives
The aim of the study was to compare differences in dosimetric, clinical and quality-of-life end points among patients treated with helical tomotherapy (HT) and segmental multileaf collimator (SMLC)-based intensity-modulated radiotherapy (IMRT) for nasopharyngeal carcinoma.
Methods
From June 2005 to August 2009, 30 consecutive patients were treated with IMRT for nasopharyngeal carcinoma to a dose of 70 Gy. 14 patients (47%) were treated using HT and 16 (53%) were treated using SMLC-based IMRT. 28 patients (93%) received concurrent chemotherapy. The patients were evenly balanced between the two radiotherapy groups with respect to clinical and pathological characteristics. Median follow-up was 30 months (range, 6–62 months).
Results
The 2-year estimates of overall survival, local–regional control and progression-free survival were 81%, 87% and 82%, respectively. There were no significant differences in any of these end points with respect to IMRT technique (p>0.05 for all). Dosimetric analysis revealed that patients treated by HT had significantly improved salivary sparing with respect to mean dose (27.3 vs 34.1 Gy, p=0.03) and volume receiving greater than or equal to 30 Gy (31.7% vs 47.3%, p=0.01) to the contralateral (spared) parotid gland. The incidence of Grade 3+ late xerostomia was 13 and 7% among patients treated with SMLC-based IMRT and HT, respectively (p=0.62). The corresponding proportion of patients who subjectively reported “too little” or “no” saliva at final follow-up was 38% and 7%, respectively (p=0.04).
Conclusion
The superior dosimetric outcome observed with HT appeared to translate into moderately improved clinical outcomes with respect to salivary sparing. Prospective trials are needed to validate this gain in the therapeutic ratio.
Radiotherapy constitutes the primary treatment modality for nasopharyngeal carcinoma. While it is well established that local control strongly correlates with dose, the proximity of these tumours to critical normal tissue structures such as the central nervous system, optic pathways and parotid glands creates inherent challenges with respect to treatment planning and radiation delivery [1-3]. As a result, intensity-modulated radiotherapy (IMRT), which relies on a computer-derived optimisation process for dose distribution based on constraints to various sensitive structures and prescribed dose to tumour targets (e.g. inverse planning), has become widely adopted as the standard technique in the radiotherapeutic management of this disease.
Helical tomotherapy (HT) is a relatively novel technique that also relies on inverse planning but utilises a rotational gantry system rather than a fixed number of beam angles, as with traditional step-and-shoot segmental multileaf collimator (SMLC)-based IMRT, for radiation delivery. While some have suggested that HT-based IMRT may offer dosimetric advantages for cancers of the head and neck, a lack of data exists on its potential utility in the treatment of nasopharyngeal carcinoma [4-6]. At our institution, decisions regarding whether to employ HT or SMLC-based IMRT for this disease have traditionally been individualised and made at the discretion of the physician. Therefore, the purpose of this analysis was to compare differences in outcome with respect to dosimetric, clinical and quality-of-life end points among a cohort of patients treated by IMRT for head and neck cancer using these different techniques.
Methods and materials
Patients and evaluation
Between June 2003 and July 2009, 30 consecutive patients with locally advanced histologically proven nasopharyngeal carcinoma were referred for radiation therapy. Table 1 outlines the clinical and disease characteristics. All patients were staged in accordance with the 2002 American Joint Commission on Cancer guidelines [7]. The median age was 56 years (range, 23–70 years). 15 men (50%) and 15 women (50%) were included.
Table 1. Clinical and disease characteristics.
| Characteristic | SMLC (%) | HT (%) | ||
| Initial KPS | ||||
| 90–100 | 10 (63) | 9 (64) | ||
| 80 | 4 (25) | 3 (21) | ||
| 70 | 1 (6) | 1 (7) | ||
| <70 | 1 (6) | 1 (7) | ||
| Ethnicity | ||||
| Asian/Pacific Islander | 10 (63) | 9 (64) | ||
| Caucasian | 5 (31) | 4 (29) | ||
| Black | 1 (6) | 0 (0) | ||
| Hispanic | 0 (0) | 1 (7) | ||
| T-category | ||||
| T1 | 1 (6) | 1 (7) | ||
| T2 | 2 (13) | 1 (7) | ||
| T3 | 3 (25) | 2 (21) | ||
| T4 | 10 (63) | 10 (71) | ||
| N-category | ||||
| N1 | 1 (6) | 1 (7) | ||
| N2 | 7 (44) | 7 (50) | ||
| N3 | 8 (50) | 6 (36) | ||
| WHO status | ||||
| I | 5 (31) | 3 (21) | ||
| II | 3 (19) | 3 (21) | ||
| III | 8 (50) | 8 (57) | ||
| Pre-therapy PET scan | ||||
| No | 8 (50) | 5 (36) | ||
| Yes | 8 (50) | 9 (64) | ||
| Concurrent chemotherapy | ||||
| No | 1 (6) | 1 (7) | ||
| Yes | 15 (94) | 13 (93) | ||
| Adjuvant chemotherapy (no. cycles) | ||||
| 0 | 9 (56) | 9 (64) | ||
| 1 | 4 (25) | 2 (14) | ||
| 2 | 2 (13) | 2 (14) | ||
| 3 | 1 (6) | 1 (7) | ||
HT, helical tomotherapy; no., number; KPS, Karnofsky performance status; PET, positron emission tomography; SMLC, segmental multileaf collimator; WHO, World Health Organization.
Pre-treatment work-up included complete history, physical examination and direct flexible fibre-optic endoscopic examination, including direct laryngoscopy, bronchoscopy and oesophagoscopy with blind and directed biopsies. Axial imaging of the head and neck with CT and MRI was performed as a component of the initial work-up. Metastatic work-up included chest X-ray and routine blood work, including liver function tests. A positron emission tomography scan was obtained for 17 (57%) patients prior to treatment. All patients received continuous-course external-beam radiation therapy. 14 (47%) patients were treated using HT and 16 (53%) patients were irradiated using SMLC–IMRT. Concurrent cisplatin-based chemotherapy was administered to 28 (93%) patients. 12 (40%) patients received adjuvant chemotherapy consisting of cisplatin and 5-fluorouracil (5-FU). Prophylactic gastrostomy tube was placed prior to initiation of radiation therapy for 24 (80%) patients.
Simulation and target volume delineation
At simulation, the head, neck and shoulders were immobilised in a hyperextended position using a perforated thermoplastic head mask with the neck supported on a Timo cushion (S-type; Med-Tec, Orange City, IA) mounted on carbon fibre board (S-type; Med-Tec). Axial images with contiguous 3-mm slice thickness without contrast were obtained on a CT simulator (Picker PQ2000; Philips Medical Systems, Andover, MA) and transferred into a contouring workstation where delineation of target and normal tissue structures was performed.
The gross tumour volume (GTV) was defined as the extent of tumour demonstrated by imaging studies and physical examination, including endoscopy. Grossly positive lymph nodes were defined as lymph nodes >1 cm. MRI registered with the CT was used to assist in defining the parapharyngeal and superior extent of tumour. Three different clinical target volumes (CTVs) were defined: (i) CTV70, which included the GTV with a 5-mm margin or slightly smaller depending on the proximity to the brain stem and optic apparatus; (ii) CTV59.4, which included the high-risk neck as well as the entire nasopharynx, retropharyngeal lymph nodes, clivus, skull base, pterygoid fossae, parapharyngeal space, inferior sphenoid sinus and posterior third of the nasal cavity and maxillary sinuses; and (iii) CTV54 for the low-risk nodal regions. Bilateral IB lymph nodes were spared in node-negative patients unless they had extensive involvement of the hard palate, nasal cavity or maxillary antrum. An additional margin, typically 3–5 mm, was added to the CTVs to compensate for the variabilities of treatment set-up and internal organ motion, resulting in corresponding planning target volumes (PTVs) of PTV70, PTV59.4 and PTV54.
Intensity-modulated radiotherapy planning
For 16 patients, radiotherapy was delivered with SMLC–IMRT using an Elekta Synergy linear accelerator (Elekta Oncology, Stockholm, Sweden) equipped with an 80 multileaf collimator (1-cm leaf width at isocentre). The number of fields used for SMLC–IMRT planning ranged from 7 to 10 (median, 9). The Pinnacle3 treatment planning system, version 8.0d (Philips Medical Systems, Highland Heights, OH), was used for SMLC–IMRT treatment planning. This software uses a convolution superposition dose calculation algorithm. For the remaining 14 patients, radiotherapy was delivered using the TomoTherapy® HI-ART treatment system (TomoTherapy Inc., Madison, WI). The planning system utilised a convolution/superposition-based dose calculation algorithm and accounted for tissue density variations. All treatment plans were evaluated via dose-volume histogram analysis and by visual inspection of selected isodose curves overlaid on axial CT slices.
Prescription and planning goals
The treatment goal was to deliver a prescribed dose of 70 Gy to the PTV70 and 60 Gy to the PTV59.4 over 33 treatments with once-daily fractionation, 5 days per week. All PTVs were treated simultaneously, with fraction sizes of 2.12, 1.8 and 1.63 Gy delivered to the PTV70, PTV59.4 and PTV54, respectively. The low neck was encompassed within the IMRT plan in all cases, and an anterior low-neck field was not used. Radiation planning goals was to encompass at least 95% of the PTVs with the prescription isodose surface while meeting the following absolute parameters: no more than 20% of PTV70 receives >110% of the prescribed dose; no more than 1% of any PTV70 and any PTV59.4 receives <93% of the prescribed dose; and no more than 1% or 1 cm3 of the tissue outside the PTVs receives >110% of the dose prescribed to the PTV70. Dose constraints with prioritisation goals that were used for IMRT planning are outlined in Table 2.
Table 2. Dose constraints for intensity-modulated radiotherapy planning.
| Structure | Constraint | Priority |
| Spinal cord | Maximum <48 Gy | High |
| Brain stem | Maximum <54 Gy | High |
| Optic chiasm | Maximum <50 Gy | High |
| Optic nerve | Maximum <54 Gy | High |
| Retina | Maximum <45 Gy | High |
| Temporal lobe | Maximum <60 Gy | Intermediate |
| Parotid gland (spared) | Mean <26 Gy or V30 <50% | Intermediate |
| Cochlea/vestibule | Maximum <50 Gy | Intermediate |
| Larynx | Mean 40 Gy | Intermediate |
| Oral cavity | Mean 35 Gy | Intermediate |
| Brachial plexus | Maximum <66 Gy | Intermediate |
| Mandible | Maximum >70 Gy | Intermediate |
| Cricopharyngeal inlet | Maximum <60 Gy | Low |
| Cervical oesophagus | Maximum <65 Gy | Low |
| Lacrimal gland | V30 <50% | Low |
Image guidance
All patients underwent daily image-guided radiotherapy (IGRT) in conjunction with treatment. Patients were placed in the treatment position on the table, which was aligned with wall-mounted lasers using external fiducial marks. Daily IGRT images were acquired volumetrically using either kilovoltage cone beam or megavoltage fan beam over a longitudinal field of view that typically ranged from C7 to 2 cm superior to the base of skull. The IGRT scanning range attempted to cover the PTV1 as previously designated. In some cases in which the entire PTV1 could not be adequately encompassed using image guidance owing to physical limitations, the superior and inferior portions of PTV1 were acquired on alternating days. After the IGRT images were reconstructed, they were fused with the treatment-planning CT images at the treatment console display using automated registration bone pre-sets followed by manual adjustments if needed. During therapy, the attending physician and radiation therapist reviewed the fused image in the sagittal, coronal and axial planes using the fusion split-screen display on a daily basis. Bony landmarks for confirming the alignment and thresholds for positioning correction were established during this initial period so that therapists were comfortable making the position adjustments that fell within guidelines. After image fusion was satisfactorily accomplished, the treatment couch was automatically repositioned for treatment delivery by the IMRT system.
Statistical analysis
The end points analysed were overall survival, local-regional control and distant metastasis-free survival. Local control was judged to have been attained if there was no evidence of tumour at the primary site based on clinical and radiographic findings at follow-up. Regional failure was recorded separately if there was evidence of a cervical or supraclavicular mass distinct from the primary site. Median follow-up was 30 months (range, 6–62 months) for the entire patient population and was 27 months (range, 6–52 months) and 34 months (range, 6–62 months) for patients treated by HT and SMLC–IMRT, respectively.
Acute and late normal tissue effects were graded according to the Radiation Therapy Oncology Group (RTOG)/European Organization for the Treatment of Cancer radiation toxicity criteria [8]. In addition, all patients completed the University of Washington quality-of-life questionnaire (UW-QOL) during each follow-up visit. Tests analysing the difference between the proportions of complications in each cohort were performed using the Fisher's exact test. The dose characteristics to critical structures were compared using a one-way analysis of variance test. Actuarial estimates of overall survival, local-regional control and distant metastasis-free survival were calculated using the Kaplan–Meier method, with comparisons among groups performed with two-sided log-rank tests [9]. All tests were two-tailed, with a probability value of <0.05 considered statistically significant.
Results
Dosimetric comparison
Table 3 illustrates the differences among various dose–volume characteristics for patients treated by SMLC–IMRT and HT. The mean dose to the contralateral (spared) parotid gland was 27.3 Gy (range, 21.7–35.3 Gy) among patients treated by HT, compared with 34.1 Gy (range, 25.1–39.5 Gy) for those treated by SMLC–IMRT (p=0.03). A statistically significant difference was also observed in the volume receiving ≥30 Gy (V30) to the contralateral parotid gland, which was 31.7 and 47.3% with HT and SMLC–IMRT, respectively (p=0.01). Using a dose constraint of V30 <50% for the contralateral parotid gland, 13 of 14 patients treated by HT were able to achieve this goal, whereas 10 of 16 patients treated by SMLC–IMRT were able to satisfy this requirement.
Table 3. Dose–volume characteristics for selected normal critical structures.
| Treatment | Contralateral parotid gland |
Spinal cord (cm3) >45 Gy | Brain stem (cm3) >54 Gy | Temporal lobes (cm3) >60 Gy | |
| V30 (%) | Mean (Gy) | ||||
| SMLC | 47.3±9.6 | 34.1±5.6 | 3.7±5.1 | 1.3±0.6 | 6.2±5.9 |
| HT | 31.7±7.5 | 27.3±4.9 | 0.7±0.6 | 0.9±0.1 | 2.3±5.0 |
HT, helical tomotherapy; SMLC, segmental multileaf collimator; V30, volume receiving ≥30 Gy.
As shown in Table 4, patients treated by HT received lower maximum doses to the auditory structures than patients treated by SMLC–IMRT. The recommended maximum dose constraints to the ipsilateral ear structures (50 Gy) were achieved in only 5 of 16 patients for the inner ear and 7 of 16 patients for the middle ear among patients treated by SMLC–IMRT. By contrast, this maximum dose constraint of 50 Gy for the ipsilateral ear structures was satisfied in 12 of 14 patients for the inner ear and 13 of 14 patients for the middle ear among patients treated by HT. HT significantly reduced maximum doses to the ipsilateral inner and middle ears from 64.8 to 49.1 Gy and from 55.0 to 45.4 Gy, respectively (p=0.01 for both). The maximum doses to the contralateral inner and middle ear were 53.0 and 48.3 Gy, respectively, using SMLC–IMRT. Although HT decreased the corresponding doses to these contralateral auditory structures to 47.4 and 46.5 Gy, respectively, the reduction was not statistically significant (p=0.33 and p=0.10 for inner and middle ear, respectively).
Table 4. Maximum dose (Gy) to auditory structures.
| Treatment | Ipsilat. inner ear | Contra. inner ear | Ipsilat. middle ear | Contra. middle ear |
| SMLC | 64.8±9.2 | 53.0±7.7 | 55.0±8.9 | 48.3±6.7 |
| HT | 49.1±7.1 | 47.4±6.9 | 45.4±7.7 | 46.5±5.1 |
contra., contralateral; HT, helical tomotherapy; ipsilat., ipsilateral; SMLC, segmental multileaf collimator; V30, volume receiving ≥30 Gy.
As shown in Table 5, maximum doses to the spinal cord, optic chiasm, brain stem and temporal lobe were greater for patients treated by SMLC–IMRT compared with HT, although none of these differences, with the exception of that for the temporal lobes (p=0.01), reached statistical significance (p<0.05 for all).
Table 5. Maximum dose (Gy) to selected normal critical normal structures.
| Treatment | Spinal cord | Optic chiasm | Brain stem | Oral cavity | Temp. lobes | Mandible |
| SMLC | 44.8±3.3 | 50.5±7.0 | 56.7±13.1 | 61.5±23.5 | 70.3±7.1 | 69.0±5.2 |
| HT | 42.1±3.0 | 47.3±6.4 | 51.1±8.4 | 63.6±14.2 | 66.7±6.8 | 69.7±6.7 |
HT, helical tomotherapy; SMLC, segmental multileaf collimator; temp., temporal lobes; V30, volume receiving ≥30 Gy.
Survival and disease control
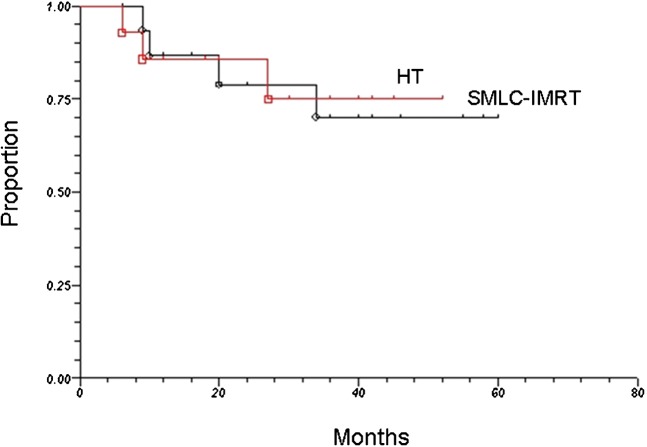
23 patients were alive at the time of this analysis, yielding a 2-year estimate of overall survival of 81%. As illustrated in Figure 1, the 2-year estimates of overall survival for patients treated by SMLC–IMRT and HT were 79 and 84%, respectively (p=0.51). Among the seven patients (four SMLC–IMRT; three HT) who died during the evaluation period, four died as a result of progressive disease at local-regional sites, two from complications related to metastatic disease and one of intercurrent disease (cerebral-vascular accident).
Figure 1.
Overall survival among patients treated by segmental multileaf collimator-based intensity-modulated radiotherapy (SMLC-IMRT) and helical tomotherapy (HT).
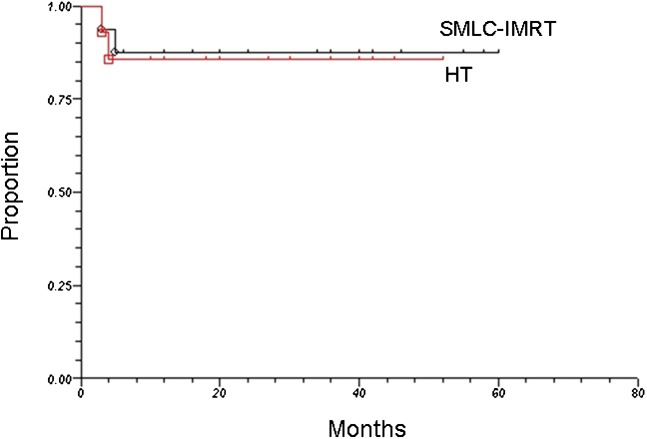
Among the entire patient population, a total of 4 patients (2 SMLC–IMRT; 2 HT) experienced progression or recurrence of local-regional disease, yielding a 2-year estimate of local-regional control of 87%. Sites of local-regional relapse included the cavernous sinus (two patients), nasopharynx (one patient), and foramen ovale (one patient). As illustrated in Figure 2, the local-regional control was 88% and 86% among patients treated by SMLC–IMRT and HT, respectively (p=0.37). Spatial evaluation of local-regional failures revealed that all patients who relapsed in the primary site or neck failed in the designated CTVs. The median time to local-regional recurrence for all patients was 4 months (range, 2–6 months).
Figure 2.
Local-regional control among patients treated by segmental multileaf collimator-based intensity-modulated radiotherapy (SMLC-IMRT) and helical tomotherapy (HT).
A total of four patients developed distant metastasis at a median time of 11 months (range, 4–16 months). Two of these were isolated first recurrences, with the remaining two cases occurring subsequent to local-regional disease failure. The 2-year estimate of progression-free survival for the entire patient population was 82%.
Toxicity
The most commonly reported Grade 3+ acute toxicity (non-haematological) was related to confluent mucositis, which occurred in 25% and 29% of the patients treated by SMLC–IMRT and HT, respectively (p=0.82). Other documented Grade 3+ acute toxicities included moist desquamation of the skin (five patients), external otitis (five patients), laryngeal oedema with hoarseness (four patients) and keratitis (one patient). There were no significant differences with respect to any of non-mucositis acute side effects among patients treated by SMLC–IMRT or HT (p>0.05 for all). No treatment-related fatalities were observed.
The incidence of Grade 3+ late toxicity was 6% and 7% among patients treated by SMLC–IMRT and HT, respectively (p=0.92). The most commonly reported Grade 3+ late effect was related to dysphagia as 13% and 14% of patients treated by SMLC–IMRT and HT, respectively, reported Grade 3+ oesophageal toxicity (liquid diet only) in the late setting (p=0.88). With respect to xerostomia, 13% and 7% of patients treated by SMLC–IMRT and HT, respectively, complained of complete dryness of mouth at any point in the late setting (p=0.62). Among the patients treated by SMLC–IMRT, 7 of 16 patients (38%) subjectively reported “too little” or “no” saliva using the UW-QOL at last follow-up compared with 1 of 14 patients (7%) treated by HT (p=0.04).
Discussion
The present study represents the first series to date reporting on outcomes after various IMRT techniques in the treatment of nasopharyngeal carcinoma. These findings are particularly relevant in view of the recently published results of RTOG trial 0225 demonstrating the feasibility and effectiveness of IMRT in the treatment of this disease [10]. Notably, both SMLC- and HT-based IMRT methods were allowed in the multi-institutional trial conducted by the RTOG, although subset analysis analysing any potential differences in outcomes between the two was not performed.
Although this was a non-randomised comparison of a single institutional experience, we were nonetheless able to identify important differences in outcome among patients treated by HT and SMLC–IMRT. Notably, the use of HT appeared to improve dose distributions to several critical structures, including the contralateral parotid gland and bilateral ear structures. More importantly, these dosimetric advantages translated into an improvement in the therapeutic ratio, as HT appeared to reduce chronic xerostomia without compromising disease control.
The most noteworthy advantage of IMRT in the treatment of nasopharyngeal cancer appears to be related to its ability to preserve salivary function [11-13]. In the only published randomised trial evaluating the influence of radiation therapy technique in nasopharyngeal carcinoma, Kam et al [14] showed that patients treated by IMRT had a significantly lower incidence of severe xerostomia and improved salivary flow rates than those treated by conventional, non-IMRT techniques. In the present series, the use of HT appeared to contribute to further reductions in toxicity. In particular, HT resulted in significant reductions to both mean dose and V30, the two most commonly cited dosimetric measures of salivary sparing, to the contralateral parotid gland. Although the magnitude of reduction was not nearly as large as commonly observed with the transition from non-IMRT to IMRT techniques, improved outcomes were nevertheless observed between those treated by SMLC- and HT-based IMRT techniques.
In the present study, a significantly greater proportion of patients treated by SMLC–IMRT complained of severe xerostomia after irradiation compared with those treated by HT. Although flow sialometry studies were not performed, others have similarly shown that the decrease in xerostomia with IMRT results in enhanced quality of life [15]. Indeed, the improvement with salivary function is of the order of 1 ml Gy−1 reduction to the mean parotid gland dose. Eisbruch et al [16] have validated the importance of mean dose to the parotid gland in the setting of IMRT and demonstrated a threshold for both stimulated (26 Gy) and unstimulated (24 Gy) salivary flow rates. Although a limitation of the present study was the failure to account for doses to the submandibular and minor salivary glands, both of which contribute to salivary function, our results nonetheless provide important evidence that the dosimetric advantages associated with HT translated into improvements in clinical outcomes with respect to the end point of xerostomia.
Another notable finding of the present study was the ability of HT to reduce dose to the ear structures. In view of several recently published studies identifying a dose–response relationship for the inner and middle ear, the importance of minimising dose to these structures is becoming increasingly recognised among those undergoing irradiation [17,18]. In a prospective study of 40 patients, Pan et al [19] showed that clinically apparent hearing loss greater than 10 dB occurred when the cochlea received mean doses in excess of 45 Gy. Honoré et al [20] similarly developed a model predictive of sensorineural hearing loss based on pre-therapy dosimetric and clinical outcome data of 20 patients treated with radiotherapy for nasopharyngeal carcinoma. Notably, when the model was adjusted for age and pre-therapy hearing level, a steep dose–response curve emerged with a threshold at approximately 40 Gy. While questions exist regarding the time course of hearing loss after irradiation, general agreement exists that doses to the inner and middle ear should be closely monitored. This may be of increasing relevance as more patients are receiving concurrent cisplatin, which has known ototoxic effects [21]. Although formal audiological testing was not performed in our study and definite toxicity data were unavailable, evaluation for iatrogenic hearing loss will be considered for these patients in the future.
The potential of HT to significantly decrease mean doses to the temporal lobes was also evident in this study. Although it remains to be determined whether the reduction in dose to this structure results in decreased toxicity on actual patients, it is worth pointing out that reported rates of cerebral necrosis after treatment of nasopharyngeal carcinoma using conventional techniques have historically ranged from 3% to 20% [22-25]. Owing to the relatively long latency period for developing this complication, however, minimal data exist on the efficacy of intensity-modulated techniques in reducing these numbers. Given the devastating nature of this side effect, however, it seems prudent that particular consideration be given to limit the dose to the central nervous system to as low as feasibly possible. Until more precise measures of correlating dosimetric parameters with clinical symptoms are identified, keeping the dose to neurological structures to a minimum is desirable.
It must be recognised that this study was a non-randomised comparison subject to various selection biases and hindered by a lack of clear criteria for the assignment to HT or SMLC–IMRT. The decision to use either technique was a complicated one, and based on factors such as physician discretion, patient request, insurance information and departmental resources. Furthermore, operator bias and the expertise of the planner may have further biased our findings. We also acknowledge that the dose and fractionation schedule used at our centre may not be standard at other institutions. As a result of these potential confounding factors, it remains difficult to definitively establish a cause–effect association between the superior dosimetric outcomes observed with HT and the improved clinical outcomes. Nonetheless, the fact that nearly all patients had locally advanced disease (with the majority presenting with T4 disease) resulted in a fairly even distribution of disease characteristics among the two cohorts. Moreover, with the development and increasing utilisation of micro-collimator techniques with leaf sizes <0.5 cm in conjunction with SMLC–IMRT, it is likely that the observed advantages associated with HT will be minimised in the future. Lastly, this study did not account for changes in patient anatomy over time and how this may have potentially affected dosimetry as well as outcomes. O'Daniel et al [26], for instance, elegantly showed that “what you plan” is not always “what you get” owing to positional and volumetric changes over a course of IMRT for head and neck cancer.
While the relatively small number of patients in this study and the limitations above preclude the drawing of definitive conclusions, our findings suggesting an improvement in the therapeutic ratio with the use of HT for nasopharyngeal cancer are nonetheless noteworthy. Based on our experiences, the current policy at our institution is to treat all patients with nasopharyngeal carcinoma with HT. Although the use of HT is associated with some limitations including the lack of room's-eye view three-dimensional dose cloud plan review, we find these acceptable in view of the dosimetric and clinical benefits discussed above. Further studies are needed to corroborate our findings and to determine whether the improvements in outcome are sustained over time.
Footnotes
Presented at the 2006 Annual Meeting of the American Society for Therapeutic Radiology and Oncology (ASTRO), Philadelphia, PA, November 2006.
References
- 1.Leibel SA, Kutcher GJ, Harrison LB, Fass DE, Burman CM, Hunt MA, et al. Improved dose distributions for 3D conformal boost treatments in carcinoma of the nasopharynx. Int J Radiat Oncol Biol Phys 1991;20:823–33 [DOI] [PubMed] [Google Scholar]
- 2.Wolden SL, Zelefsky MJ, Kraus DH, Rosenzweig KE, Chong LM, Shaha AR, et al. Accelerated concomitant boost radiotherapy and chemotherapy for advanced nasopharyngeal carcinoma. J Clin Oncol 2001;19:1105–10 [DOI] [PubMed] [Google Scholar]
- 3.Perez CA, Devineni VR, Marcial-Vega V, Marks JE, Simpson JR, Kucik N. Carcinoma of the nasopharynx: factors affecting prognosis. Int J Radiat Oncol Biol Phys 1992;23:271–80 [DOI] [PubMed] [Google Scholar]
- 4.Bauman G, Yartsev S, Rodrigues G, Lewis C, Venkatesan VM, Yu E, et al. A prospective evaluation of helical tomotherapy. Int J Radiat Oncol Biol Phys 2007;68:632–41 [DOI] [PubMed] [Google Scholar]
- 5.Fiorino C, Dell'Oca I, Pierelli A, Broggi S, De Martin E, Di Muzio N, et al. Significant improvement in normal tissue sparing and target coverage for head and neck cancer by means of helical tomotherapy. Radiother Oncol 2006;78:276–82 [DOI] [PubMed] [Google Scholar]
- 6.Sheng K, Molloy JA, Read PW. Intensity-modulated radiation therapy (IMRT) dosimetry of the head and neck: a comparison of treatment plans using linear accelerator-based IMRT and helical tomotherapy. Int J Radiat Oncol Biol Phys 2006;65:917–23 [DOI] [PubMed] [Google Scholar]
- 7.Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, et al. AJCC cancer staging manual. 6th edn New York, NY: Springer–Verlag; 2002 [Google Scholar]
- 8.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;31:1341–6 [DOI] [PubMed] [Google Scholar]
- 9.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:547–81 [Google Scholar]
- 10.Lee N, Harris J, Garden AS, Straube W, Glisson B, Xia P, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: Radiation Therapy Oncology Group Phase II Trial 0225. J Clin Oncol 2009;27:3684–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao KS, Majhail N, Huang CJ, Simpson JR, Perez CA, Haughey B, et al. Intensity-modulated radiation therapy reduces late salivary toxicity without compromizing tumor control in patients with oropharyngeal carcinoma: a comparison with conventional techniques. Radiother Oncol 2001;61:275–80 [DOI] [PubMed] [Google Scholar]
- 12.Jabbari S, Kim HM, Feng M, Lin A, Tsien C, Elshaikh M, et al. Matched case-control study of quality of life and xerostomia after intensity-modulated radiotherapy or standard radiotherapy for head and neck cancer of unknown primary: toxicity and preliminary efficiency. Int J Radiat Oncol Biol Phys 2008;70:1100–717980501 [Google Scholar]
- 13.Braam PM, Terhaard CH, Roesink JM, Raaijmakers CP. Intensity-modulated radiotherapy significantly reduces xerostomia compared with conventional radiotherapy. Int J Radiat Oncol Biol Phys 2006;66:975–80 [DOI] [PubMed] [Google Scholar]
- 14.Kam MK, Leung SF, Zee B, Chau RM, Suen JJ, Mo F, et al. Prospective randomized study of inensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol 2007;25:4873–9 [DOI] [PubMed] [Google Scholar]
- 15.Scrimger R, Kanji A, Parliament M, Warkentin H, Field C, Jha N, et al. Correlation between saliva production and quality of life measurements in head and neck cancer patients treated with intensity-modulated radiotherapy. Am J Clin Oncol 2007;30:271–7 [DOI] [PubMed] [Google Scholar]
- 16.Eisbruch A, Ten Haken RK, Kim HM, Marsh LH, Ship JA. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys 1999;45:577–87 [DOI] [PubMed] [Google Scholar]
- 17.Ho WK, Wei WI, Kwong DL, Sham JS, Tai PT, Yuen AP, Au DK. Long-term sensorineural hearing deficit following radiotherapy in patients suffering from nasopharyngeal carcinoma: a prospective study. Head Neck 1999;21:547–53 [DOI] [PubMed] [Google Scholar]
- 18.Chen WC, Jackson A, Budnick AS, Pfister DG, Kraus DH, Hunt MA, et al. Sensorineural hearing loss in combined modality treatment of nasopharyngeal carcinoma. Cancer 2006;106:820–9 [DOI] [PubMed] [Google Scholar]
- 19.Pan CC, Eisbruch A, Lee JS, Snorrason RM, Ten Haken RK, Kileny PR. Prospective study of inner ear radiation dose and hearing loss in head-and-neck cancer patients. Int J Radiat Oncol Biol Phys 2005;61:1393–402 [DOI] [PubMed] [Google Scholar]
- 20.Honoré HB, Bentzen SM, Møller K, Grau C. Sensorineural hearing loss after radiotherapy for nasopharyngeal carcinoma: individualized risk estimation. Radiother Oncol 2002;65:9–16 [DOI] [PubMed] [Google Scholar]
- 21.Ho WK, Wei WI, Kwong DL, et al. Long-term sensorineural hearing loss in patients treated for nasopharyngeal carcinoma: a prospective study of the effect of radiation and cisplatin treatment. Int J Radiat Oncol Biol Phys 1996;36:281–9 [DOI] [PubMed] [Google Scholar]
- 22.Yeh SA, Tang Y, Lui CC, Huang YJ, Huang EY. Treatment outcomes and late complications of 849 patients with nasopharyngeal carcinoma treated with radiotherapy alone. Int J Radiat Oncol Biol Phys 2005;62:672–9 [DOI] [PubMed] [Google Scholar]
- 23.Jen YM, Hsu WL, Chen CY, Hwang JM, Chang LP, Lin YS, et al. Different risks of symptomatic brain necrosis in NPC patients treated with different altered fractionated radiotherapy techniques. Int J Radiat Oncol Biol Phys 2001;51:344–8 [DOI] [PubMed] [Google Scholar]
- 24.Lee AW, Foo W, Chappell R, Fowler JF, Sze WM, Poon YF, et al. Effect of time, dose, and fractionation on temporal lobe necrosis following radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 1998;40:35–42 [DOI] [PubMed] [Google Scholar]
- 25.Lee AW, Kwong DL, Leung SF, Tung SY, Sze WM, Sham JS, et al. Factors affecting risk of symptomatic temporal lobe necrosis: significance of fractional dose and treatment time. Int J Radiat Oncol Biol Phys 2002;53:75–85 [DOI] [PubMed] [Google Scholar]
- 26.O'Daniel JC, Garden AS, Schwartz DL, Wang H, Ang KK, Ahamad A, et al. Parotid gland dose in intensity-modulated radiotherapy for head and neck cancer: is what you plan what you get? Int J Radiat Oncol Biol Phys 2007;69:1290–6 [DOI] [PMC free article] [PubMed] [Google Scholar]