Abstract
Objective
To determine the role of abdominal CT in assessment of severity and prognosis of patients with acute gastrointestinal (GI) graft-vs-host disease (GVHD).
Methods
During 2000–2004, 41 patients with a clinical diagnosis of acute GI-GVHD were evaluated. CTs were examined for intestinal and extra-intestinal abnormalities, and correlated with clinical staging and outcome.
Results
20 patients had GVHD clinical Stage I–II and 21 had Stage III–IV. 39 (95%) had abnormal CT appearances. The most consistent finding was bowel wall thickening: small (n=14, 34%) or large (n=5, 12%) bowel, or both (n=20, 49%). Other manifestations included bowel dilatation (n=7, 17%), mucosal enhancement (n=6, 15%) and gastric wall thickening (n=9, 38%). Extra-intestinal findings included mesenteric stranding (n=25, 61%), ascites (n=17, 41%), biliary abnormalities (n=12, 29%) and urinary excretion of orally administered gastrografin (n=12, 44%). Diffuse small-bowel thickening and any involvement of the large bowel were associated with severe clinical presentation. Diffuse small-bowel disease correlated with poor prognosis. 8 of 21 patients responded to therapy, compared with 15 of 20 patients with other patterns (p=0.02), and the cumulative incidence of GVHD-related death was 62% and 24%, respectively (p=0.01). Overall survival was not significantly different between patients with diffuse small-bowel disease and patients with other patterns (p=0.31). Colonic disease correlated with severity of GVHD (p=0.04), but not with response to therapy or prognosis (p=0.45).
Conclusion
GVHD often presented with abdominal CT abnormalities. Diffuse small-bowel disease was associated with poor therapeutic response. CT may play a role in supporting clinical diagnosis of GI GVHD and determining prognosis.
Allogeneic stem-cell transplantation (SCT) has been used increasingly to treat haematopoietic disorders and haematological malignancies [1,2]. Among the complications of SCT, graft-vs-host disease (GVHD) is one of the major causes of morbidity and mortality [3-5]. Intestinal GVHD is one of the most frequent features of acute GVHD. Gastrointestinal (GI) symptoms include abdominal pain, nausea, vomiting and profuse diarrhoea [5-8]. The diagnosis and grading of the disease are based on a spectrum of clinical and laboratory features. Clinical parameters such as the quantity of diarrhoea are used to determine the clinical severity of GI GVHD [9]. These are, however, not very accurate, as assessment of the volume of diarrhoea is inconvenient and inaccurate. Endoscopic evaluation, with histological examination of biopsy specimens, can be useful for diagnosing and staging intestinal GVHD [10-12]. However, GI biopsies may be hazardous in patients with severe thrombocytopenia, coagulopathy and granulopenia [13]. Moreover, both endoscopic evaluation and histology can underestimate the severity of the disease [14].
Recently, non-invasive methods have been used to assess the extent and severity of intestinal GVHD, including CT [15-20], high-resolution ultrasonography [21,22], MRI [23] and positron emission tomography with fluorodeoxyglucose (PET-FDG) [24]. Abdominal CT has been the main modality, showing abnormal findings in gastrointestinal GVHD [16,25] which correlate with both pathological [18] and clinical grading [20]. No study has as yet tried to correlate these CT findings with the outcome of the disease. This study was therefore designed to determine the role of abdominal CT in the assessment of severity and prognosis of patients with acute intestinal GVHD.
Methods and materials
Study population
This is a retrospective analysis of 41 patients with haematological malignancies who had undergone allogeneic stem-cell transplantation and presented with a clinical diagnosis of acute GVHD of the GI tract. There were 285 allogeneic transplants performed during the study period. 74 of these patients had a clinical diagnosis of GVHD involving the GI tract and 41 participated in the study. Patients were evaluated by abdominal CT early after presentation. All patients were treated according to institutional review board approved protocols.
Clinical staging and treatment
Clinical staging of the severity of GI GVHD was based on the severity of nausea and abdominal pain, and on the volume of diarrhoea, and was classified into four stages according to Glucksberg criteria (1974) [9]: Stage I—diarrhoea volume 500–1000 cm3 d−1, or persistent nausea; Stage II—diarrhoea volume 1000–1500 cm3 d–1; Stage III—diarrhoea volume >1500 cm3 d–1; Stage IV—severe abdominal pain and/or ileus. Infection as the cause of GI symptoms was ruled out by repeat stool cultures and testing for Clostridium difficile toxin. Involvement of other organs was also determined, but was not the primary goal of this study. The diagnosis was confirmed by GI biopsies when appropriate. All patients were given ciclosporin A and a short course of methotrexate for GVHD prevention after transplantation. Treatment of acute GVHD included high-dose methylprednisone (2 mg kg–1) intravenously and tapered according to response. Mycophenolate mofetil was added for steroid-resistant or dependent cases. Patients were followed routinely and response to therapy was recorded. The cause of death was coded as GVHD if there was no response to treatment or if infection occurred in the context of aggressive immunosuppressive therapy.
CT studies and interpretations
CT studies were performed on a helical CT (Twin CT dual slice; Elscint, Haifa, Israel; or Mx 8000 multislice; Philips, Cleveland, OH) with slice thickness of 2.5–6.5 mm. All patients received 1500 ml of diluted oral gastrografin. 23 (56%) had pre- and post-intravenous contrast-enhanced studies (1.5 ml kg–1), 14 (34%) had post-contrast studies only (they underwent unenhanced scans on previous studies) and 4 (10%) had unenhanced scans only (allergy or abnormal renal function tests). Scanning was obtained 60–70 s after contrast injection for maximal mucosal enhancement.
The CT studies were evaluated by a radiologist experienced in abdominal CT (SA, 21 years' experience) who was blinded to the clinical data. CT scans were evaluated for intestinal and extra-intestinal abnormalities.
Intestinal CT abnormalities included bowel wall thickening, defined as >3 mm, and bowel wall dilatation, defined as region of small bowel with a diameter >2.5 cm, or of large bowel >8.0 cm. Extent of bowel involvement was defined as segmental (≤40 cm) or diffuse (>40 cm).
Intestinal mucosal enhancement and attenuation patterns of bowel wall were assessed as well. The latter included the “water halo sign” (defined as a line of decreased attenuation within the bowel wall [16]), the “accordion sign” (defined as broad transverse bands in the colon that trap oral contrast [26]) and pneumatosis intestinalis. Oesophageal and gastric wall were evaluated only when good distension of these organs was achieved as determined by visual assessment. Intramural air was also noted.
Extra-intestinal CT abnormalities evaluated included mesenteric fat stranding, ascites, biliary abnormalities (distended gall bladder, wall thickening and wall enhancement), periportal oedema and free air in the abdominal cavity. Urinary excretion of orally ingested gastrografin (on unenhanced scans) was noted as well [27].
CT findings were correlated with clinical staging, response to therapy and cause of death.
Statistical analysis
The primary objective of the study was to correlate CT findings with GI GVHD stage. CT findings were reported as crude percentages. Means were reported with standard deviations and medians with interquartile range, when appropriate. The association of CT findings and clinical severity was performed using χ2 analysis. This was a retrospective study and the sample size was determined by patient availability during the study period. Power analysis shows that for α=0.05 and a power of 0.80, 26 patients in each group are required to detect a large-sized difference (of 50%). More specifically, when comparing proportions of a specific CT finding in two groups of mild and severe GVHD, with 20 patients in each group, there is an 80% probability of detecting a difference of 45%. A similar approach was used to compare responses to therapy among different patient groups by CT patterns. Survival analysis was a secondary objective. Overall survival was calculated from the day of the CT study until death or last follow-up. The probability of overall survival was estimated and plotted using the Kaplan–Meier product-limit method. GVHD-related death was estimated using cumulative incidence analysis, with death related to other causes considered a competing risk. The affect of radiological patterns on survival probabilities was studied with the log-rank test. A sample size of 41 patients can detect a difference at 0.05 significance level if the true hazard rate is 2.7, assuming an accrual period of 60 months, follow-up of 24 months and median survival of 12 months. Thus only large difference in survival rates could be detected. Multivariate analysis could not be performed owing to the limited size of the patient group.
Results
Patient characteristics
The study included 41 patients with various haematological malignancies (27 men, 14 women; median age 51 years, interquartile range 39–55 years). All patients had a clinical diagnosis of acute GI GVHD diagnosed during a 5-year period (2000–2004 inclusive). Patient characteristics are outlined in Table 1. In all, 7 patients had acute GI GVHD in clinical Stage I, 13 patients had Stage II and 21 patients had Stage III–IV.
Table 1. Patient characteristicsa.
| Characteristic | Results |
| Age (years) | 51 (39–55) |
| Gender | |
| Male | 27 (66) |
| Female | 14 (34) |
| Diagnosis | |
| AML/MDS | 13 (32) |
| ALL | 6 (15) |
| MM | 9 (22) |
| NHL | 6 (15) |
| CLL | 5 (12) |
| CML | 2 (5) |
| Donor | |
| HLA-matched sibling | 26 (63) |
| 1-Ag mismatched related | 3 (7) |
| Matched unrelated | 12 (29) |
| Conditioning regimen | |
| Myeloablative | 11 (27) |
| Reduced-intensity | 30 (73) |
| Staging of GI GVHD | |
| Stage I | 7 (17) |
| Stage II | 13 (32) |
| Stage III–IV | 21 (51) |
AML/MDS, acute myeloid leukaemia/myelodysplastic syndrome; ALL, acute lymphoblastic leukaemia; HLA, human leukocyle antigen; MM, multiple myeloma; NHL, non-Hodgkin's lymphoma; CLL, chronic lymphocytic leukaemia; CML, chronic myeloid leukaemia.
aData are given as median (interguartile range) and number (percentage).
CT findings
Table 2 outlines the abnormal findings detected on abdominal CT. Abnormal intestinal findings were observed in 39 (95%) of the 41 patients and extra-intestinal findings were detected in 34 (83%). Thickening of the bowel wall (Figures 1 and 2) was the most common finding, occurring in all 39 patients with abnormal CT findings. The small bowel wall measured 4–12 mm (mean 6.4±1.9 mm) and the large bowel wall 4–18 mm (mean 8.7±3.8 mm). Involvement of both small and large bowel was more frequently seen (20 patients, 49%) than involvement of only small bowel (14 patients, 34%) or large bowel (5 patients, 12%). Bowel dilatation was seen in 18 patients (44%) and was most often present in the small bowel, measuring 2.6–3.9 cm (mean 2.9±0.5 cm). Only 1 patient (2%) had dilatation of the large bowel. Diffuse involvement of small bowel was more often seen than segmental involvement. Oesophageal wall thickening was observed in 2 of 14 evaluable patients with distended oesophagus on CT (14%), and gastric wall thickening (Figure 3) was observed in 9 of 24 evaluable patients with fully distended stomach on CT (38%).
Table 2. Clinical stages and CT findingsa,b.
| CT findings | All patients (n=41) | Stage I–II (n=20) | Stage III–IV (n=21) | p-value |
| Intestinal | ||||
| Small bowel | ||||
| Wall thickening | 14 (34) | 9 (45) | 5 (24) | 0.15 |
| Dilatation | 9 (22) | 4 (20) | 5 (24) | 0.77 |
| Large bowel | ||||
| Wall thickening | 5 (12) | 1 (5) | 4 (19) | 0.17 |
| Dilatation | 1 (2) | – | 1 (5) | 0.32 |
| Small and large bowel | ||||
| Wall thickening | 20 (49) | 8 (40) | 12 (57) | 0.27 |
| Dilatation | 8 (20) | 3 (15) | 5 (24) | 0.48 |
| Any small bowel thickening | 34 (83) | 17 (85) | 17 (81) | 0.73 |
| Segmental pattern | 13 (32) | 12 (60) | 1 (5) | 0.001 |
| Diffuse pattern | 21 (51) | 5 (25) | 16 (76) | 0.04 |
| Any large bowel thickening | 25 (61) | 9 (45) | 16 (76) | – |
| Normal scan | 2 (5) | 2 (10) | – | 0.14 |
| Extra-intestinal | ||||
| Mesenteric stranding | 25 (61) | 12 (60) | 13 (62) | 0.90 |
| Ascites | 17 (41) | 8 (40) | 9 (43) | 0.85 |
| Biliary abnormalities | 12 (29) | 7 (35) | 5 (24) | 0.43 |
| Urinary excretion of orally ingested gastrografinc | 12 (44) | 5 (42) | 7 (47) | 0.80 |
| Periportal oedema | 9 (22) | 5 (25) | 4 (19) | 0.65 |
| Free abdominal air | 2 (5) | – | 2 (10) | 0.16 |
| Pneumatosis intestinalis | 1 (2) | – | 1 (5) | 0.32 |
aSeveral patients had more than one finding.
bData are given as number (percentage) unless otherwise specified.
cOf 27 patients given oral gastrografin.
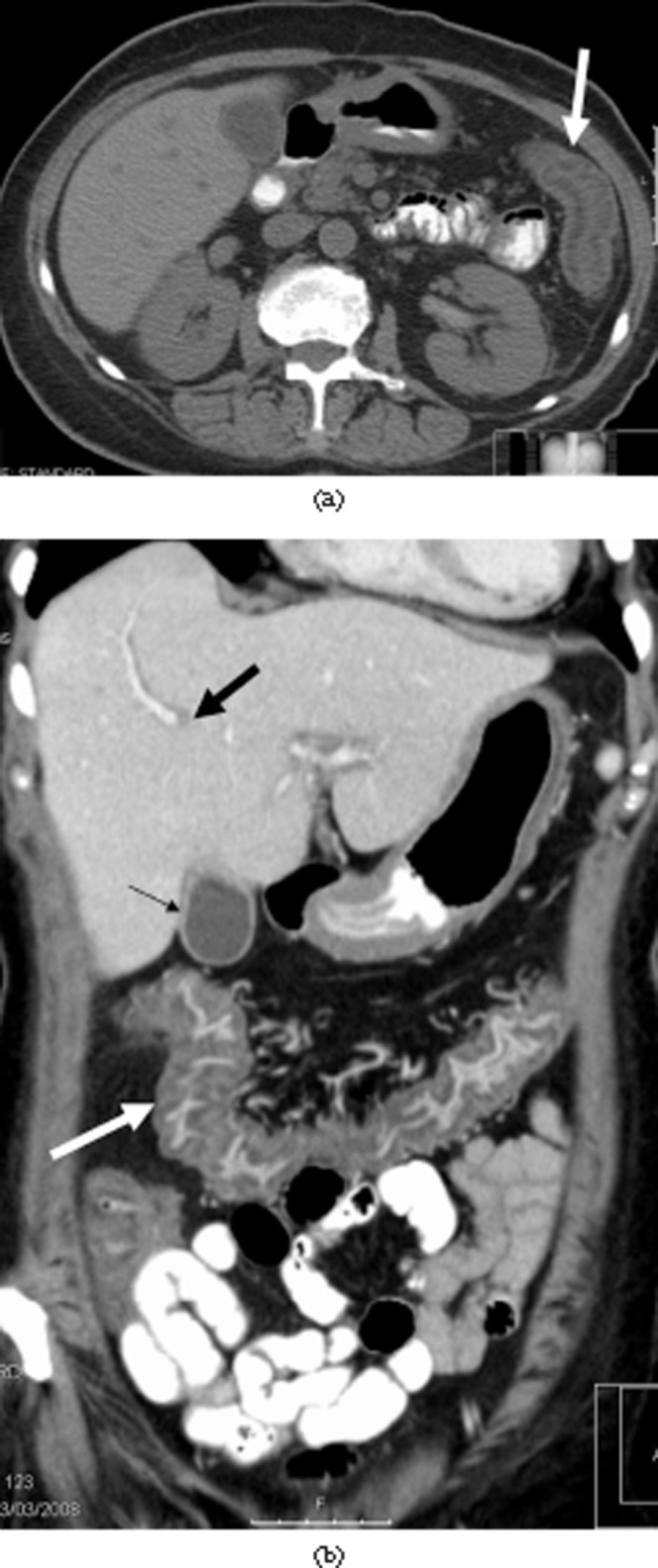
Figure 1.
Diffuse small-bowel involvement in graft-vs-host disease (GVHD). A 48-year-old man with clinical Grade IV GVHD. Patient died of GVHD. (a) Unenhanced scan shows marked small-bowel wall thickening (arrow). (b) Enhanced study shows the “water halo” sign in small-bowel wall (arrow).
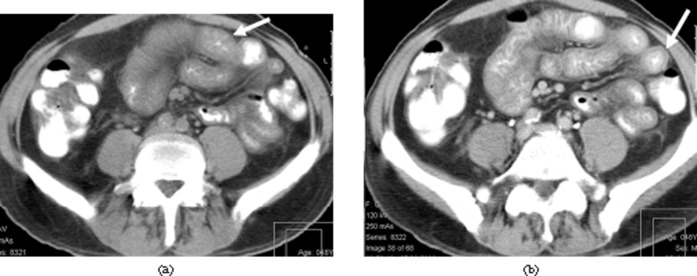
Figure 2.
Small and large bowel involvement. A 41-year-old man with clinical Grade IV graft-vs-host disease (GVHD). Patient died of GVHD. Unenhanced scan shows bowel wall thickening (thin white arrows), more severe in the large bowel (long arrow). Orally ingested gastrografin is present in the renal pelvis on both sides (thick white arrows).
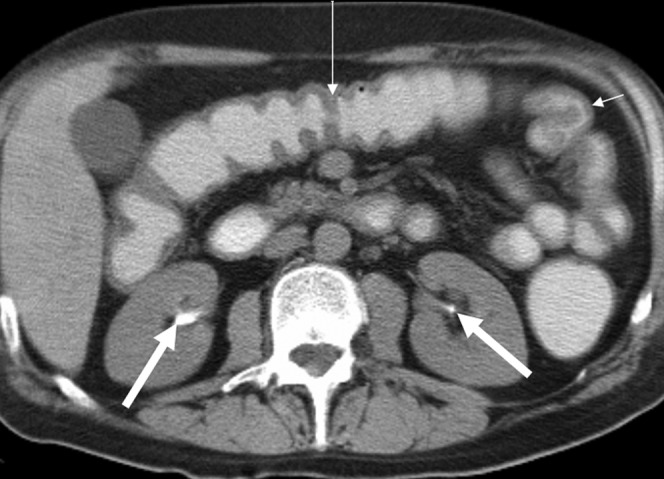
Figure 3.
Gastric involvement. A 24-year-old woman with clinical Grade IV graft-vs-host disease. Patient died from relapse of underlying disease, non-Hodgkin's lymphoma. Unenhanced study shows gastric wall oedema and thickening (thick black arrow). Contrast material is seen in the renal pelvis on the right (thick white arrow). Thickening of gall bladder wall is seen as well (thin black arrow).
The “water halo sign” was noted in 17 patients (41%) in the small bowel (Figure 1b) and large bowel (Figure 4a). The “accordion sign” was observed in 8 (20%) patients (Figure 5). Mucosal enhancement was present in 6 of the 37 (16%) who had enhanced studies (Figure 4b). Pneumatosis intestinalis was observed in one patient (2%).
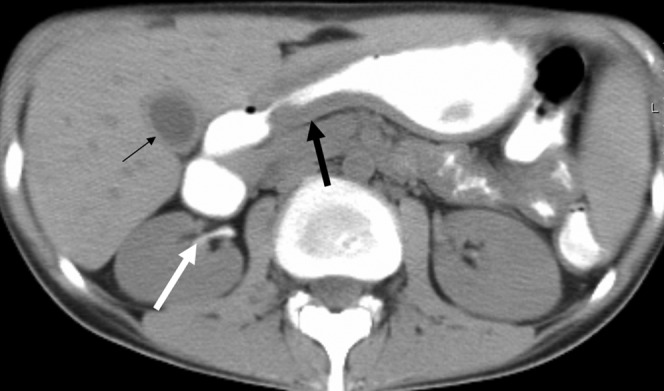
Figure 4.
Intestinal and extra-intestinal involvement. A 59-year-old woman with Grade IV graft-vs-host disease (GVHD). Patient died of GVHD. (a) Unenhanced scan shows the “water halo” sign in the large bowel (arrow). (b) Contrast-enhanced coronal reformation demonstrates large-bowel wall thickening and marked mucosal enhancement (thick white arrow). Gallbladder wall thickening (thin black arrow) and periportal oedema (thick black arrow) are seen as well.
Figure 5.
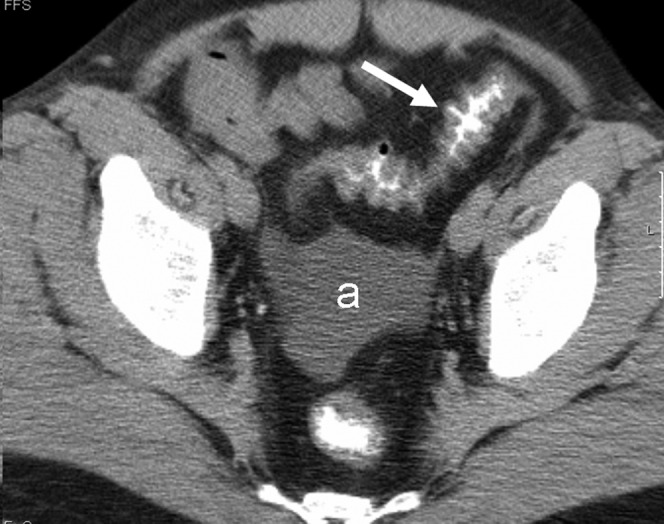
Extra-intestinal findings. A 34-year old-man with Grade IV graft-vs-host disease (GVHD). Patient died of GVHD. A scan through the pelvis shows the “accordion” sign in the large bowel (arrow) and moderate ascitis (a).
Extra-intestinal findings included mesenteric stranding in 25 (61%) patients, orally ingested gastrografin observed in the urinary tract in 12 (44%) patients out of the 27 who had pre-contrast studies (Figures 2 and 3), ascites in 17 (41%) patients (Figure 5), biliary abnormalities in 12 (29%) (Figure 3) and periportal edema in 9 (22%) (Figure 4b). Abundant free intraperitoneal air was seen in 2 (5%) patients, resulting (probably) from occult intestinal perforation. Retroperitoneal air was present in the 1 (2%) patient with pneumatosis intestinalis of the colon.
CT findings and severity of gastrointestinal graft-vs-host disease
The correlation between clinical stages and CT findings is presented in Table 2. The two patients with normal bowel on CT had Stage I clinical disease. There was no difference between patients with clinical Stage I–II and clinical Stage III–IV in the overall involvement of the small bowel. However, 16 (76%) of 21 patients with clinical Stage III–IV had diffuse pattern of small-bowel disease (76%), compared with only 5 (25%) of 20 patients with clinical Stage I–II (p=0.001). Large bowel involvement was more common in Stage III–IV. 16 patients (76%) with clinical Stage III–IV had large bowel involvement, compared with only 9 (45%) patients with clinical Stage I–II (p=0.04). Nodular haustral thickening was observed more often in clinical Stages III–IV; however, the small number of patients (8) could not be statistically evaluated.
Extra-intestinal findings including mesenteric stranding, biliary abnormalities and urinary excretion of orally ingested gastrografin were seen in both groups and were not statistically different.
CT findings and prognosis
23 (56%) patients initially responded to immunosuppressive therapy. With a median follow-up of 27 months (interquartile range 17–33 months), 11 patients were alive and 30 had died; 16 patients died from acute GVHD, 11 of relapse, 2 as a result of unrelated infections and 1 of head trauma. No patient was lost to follow-up. The 2-year overall survival was 24% [95% confidence interval (CI), 11–38]. 8 (38%) of the 21 patients with diffuse small-bowel involvement responded to therapy, compared with 15 (75%) of 20 patients with other patterns of involvement (p=0.02). 12 patients with diffuse intestinal involvement died as a direct result of acute GVHD, a cumulative incidence of 62% (95% CI, range 44–88), compared with 4 patients with other patterns, a cumulative incidence of 24% (95% CI, range 10–58; Figure 6; hazard ratio 3.1, p=0.01). Involvement of the large bowel that was correlated with a severe clinical stage was not associated with a lower response rate; 14 (56%) of 25 patients with large-bowel disease responded, compared with 9 (56%) of 16 with an uninvolved colon (p=0.99). Colonic disease was not associated with GVHD-related death (cumulative incidence 42% compared with 50%, respectively, p=0.45). The different CT patterns were not associated with a different overall survival because patients with GVHD had a lower cumulative incidence of relapse than patients without GVHD (Figure 7; p=0.31).
Figure 6.
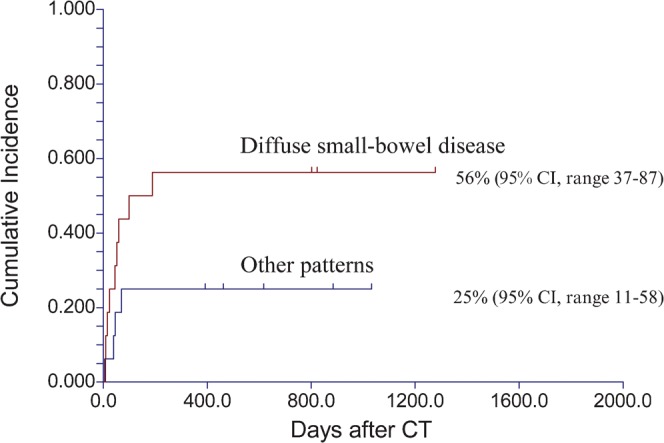
Cumulative incidence of graft-vs-host disease (GVHD)-associated mortality. Patients with diffuse intestinal involvement had a higher risk for death as a direct result of GVHD than patients with other patterns of intestinal involvement. CI, confidence interval.
Figure 7.
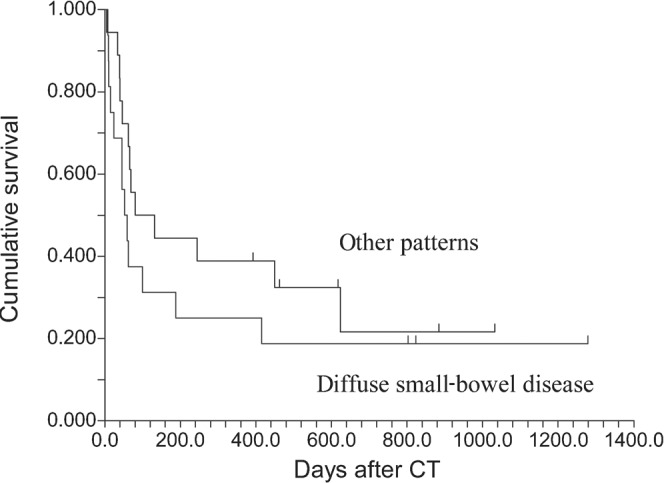
Overall survival from CT study. Similar survival with different patterns of intestinal involvement.
Discussion
The principal findings of this study were that abnormal findings on CT, both intestinal and extra-intestinal, were present in 95% of patients with GVHD, and that some of these findings correlated with the severity of the disease and were indicators of a poor prognosis.
The study included a relatively large number of patients, and had a relatively long-term follow-up. Although previous series have been reported, they had relatively few patients [18,20]. However, despite that, the study was only powered to detect CT findings that have a very large difference in incidence between different GVHD groups. The study could not rule out findings that are of smaller difference in incidence, but that are still clinically relevant.
Our results are similar to those reported recently by Brodoefel et al [20], and earlier by Kalantari et al [18], Jones et al [15] and Benya et al [21], concerning bowel wall thickening. All these authors reported bowel wall thickening as a prominent feature in their studies. Indeed, this was the most common finding seen in our patients, more often present in the small than in the large bowel.
In addition to bowel wall thickening, we observed the “water halo” sign in both the small and large bowel. This has been previously described in GVHD, believed to represent the inflammatory process in the bowel wall [16,28,29]. This finding is, however, non-specific and can be seen in other inflammatory bowel conditions [30]. We also noted the “accordion sign” in the colonic wall, originally described in pseudomembranous enterocolitis [26], thought to be due to nodular haustral thickening, which may predict severe clinical disease [31]. Although it has not been previously described in GVHD, we believe that this represented the markedly thickened folds of the colon found in these patients.
A feature in our series differing from some previous reports was the less frequent occurrence of bowel dilatation. Thus Donnelly and Morris [16] claim that multiple fluid-filled dilated loops of bowel are a typical CT finding in children with GVHD, as both large- and small-bowel dilatation were detected in 94% of their patients. We found such dilatation in only 41% of the patients, all but one in the small bowel. Our results are, however, similar to those recently reported by Brodoefel et al [20], who found dilatation of the small bowel in 30% and large bowel in 10%, and those previously reported by Kalantari et al [18], who described dilatation on CT in 23% of their patients. We therefore feel that bowel dilatation is a less typical feature in GVHD than bowel wall thickening.
In contradiction to previous reports only 6 (16%) of our patients showed intestinal mucosal enhancement. This sign was reported in 100% of children by Donnelly and Morris [16] and in 90% by Bodoefel et al [20]. However, such enhancement was detected in only 54% of adults with GVHD reported by Kalantari et al [18]. The relative rarity of mucosal enhancement in our series may be a technical problem as almost all our patients received oral gastrografin as well, which may have obscured the phenomenon of enhancement, as was the case with Kalantari's patients. Indeed, the patients studied by Brodoefel et al [20] were given 500 ml of water to mark the gastrointestinal tract.
Pneumatosis intestinalis was observed in only one of our patients. It has been rarely reported in patients with GVHD, believed to be a benign entity related to high-dose steroids and chemotherapy [17,32]. Occasionally it is asymptomatic and detected incidentally. Gas may also be detected in the retroperitoneum or peritoneal cavity [33], as was the case in our patient.
Regarding the extra-intestinal findings, most of our findings are similar to those previously described [18,20]. Mesenteric stranding, biliary abnormalities, ascites and periportal oedema were seen frequently in our patients. Urinary excretion of orally ingested gastrografin has not been previously reported in GVHD. This phenomenon has been previously described in various inflammatory conditions of the bowel [27]. Such absorption occurs through a damaged intestinal mucosa, and is detected on CT in the urinary tract. We assume that the damaged mucosa in GVHD leads to an increased permeability of the intestinal mucosa, allowing for absorption of the orally ingested gastrografin. Gastrografin is routinely administered to patients undergoing abdominal CT. However, its use in GVHD may be debated. It may be helpful in patients who are not given intravenous contrast for several reasons, but bowel wall enhancement after intravenous contrast injection may be obscured. The finding of urinary excretion of orally ingested gastrografin may be helpful in the diagnosis of GVHD, but is not common and may not directly relate to GVHD severity.
It should be noted that the CT findings described in recent transplant recipients may also follow infection, inflammatory bowel disease and radiation enteritis [19,25]. Usually, however, the overall extent of bowel involvement tends to be greater in GVHD [28,29]. Neutropenic enterocolitis tends to involve the right-sided colon, and is more frequently associated with pneumatosis than GVHD [25].
Severity and prognosis of GI GVHD are determined by clinical grading supported by histopathological findings. Grades I and II are mild forms, while Grades III–IV can be life-threatening [34]. Immunosuppressive drugs and steroids are used to treat this complication, contributing to an increased risk of serious infections [35]. Between 10 and 40% of patients with acute GVHD die of the disease or its complications. Consequently, grading and management of intestinal GVHD are important in the prevention of morbidity and mortality of the patients.
Current grading systems of acute GVHD cannot effectively identify patients with poor prognosis at the onset of acute GVHD [35,36]. Intestinal biopsies may underestimate the grade of the disease; moreover, such biopsies carry increased risks in these patients, who are often thrombocytopenic [13,25].
CT has been proven to be reliable in the staging of GVHD. Thickening of the wall of the distal oesophagus with an increased number of affected bowel segments was associated with high-grade GVHD on pathological inspection [18]. In a recent study, a designated CT score was described, integrating multiple abdominal features; increased bowel wall thickness and mucosal attenuation in unenhanced scans were significantly related to clinical and pathological grading [20].
In this study, diffuse small bowel disease and any colonic involvement were significantly correlated (p=0.001) with severe clinical GVHD. Intestinal marked nodular haustral thickening, and extra-intestinal abnormalities such as mesenteric stranding, biliary abnormalities and urinary excretion of orally ingested gastrografin, were also more often observed in the severe clinical grades, and may predict a more severe clinical disease, but this did not reach statistical significance, possibly owing to under power.
Furthermore, diffuse small-bowel disease was associated with a poor prognosis, while colonic disease, which may have also presented clinically as severe GVHD, had a better prognosis. However, caution is needed when interpreting prognosis and survival data. This is a retrospective study and only factors with very high hazard ratios could be detected owing to limited sample size. Prognosis is determined by multiple factors that are not adjusted for in this study, such as age, donor type, comorbidities etc. Multivariate analysis could not be performed in this limited patient group. Larger prospective studies are needed to confirm these observations and to support the assumption that GVHD pattern assessment by abdominal CT may help the clinician in selecting more intensive treatment regimes for patients with poorer prognosis. Our results are somewhat different from those recently reported by Ketelsen et al, who described a better prognosis in patients with involvement of the entire gastrointestinal tract [37].
Our study is limited in that a biopsy was not done in all the patients, as it was often contra-indicated, owing to their clinical condition. Also, all the patients included in the study had a clinical diagnosis of GI GVHD, with no comparison with patients after SCT with GI symptoms due to other aetiologies. One may, however, make a presumptive diagnosis of GVHD if bowel abnormalities are seen on CT, cultures are negative and GI symptoms are present. We did not examine these patients on other CT scanners; however, as this is a common standard technology, we assume that these measurements will be reproducible on other scanners.
In conclusion, CT can play a role in supporting the clinical diagnosing of GI GVHD, and in the assessment of severity and prognosis. CT findings may help the clinician in determining the therapeutic approach.
References
- 1.Martin PJ, Hansen JA, Strob R, Thomas ED. Human marrow transplantation: an immunological perspective. Adv Immunol 1987;40:379–438 [DOI] [PubMed] [Google Scholar]
- 2.Beatty PG, Dahlberg S, Mickelson EM, Nisperos B, Opelz G, Martin PJ, et al. Probability of finding HLA-matched unrelated marrow donors. Transplantation 1988;45:714–18 [DOI] [PubMed] [Google Scholar]
- 3.Armitage JO. Bone marrow transplantation. N Engl J Med 1994;330:827–38 [DOI] [PubMed] [Google Scholar]
- 4.Thomas ED. Bone marrow transplantation: a review. Semin Hematol 1999;36:95–103 [PubMed] [Google Scholar]
- 5.Tabbara IA, Zimmerman K, Morgan C, Nahleh Z. Allogeneic hematopoietic stem cell transplantation. Complications and results. Arch Intern Med 2002;162:1558–66 [DOI] [PubMed] [Google Scholar]
- 6.Ferrara JL, Deeg HJ. Graft-versus-host disease. N Engl J Med 1991;324:667–74 [DOI] [PubMed] [Google Scholar]
- 7.Deeg HJ, Antin JH. The clinical spectrum of acute graft-versus-host disease. Semin Hematol 2006;43:24–31 [DOI] [PubMed] [Google Scholar]
- 8.Schulenburg A, Turetschek K, Wrba F, Vogelsang H, Greinix HT, Keil F, et al. Early and late gastrointestinal complications after myeloablative and nonmyeloablative stem cell transplantation. Ann Hematol 2004;83:101–6 [DOI] [PubMed] [Google Scholar]
- 9.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974;18:295–304 [DOI] [PubMed] [Google Scholar]
- 10.Sale GE, Shulman HM, McDonald GB, Thomas ED. Gastrointestinal graft-versus-host disease in man. A clinicopathologic study of the rectal biopsy. Am J Surg Pathol 1979;3:291–9 [DOI] [PubMed] [Google Scholar]
- 11.Cruz-Correa M, Poonawala A, Abraham SC, Wu TT, Zahurak M, Vogelsang G, et al. Endoscopic findings predict the histologic diagnosis in gastrointestinal graft-versus-host disease. Endoscopy 2002;34:808–13 [DOI] [PubMed] [Google Scholar]
- 12.Nydegger A, Catto-Smith AG, Tiedemann K, Hardikar W. Diagnosis of gastrointestinal graft-versus-host disease – is rectal biopsy enough? Pediatr Blood Cancer 2007;48:561–6 [DOI] [PubMed] [Google Scholar]
- 13.Epstein RJ, McDonald GB, Sale GE, Shulman HM, Thomas ED. The diagnostic accuracy of the rectal biopsy in acute graft-versus-host disease: a prospective study of thirteen patients. Gastroenterology 1980;78:764–71 [PubMed] [Google Scholar]
- 14.Ponec RJ, Hackman RC, McDonald GB. Endoscopic and histologic diagnosis of intestinal graft-versus-host disease after marrow transplantation. Gastrointest Endosc 1999;49:612–21 [DOI] [PubMed] [Google Scholar]
- 15.Jones B, Fishman EK, Kramer SS, Siegelman SS, Saral R, Beschorner WE, et al. Computed tomography of gastrointestinal inflammation after bone marrow transplantation. AJR Am J Roentgenol 1986;146:691–5 [DOI] [PubMed] [Google Scholar]
- 16.Donnelly LF, Morris CL. Acute graft-versus-host disease in children: abdominal CT findings. Radiology 1996;199:265–8 [DOI] [PubMed] [Google Scholar]
- 17.Coy DL, Ormazabal A, Godwin JD, Lalani T. Imaging evaluation of pulmonary and abdominal complications following hematopoietic stem cell transplantation. Radiographics 2005;25:305–18 [DOI] [PubMed] [Google Scholar]
- 18.Kalantari BN, Mortele KJ, Cantisani V, Ondategui S, Glickman JN, Gogate A, et al. CT features with pathologic correlation of acute gastrointestinal graft-versus-host disease after bone marrow transplantation in adults. AJR Am J Roentgenol 2003;181:1621–5 [DOI] [PubMed] [Google Scholar]
- 19.Schmit M, Bethge W, Beck R, Faul C, Claussen CD, Horger M. CT of gastrointestinal complications associated with hematopoietic stem cell transplantation. AJR Am J Roentgenol 2008;190:712–9 [DOI] [PubMed] [Google Scholar]
- 20.Brodoefel H, Bethge W, Vogel M, Fenchel M, Faul C, Wehrmann M, et al. Early and late-onset acute GvHD following hematopoietic cell transplantation: CT features of gastrointestinal involvement with clinical and pathological correlation. Eur J Radiol 2010;73:594–600 [DOI] [PubMed] [Google Scholar]
- 21.Benya EC, Sivit CJ, Quinones RR. Abdominal complications after bone marrow transplantation in children: sonographic and CT findings. AJR Am J Roentgenol 1993;161:1023–7 [DOI] [PubMed] [Google Scholar]
- 22.Gorg C, Wollenberg B, Beyer J, Stolte MS, Neubauer A. High-resolution ultrasonography in gastrointestinal graft-versus-host disease. Ann Hematol 2005;84:33–9 [DOI] [PubMed] [Google Scholar]
- 23.Mentzel HJ, Kentouche K, Kosmehl H, Gruhn B, Vogt S, Sauerbrey A, et al. US and MRI of gastrointestinal graft-versus-host disease. Pediatr Radiol 2002;32:195–8 [DOI] [PubMed] [Google Scholar]
- 24.Stelljes M, Hermann S, Albring J, Kohler G, Franzius C, Poremba C, et al. Clinical molecular imaging in intestinal graft-versus-host disease: mapping of disease activity, prediction, and monitoring of treatment efficiency by positron emission tomography. Blood 2008;111:2909–18 [DOI] [PubMed] [Google Scholar]
- 25.Kirkpatrick IDC, Greenberg HM. Gastrointestinal complications in the neutropenic patient: characterization and differentiation with abdominal CT. Radiology 2003;226:668–74 [DOI] [PubMed] [Google Scholar]
- 26.Fishman EK, Kavuru M, Jones B, Kuhlman JE, Merine DS, Lillimoe KD, et al. Pseudomembranous colitis: CT evaluation of 28 cases. Radiology 1991;180:57–60 [DOI] [PubMed] [Google Scholar]
- 27.Apter S, Gayer G, Amitai M, Hertz M. Urinary excretion of orally ingested gastrografin on CT. Abdom Imaging 1998;23:297–300 [DOI] [PubMed] [Google Scholar]
- 28.Horton HM, Corl FM, Fishman EK. CT of nonneoplastic diseases of the small bowel: spectrum of disease. J Comput Assist Tomogr 1999;23:417–28 [DOI] [PubMed] [Google Scholar]
- 29.Horton KM, Corl FM, Fishman EK. CT evaluation of the colon: inflammatory disease. RadioGraphics 2000;20:399–418 [DOI] [PubMed] [Google Scholar]
- 30.Thoeni RF, Cello JP. CT imaging of colitis. Radilogy 2006;240:623–38 [DOI] [PubMed] [Google Scholar]
- 31.Boland GW, Lee MJ, Cats AM, Ferraro MJ, Matthia AR, Mueller PR. Clostridium difficile colitis: correlation of CT findings with severity of clinical disease. Clin Radiol 1995;50:153–6 [DOI] [PubMed] [Google Scholar]
- 32.Day DL, Ramsay NKC, Letourneau JG. Pneumatosis intestinalis after bone marrow transplantation. AJR Am J Roentgenol 1988;151:85–7 [DOI] [PubMed] [Google Scholar]
- 33.Bates FT, Gumey JW, Goodman LR, Santamaria JJ, Hansen RM, Ash RC. Pneumatosis intestinalis in bone marrow transplantation patients: diagnosis on routine chest radiographs. AJR Am J Roentgenol 1989;152:991–4 [DOI] [PubMed] [Google Scholar]
- 34.Vogelsang GB, Lee l, Bensen-Kennedy DM. Pathogenesis and treatment of graft–versus-host disease after bone marrow transplantation. Annu Rev Med 2003;54:29–52 [DOI] [PubMed] [Google Scholar]
- 35.Cahn JY, Klein JP, Lee SJ, Milpied N, Blaise D, Antin JH, et al. Prospective evaluation of 2 acute graft-versus-host (GVHD) grading systems: a joint Societe Francaise de Greffe de Moelle, et al. Therapie Cellulaire (SFGM-TC), Dana Farber Cancer Intitute (DFCI), and international Bone Marrow Transplant registry (IBMTR) prospective study. Blood 2005;106:1495–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee KH, Choi SJ, Lee JH, Lee JS, Kim WK, Lee KB, et al. Prognostic factors identifiable at the time of onset of acute graft-versus-host disease after allogeneic hematopoietic cell transplantation. Haematologica 2005;90:939–48 [PubMed] [Google Scholar]
- 37.Ketelsen D, Vogel W, Bethge W, Faul C, Claussen CD, Horger M. CT-analysis of the course of gastrointestinal graft-versus-host disease- Patterns of involvement. Eur J Radiol 2011;79:36–41 [DOI] [PubMed] [Google Scholar]