Abstract
Objective
The aim of this study was to correlate the apparent diffusion coefficient (ADC) value of breast cancer with prognostic factors.
Methods
335 patients with invasive ductal carcinoma not otherwise specified (IDC NOS) and ductal carcinoma in situ (DCIS) who underwent breast MRI with diffusion-weighted imaging were included in this study. ADC of breast cancer was calculated using two b factors (0 and 1000 s mm–2). Mean ADCs of IDC NOS and DCIS were compared and evaluated. Among cases of IDC NOS, mean ADCs were compared with lymph node status, size and immunochemical prognostic factors using Student's t-test. ADC was also correlated with histological grade using the Kruskal–Wallis test.
Results
Mean ADC of IDC NOS was significantly lower than that of DCIS (p<0.001). However, the mean ADC of histological grade of IDC NOS was not significantly different (p=0.564). Mean ADC of oestrogen receptor (ER)-positive or progesterone receptor (PR)-positive cancer was significantly lower than that of ER-negative or PR-negative cancer (p=0.003 vs p=0.032). Mean ADC of Ki-67 index-positive cancer was significantly lower than that of Ki-67 index-negative cancer (p=0.028). Mean ADC values of cancers with increased microvascular density (MVD) were significantly lower than those of cancer with no MVD increase (p=0.009). No correlations were observed between mean ADC value and human growth factor receptor 2 expression, tumour size and lymph node metastasis.
Conclusion
Low ADC value was correlated with positive expression of ER, PR, increased Ki-67 index, and increased MVD of breast cancer.
Breast MRI is an established supplemental technique to mammography and ultrasonography for evaluation of suspicious breast lesions. Diffusion-weighted MRI (DWI) has recently been integrated into the standard breast MRI for discrimination of benign and malignant breast lesions obtained with dynamic contrast-enhanced MRI [1-13]. DWI is a non-invasive technique that represents the biological character of the mainly Brownian movement of protons in bulk water molecules in vivo. Apparent diffusion coefficient (ADC) values are quantified by measurement of mean diffusivity along three orthogonal directions, which are affected by cellularity of the tissue, fluid viscosity, membrane permeability and blood flow [7,9-11]. Microstructural characteristics, including water diffusion and blood microcirculations in capillary networks, were associated with ADC value. Decreased movement of molecules in highly cellular tissue showed correlation with a low ADC value [3,4]. Several studies of DWI of the breast have reported significantly lower ADC values in malignant tumours, compared with benign breast lesions and normal tissue [1-3,5-11,14]. Classic prognostic markers, including tumour size and grade, and lymph node status in patients with breast cancer, and molecular markers, including oestrogen receptor (ER), progesterone receptor (PR), Ki-67 index, human growth factor receptor 2 (HER2) protein and angiogenic molecular markers, have been reported [1,15,16]. Few studies have examined the correlation between ADC values and prognostic factors [1,8]. The purpose of this study is to compare ADC values of DWI of breast cancer with prognostic factors.
Methods and material
Patients
Our institutional review board approved the study and waived patient informed consent because of the retrospective design. Between December 2005 and November 2010, 731 consecutive patients underwent MRI with diffusion in our institution. Cases involving previous excisional biopsy (n=84), neoadjuvant chemotherapy (n=82), evaluation for screening owing to mammoplasty (n=75), no pathological confirmation (n=27), benign lesion (n=31) and male patient (n=1) were excluded. 64 lesions were excluded because the ADC value was not evaluated or because of technical issues with DWI acquisition resulting in failure of lesion detection. 34 lesions were excluded because they were confirmed to be a special type of invasive ductal carcinoma (IDC), including mucinous, medullary, papillary and infiltrative lobular carcinoma. Finally, 335 breast lesions in 333 consecutive patients (2 patients with bilateral breast cancer) with pathology-proven lesions, with IDC not otherwise specified (NOS; n=231), IDC NOS with ductal carcinoma in situ (DCIS; n=59) and pure DCIS (n=45), were evaluated retrospectively. Patients ranged in age from 24 to 85 years, with a mean age of 50.6 years.
Breast MRI
All MRI was performed with a 1.5 T scanner (Sonata; Siemens, Erlangen, Germany). All patients were examined in the prone position using a breast array coil. An axial, fat-suppressed, T2 weighted fast spin-echo sequence [repetition time (TR)/echo time (TE)=6640/101, 30 slices with a field of view (FOV) of 320 mm, a matrix of 512×256, number of excitations (NEX)=2, a 3.5-mm section thickness with a 0.7-mm intersection gap, an acquisition time of 3 min 54 s] and a T1 weighted spin-echo sequence were obtained. Axial DW-MRI with single-shot echo-planar imaging (EPI; b=0 and 1000 s mm–2, TR/TE=5000/110, an FOV of 320 mm, a matrix of 128×128, NEX=3, a 3.5-mm slice thickness with a 0.7-mm slice gap and an acquisition time of 1 min 7 s) was performed. A contrast-enhanced axial T1 weighted three-dimensional fast low-angle shot (FLASH) sequence with fat suppression (TR/TE=4.36/1.5, a flip angle of 12°, a 1.5-mm section thickness with no gap, an acquisition time of 1 min 20 s, an FOV of 320 mm and a matrix of 330×512) was obtained prior to injection of contrast medium, and then every 80 s, repeated five times, after a bolus injection of 0.1 mmol kg–1 gadodiamide (Omniscan, GE Healthcare, Carrigtwohill, Ireland). Standard subtraction images were created from the non-enhanced and early and late contrast-enhanced FLASH sequences. The largest malignancies were measured by dynamic enhanced subtraction MRI. Sizes of the IDC NOS ranged from 0.7 to 9.8 cm (mean size 2.09 cm).
Diffusion image acquisition and ADC analysis
DWI was obtained along each of the x-, y- and z-axes. ADC value was calculated according to the formula ADC=[1/(b2−b1)] Ln(S2/S1), where S1 and S2 are the signal intensities in the regions of interest (ROIs) obtained by two gradient factors, b2 and b1 (b1=0 and b2=1000 s mm–2). For measurement of the ADC value, one radiologist with 10 years of experience in breast imaging manually placed a ROI with a diameter of 5–10 mm2. Care was taken to avoid areas of T2 shine-through, such as cystic or necrotic portions of the tumour shown as high-signal intensity on T2 weighted images and ADC maps. When comparing with dynamic contrast enhanced MR images, the enhancing solid portion was used to site ADC measurements. A ROI at the corresponding location was manually defined on averaged DW images to include the area of hyperintensity. The ADC value was automatically calculated when the ROI was drawn. Three measurements where the ADC value was shown to be lower were selected on the ADC map, and were averaged and used as the ADC value.
Histological analysis
The method described by Elston and Ellis [17] was used for assessment of histological grades of IDC NOS using a numerical scoring system for tubule formation, pleomorphism and mitotic count. The total score could range from 3 to 9, with a total score of 3–5 representative of grade 1, a total score of 6 or 7 representative of grade 2 and a total score of 8 or 9 representative of grade 3. Lymph node specimens were obtained by sentinel lymph node (SLN) resection followed by immediate lymph node dissection if one or more SLNs were positive and were histologically assessed on routinely stained sections. The presence of a metastasis was regarded as a positive finding.
In addition, immunohistochemical analysis was performed for ER, PR, HER2, Ki-67 and microvascular density (MVD). The status of ER and PR was considered to be negative if expression was <10% and positive if expression was ≥10%. Results for HER2 expression were scored as negative, 1+, 2+ or 3+, according to the manufacturer's recommendations. Tumours with 0 or 1+ were classified as HER2 negative and 2+ or 3+ were HER2 positive. Ki-67 staining of ≥20% was considered positive expression and <20% was considered negative expression. Microvascular density (MVD) was assessed by counting structures stained with CD 31 in three microscopic fields at ×200 magnification by determination of the average number of structures in the most vascularised areas at the periphery of the tumour [18]. Staining of CD 31 was scored as 0, 1+, 2+ or 3+ and considered positive when vascularity was scored as 1+, 2+ or 3+.
Statistical analysis
For evaluation of differentiation between DCIS and IDC NOS, the t-test was used for analysis of mean ADC values between pure DCIS and IDC NOS. Mean ADC for histological grade of IDC NOS was also analysed using the Kruskal–Wallis test.
Among cases of IDC NOS, tumour sizes measured by dynamic enhanced subtraction MRI were divided using the cut-off value of 2 cm and analysed with mean ADC values by t-test. Mean ADCs were compared with lymph node metastasis, ER status, PR status, extent of HER2 expression, Ki-67 index and MVD, respectively, by t-test. SPSS v. 14.0 software (SPSS, Chicago, IL) was used for statistical analyses of data.
Results
Mean ADC value of IDC NOS (0.907×10−3±0.160 mm2 s–1) was significantly lower than that of DCIS (1.113×10−3±0.231×10−3 mm2 s–1, p<0.001; Figure 1). However, mean ADC value of histological grade of IDC NOS was not significantly different (p=0.564; Table 1). Mean ADC of ER-positive cancers (0.885×10−3±0.152×10−3 mm2 s–1) was significantly lower than that of ER-negative cancers (0.941×10−3±0.168×10−3 mm2 s–1, p=0.003). Mean ADC of PR-positive cancers (0.888×10−3±0.148×10−3 mm2 s–1) was significantly lower than that of PR-negative cancers (0.928×10−3±0.171×10−3 mm s–1, p=0.032). Mean ADC of Ki-67 index-positive cancers (0.890×10−3±0.164×10−3 mm2 s–1) was significantly lower than that of Ki-67 index-negative cancers (0.933×10−3±0.152×10−3 mm2 s–1, p=0.028). Mean ADC value of cancers with increased MVD (0.894×10−3±0.147×10−3 mm2 s–1) was significantly lower than that of cancer with no MVD increased (0.954×10−3±0.154×10−3 mm2 s–1, p=0.009). lymph node (LN) statues between metastasis negative (0.919×10−3±0.166×10−3 mm2 s–1) and positive (0.885×10−3±0.147×10−3 mm2 s–1) showed borderline significance (p=0.090; Figures 2 3). However, HER2 expression status and tumour size showed no statistically significant correlation with mean ADC (Table 2).
Figure 1.

MR images of a 46-year-old woman with ductal carcinoma in situ (arrow) on (a) contrast-enhanced FLASH early subtraction image, (b) diffusion-weighted image (b=1000 s mm–2) and (c) ADC map (1.324×10–3 mm2 s–1).
Table 1. Correlation of ADC with IDC NOS and DCIS.
| Pathology | ADC value |
p-value |
| (×10−3 mm2 s−1) | ||
| DCIS (n=45) | 1.113±0.231 | |
| IDC NOS (n=290) | 0.907±0.160 | <0.001 |
| Grade 1 (n=14) | 0.914±0.222 | |
| Grade 2 (n=214) | 0.911±0.161 | |
| Grade 3 (n=62) | 0.891±0.142 | 0.564 |
ADC, apparent diffusion coefficient; DCIS, ductal carcinoma in situ. IDC NOS, invasive ductal carcinoma not otherwise specified.
ADC value is expressed as mean±standard deviation.
Figure 2.

A 53-year-old woman with invasive ductal carcinoma, histological grade 1 (arrow). Immunohistochemical staining for oestrogen receptors and progesterone receptors showed positivity. HER2 gene expression showed positivity. Ki-67 index showed a positive increase of over 20%. Microvascular density showed mild increase. Lymph node metastasis revealed negativity. (a) Contrast-enhanced FLASH early subtraction image. (b) Diffusion-weighted image (b=1000 s mm–2), and (c) ADC map (0.754×10–3 mm2 s–1).
Figure 3.
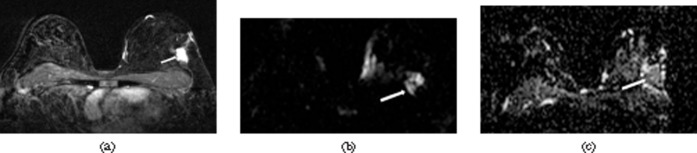
A 53-year-old woman with invasive ductal carcinoma, histological grade 3 (arrow). Immunohistochemical staining for oestrogen receptors and progesterone receptors showed negativity. HER2 gene expression showed negativity. Ki-67 index showed a negative increase of over 20%. Microvascular density showed a mild increase. Lymph node metastasis revealed positive metastasis. (a) Contrast-enhanced FLASH early subtraction image, (b) diffusion-weighted image (b=1000 s mm–2) and (c) ADC map (0.796×10–3 mm2 s–1).
Table 2. Correlation of ADC with prognostic factors in IDC NOS.
| Prognostic factors | No. of cases(n=290) | ADC value |
|
| (×10−3 mm2 s−11) | p-value | ||
| Oestrogen receptor | Negative (n=113) | 0.941±0.168 | |
| Positive (n=177) | 0.885±0.152 | 0.003 | |
| Progesterone receptor | Negative (n=135) | 0.928±0.171 | |
| Positive (n=155) | 0.888±0.148 | 0.032 | |
| HER2 | Negative (n=149) | 0.911±0.158 | |
| Positive (n=141) | 0.903±0.164 | 0.677 | |
| Ki-67 | <20% (n=112) | 0.933±0.152 | |
| >20% (n=178) | 0.890±0.164 | 0.028 | |
| MVD | Not increased (n=63) | 0.954±0.167 | |
| Increased (n=227) | 0.894±0.156 | 0.009 | |
| Size | <2 cm (n=133) | 0.918±0.162 | |
| >2 cm (n=157) | 0.898±0.159 | 0.294 | |
| LN metastasis | Negative (n=188) | 0.919±0.166 | |
| Positive (n=102) | 0.885±0.147 | 0.090 |
ADC, apparent diffusion coefficient; HER2, human growth factor receptor 2; IDC NOS, invasive ductal carcinoma not otherwise specified; LN, lymph node; MVD, microvascular density.
Discussion
The ADC is a quantifiable value that provides a measurement of signal attenuation and is affected by microscopic motion, including molecular diffusion of water and blood microcirculation in the capillary network [1-3,19-21]. Water diffusion is greatly influenced by factors such as cellularity, fluid viscosity, intra- and extracellular membrane permeability, active transport, flow and structural directionality [1,9,22]. Several studies have reported that malignant tumours usually show higher signal intensity on DWI, compared with benign lesions and normal fibroglandular tissue, resulting in lower ADC values [2,3,5,7,9-12,21]. It has been suggested that the decreased ADC value in malignant tumours may be due to their increased cellularity, larger nuclei with more abundant macromolecular proteins, and less extracellular space [5,20]. Several studies have reported that ADC values appear to be lower in invasive tumours than in carcinoma in situ [2,3,5,7,9-11,13,21]. In our study, the ADC value was significantly lower in IDC NOS than in DCIS. IDC features densely packed tumour cells, which inhibit the effective motion of water molecules and restrict diffusion, compared with DCIS, showing lower ADC values [4,21]. Few studies have analysed the relationship between tumour grading and ADC values; high-grade tumours are likely to have more limited water diffusion than low-grade tumours, based on cancer cell morphology and arrangements of the extracellular matrix, leading to non-conclusive results [1,21]. However, some authors have not demonstrated a direct relationship between cellularity and ADC values or between cellularity and tumour grade [4,22]. In our results, tumour grading among IDC NOS was not statistically significant compared with ADC values.
Few studies have analysed the relationship between prognostic factors and ADC values [1,8,23]. Razek et al [23] reported an association of lower ADC values with pathological prognostic factors, including higher histological grade, larger tumour size and presence of axillary lymph nodes. Jeh et al [8] reported an association of low ADC value with positive expression of ER and negative expression of HER2 in 107 women with IDC. Their study showed no significant correlation of ADC values with other prognostic factors (i.e. PR expression, Ki-67 expression, epidermal growth factor receptor, tumour size, LN metastasis and histological grade). However, they obtained ADC values from 1.5 and 3 T MRI machines with different b-values. Different magnetic fields of MR and different b-values may influence different ADC values [1]. In our study, several prognostic factors of biological markers, including ER status, PR status, Ki-67 index and MVD, show a statistically significant difference in ADC values; however, pathological prognostic factors, including tumour size, grade and lymph node status were not significant. Oestrogen receptors and progesterone receptors are intracellular steroid hormone receptor proteins, which have been used as indicators of prognosis and as a guide to hormone and endocrine therapy [16,24]. ER was the confounding factor that influenced PR. Overexpression of the PR status is an indication of the ER pathway, even in cases in which overexpression of ER was reported to be negative [1,24]. Some studies have reported that the ER affected the ADC value because of inhibition of the angiogenic pathway and induced a decrease in perfusion [1,25]. Another study reported that ER-positive tumours showed high cellularity [26]. This finding corresponds with our results showing that ER-positive cancer and PR-positive cancer showed lower mean ADC values, compared with negative ones. Ki-67 index is a nuclear antigen appearing during the proliferative phase, which represents tumour proliferation and significant correlation with high mitotic counts, usually using 20% as the cut-off for defining high and low proliferation indices [1,27]. High Ki-67 index is associated with poor prognostic differentiation and with lymph node metastasis [24]. In our study, mean ADC value was significantly lower in cases of Ki-67 index positive IDC NOS, compared with negative cases. The result could be an indication that increased Ki-67 index is a marker of increased cellularity and correlated with lower ADC values. In vivo, the ADC value is affected not only by microscopic motion from diffusion but also perfusion. The perfusion effect would cause significantly greater artificially increased ADC in malignant lesions owing to increasing microvessel count of tumour angiogenesis [1,2,22,28]. According to several studies, the ADC value was significantly lower in malignant than in benign lesions, indicating that this effect might be mainly owing to the effect of high cell density overcoming the opposite effects of perfusion [1,5,14]. In our study, mean ADC value was significantly lower in increasing MVD, compared with cancer with no MVD increase. A decrease in the ADC is expected with increased intracellular tissue caused either by cell swelling or increased cellular density rather than vascular perfusion. HER2-positive expression had a more malignant phenotype accompanied by cell proliferation, invasion and metastasis [1,2,8]. However, in our study, no statistically significant difference was observed between the negative and positive group of IDC NOS. Partridge et al [7] reported that malignant tumours had significantly lower ADC values, compared with benign lesions, for both masses and lesions with non-mass-like enhancement, and that the diagnostic performance of DWI based on ADC thresholds was comparable for both lesion types. They also reported that no association was identified between lesion size and ADC values. In our study, no significant difference in ADC values with size of mass was noted among IDC NOS. Using a lower b-value, the image would be more affected by a perfusion-induced slightly increased ADC value in malignant lesions due to tumour angiogenesis. By contrast, using higher b-values, the image may be distorted because it has a long TE [1-3]. Our study used b=1000 s mm–2 for calculating ADC. At present there is no consensus regarding optimal b-values in the diagnosis of breast cancer, and further study will be needed in the future [29]. A single 1.5 T machine was used in performance of our study, using b-values of 1000 s mm–2 in a relatively large number of IDC NOS. However, our study had several limitations. Our study was a retrospective review and imaging was performed at 1.5 T for evaluation of ADC values. Therefore, there is a possibility that small malignancies may have been missed. In 3 T MRI, signal-to-noise ratio and the contrast-to-noise ratio of ADC images was higher with 3 T than with 1.5 T and may better show small or diffuse lesions.
In conclusion, we found ADC values correlated with several biological markers of disease. Low ADC was associated with positive ER and PR expression, increased Ki-67 and increased MVD. ADC values may be of use in differentiating between DCIS and invasive breast carcinoma, and in providing prognostic information about disease.
References
- 1.Kim SH, Cha ES, Kin HS, Kang BJ, Choi JJ, Jung JH, et al. Diffusion-weighted imaging of breast cancer: correlation of the apparent diffusion coefficient value with prognostic factors. J Magn Reson Imaging 2009;30:615–20 [DOI] [PubMed] [Google Scholar]
- 2.Woodhams R, Matsunaga K, Iwabuchi K, Kan S, Hata H, Kuranami M, et al. Diffusion-weighted Imaging of malignant breast tumors. J Comput Assist Tomogr 2005;29:644–9 [DOI] [PubMed] [Google Scholar]
- 3.Kinoshita T, Yashiro N, Ihara N, Funatu H, Fukuma E, Narita M. Diffusion-weighted half-fourier single-shot turbo spin echo imaging in breast tumors: differentiation of invasive ductal carcinoma from fibroadenoma. J Comput Assist Tomogr 2002;26:1042–6 [DOI] [PubMed] [Google Scholar]
- 4.Yoshikawa MI, Ohsumi S, Sugata S, Kataoka M, Takashima S, Mochizuki T, et al. Relation between cancer cellularity and apparent diffusion coefficient values using diffusion-weighted magnetic resonance imaging in breast cancer. Radiat Med 2008;26:222–6 [DOI] [PubMed] [Google Scholar]
- 5.Guo Y, Cai YQ, Cai ZL, Gau YG, An NY, Ma L, et al. Differentiation of clinical benign and malignant lesions using diffusion-weighted imaging. J Magn Reson Imaging 2002;16:172–8 [DOI] [PubMed] [Google Scholar]
- 6.Park MJ, Cha ES, Kang BJ, Ihn YK, Baik JH. The role of diffusion-weighted imaging and the apparent diffusion coefficient (ADC) values for breast tumors. Korean J Radiol 2007;8:390–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Partridge SC, Mullins CD, Kurland BF, Allain MD, DeMarini WB, Eby PR, et al. Apparent diffusion coefficient values for discriminating benign and malignant breast MRI lesions: effects of lesion type and size. AJR Am J Roentgenol 2010;194:1664–73 [DOI] [PubMed] [Google Scholar]
- 8.Jeh SK, Kim SH, Kim HS, Kang BJ, Jeong SH, Yim HW, et al. Correlation of the apparent diffusion coefficient value and dynamic magnetic resonance imaging findings with prognostic factors in invasive ductal carcinoma. J Magn Reson Imaging 2011;33:102–9 [DOI] [PubMed] [Google Scholar]
- 9.Marini C, Iacconi C, Giannelli M, Cilotti A, Moretti M, Bartolozzi C. Quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesion. Eur Radiol 2007;17:2646–55 [DOI] [PubMed] [Google Scholar]
- 10.Rubesova E, Grell AS, De Maertelaer V, Metens T, Chao SL, Lemort M. Quantitative diffusion imaging in breast cancer: a clinical prospective study. J Magn Reson Imaging 2006;24:319–24 [DOI] [PubMed] [Google Scholar]
- 11.Sinha S, Lucas-Quesada FA, Sinha U, DeBruhl N, Bassett LW. In vivo diffusion-weighted MRI of the breast: potential for lesion characterization. J Magn Reson Imaging 2002;15:693–704 [DOI] [PubMed] [Google Scholar]
- 12.Yabuuchi H, Matsuo Y, Okafuji T, Kamitani T, Soeda H, Setoguchi T, et al. Enhanced mass on contrast-enhanced breast MR imaging: lesion characterization using combination of dynamic contrast enhanced and diffusion-weighted MR images. J Magnet Reson Imaging 2008;28:1157–65 [DOI] [PubMed] [Google Scholar]
- 13.Kuroki Y, Nasu K, Kuroki S, Murakami K, Hayashi T, Sekguchi R, et al. Diffusion-weighted imaging of breast cancer with the sensitivity encoding technique: analysis of the apparent diffusion coefficient value. Magn Reson Med Sci 2004;3:79–85 [DOI] [PubMed] [Google Scholar]
- 14.Hatakenaka M, Soeda H, Yabuuchi H, Matsuo Y, Kamitani T, Oda Y, et al. Apparent diffusion coefficients of breast tumors: clinical application. Magn Reson Med Sci 2008;7:23–9 [DOI] [PubMed] [Google Scholar]
- 15.Makkat S, Luypaert R, Stadnik T, Bourgain C, Sourbron S, Dujardin M, et al. Deconvolution-based dynamic contrast enhanced MR imaging of breast tumors: correlation of tumor blood flow with human epidermal growth factor receptor 2 status and clinicopathologic findings—preliminary results. Radiology 2008;249:471–82 [DOI] [PubMed] [Google Scholar]
- 16.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype. Cancer 2007;109:1721–8 [DOI] [PubMed] [Google Scholar]
- 17.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer I. The value of histological grade in breast cancer; experience from a large study with long-term follow-up. Histopathology 1991;19:403–10 [DOI] [PubMed] [Google Scholar]
- 18.Szabo BK, Aspelin P, Wiberg MK, Tot T, Bone B. Invasive breast cancer: correlation of dynamic MR features with prognostic factors. Eur Radiol 2003;13:2425–35 [DOI] [PubMed] [Google Scholar]
- 19.Pererira FPA, Martins G, Figueiredo E, Domingues MNA, Domingues RC, da Fonseca LMB, et al. Assessment of breast lesions with diffusion-weighted MRI: Comparing the use of different b values. AJR Am J Roentgenol 2009;193:1030–5 [DOI] [PubMed] [Google Scholar]
- 20.Tsushima Y, Takahashi-Taketomi A, Endo K. Magnetic resonance (MR) differential diagnosis of breast tumor using apparent diffusion coefficient (ADC) on 1.5-T. J Magn Reson Imaging 2009;30:249–55 [DOI] [PubMed] [Google Scholar]
- 21.Costantini M, Belli P, Rinaldi P, Bufi E, Giardina G, Franceschini G, et al. Diffusion-weighted imaging in breast cancer: relationship between apparent diffusion coefficient and tumor aggressiveness. Clin Radiol 2010;65:1005–12 [DOI] [PubMed] [Google Scholar]
- 22.Buadu LD, Murakami J, Murayama S, Hashiguchi N, Sakai S, Masuda K, et al. Breast lesions: correlation of contrast medium enhancement patterns on MR images with histopathologic findings and tumor angiogenesis. Radiology 1996;200:639–49 [DOI] [PubMed] [Google Scholar]
- 23.Razek AA, Gaballa G, Denewer A, Nada N. Invasive ductal carcinoma: correlation of apparent diffusion coefficient value with pathological prognostic factors. NMR Biomed 2010;23:619–23 [DOI] [PubMed] [Google Scholar]
- 24.Donegan WL. Tumor-related prognostic factors for breast cancer. CA Cancer J Clin 1997;47:28–51 [DOI] [PubMed] [Google Scholar]
- 25.Ludovini V, Sidoni A, Pistola L, Bellezza G, De Angelis V, Gori S, et al. Evaluation of the prognostic role of vascular endothelial growth factor and microvessel density in stages I and II breast cancer patients. Breast Cancer Res Treat 2003;81:159–68 [DOI] [PubMed] [Google Scholar]
- 26.Black R, Prescott R, Bers K, Hawkins A, Stewart H, Forrest P. Tumor cellularity, estrogen receptors and prognosis in breast cancer. Clin Oncol 1983;9:311–18 [PubMed] [Google Scholar]
- 27.Ding SL, Sheu LF, Yu JC, Yang TL, Chen B, Leu FJ, et al. Expression of estrogen receptor-alpha and Ki67 in relation to pathological and molecular features in early-onset infiltrating ductal carcinoma. J Biomed Sci 2004;11:911–19 [DOI] [PubMed] [Google Scholar]
- 28.Bogner W, Gruber S, Pinker K, Grabner G, Stadlbauer A, Weber M, et al. Diffusion-weighted MR for differentiation breast lesions at 3.0T: how does selection of diffusion protocols affect diagnosis? Radiology 2009;253:341–51 [DOI] [PubMed] [Google Scholar]
- 29.Tozaki M, Fukuma E. 1H MR spectroscopy and diffusion-weighted imaging of the breast: are they useful tools for characterizing breast lesions before biopsy? AJR Am J Roentgenol 2009;193:840–9 [DOI] [PubMed] [Google Scholar]


