Abstract
Objective
The purpose of this study was to evaluate the radiological and clinical findings of invasive pulmonary aspergillosis (IPA) after liver transplantation.
Methods
This study included 25 consecutive liver transplant recipients with histologically confirmed IPA after liver transplantation. Radiological examinations performed for diagnosis were available in all patients. Clinical findings and changes in clinical response and radiological findings after treatment were also evaluated.
Results
3 main radiological findings were identified: nodules, 64% (16/25); masses, 36% (9/25); and consolidations in a patchy pattern, 20% (5/25). A tree-in-bud pattern was found in 12% (3/25) of patients. In 8 (32%) of 25 patients, we found a combination of 2 or more of these signs: 5 (20%) patients presented with concurrent nodules accompanied by patchy consolidations and/or tree-in-bud, and 3 (12%) patients showed masses accompanied by large consolidations. A halo sign was observed in 20 (80%) of 25 patients. Hypodense sign and cavitary lesions were encountered in 17 (68%) of 25 patients. Follow-up radiological findings after treatment showed improvement in 18 patients, no change in 4 patients and progression in 3 patients. There were three aspergillosis-associated deaths during the follow-up period. The onset time of IPA was a median of 31 days after transplantation. The most common symptom at diagnosis was fever (n=15). None of the 25 patients had leukopaenia at the time of the diagnosis of IPA.
Conclusions
The most common radiological findings of IPA after liver transplantation are multiple nodules with or without halo sign, masses and consolidations, which usually appear about 1 month after transplantation.
Liver transplantation has become a commonly accepted treatment method for patients with end-stage liver disease [1-3]. According to the World Transplant Registry, around 20 000 liver transplants are performed annually all over the world [2]. The increased numbers of liver transplant patients mean increased numbers of immunocompromised patients who have high rates of infection-related morbidity and mortality [1,3]. Despite being less common than bacterial infections, fungal infections are associated with the highest incidence of infection-related mortality [4-6]. The Aspergillus genus is one of the most common types of fungal disease in liver transplant patients. Although the radiological and clinical findings of invasive pulmonary aspergillosis (IPA) in patients with haematopoietic stem cell transplantation (HSCT), acquired immunodeficiency syndrome (AIDS), non-AIDS immunocompromised status and immunocompetent status have been studied, the radiological and clinical manifestations of IPA have rarely been reported in a large number of liver transplant patients [7-10]. Therefore, the purpose of our study was to assess the radiological and clinical findings of IPA in liver transplant recipients.
Methods and materials
Patients
From January 2003 to January 2010, the total number of liver transplant patients at our institution was 2150. 2018 out of 2150 transplant recipients had complete follow-up and 132 were lost to follow-up. 2018 follow-up transplant recipients had abnormal chest radiographs during post-transplant hospitalisation and post-transplant outpatient surveillance. 1127 CT examinations were performed based on suspicious radiographic findings. Three patients had suspected IPA but did not have a biopsy because of the patients' refusal of the procedure. Seven patients had suspected IPA but a negative biopsy. All patients with both definite and probable IPA were included in our study. Serum and bronchoalveolar lavage (BAL) galactomannan (GM) assays were performed in these patients. GM assay (Platelia Aspergillus; Bio-Rad Laboratories, Hercules, CA) was performed according to the manufacturer's recommendations for testing serum and BAL samples. Both serum and BAL GM samples were considered positive when optical density index value was ≥0.5 ng ml–1. 25 of 43 patients with positive GM tests were histologically confirmed as having IPA.
We identified study cases based on pathology results. We also reviewed cases with imaging findings that initially suggested invasive Aspergillus but were proven to represent an infection from another organism.
The mycoses Study Group diagnostic criteria were employed for diagnosis: “definite IPA” was defined as histological evidence of Aspergillus hyphae upon biopsy with tissue destruction and/or tissue invasion; and “probable IPA” was defined as clinical and radiological features suggestive of invasive aspergillosis and culture evidence of Aspergillus from a significant sample (BAL, lung biopsy or transthoracic fluoroscopic or CT-guided needle aspiration, two positive sputum cultures or cytology smears) [2,7].
Our final study population comprised 25 consecutive patients with histologically confirmed IPA that developed following liver transplantation. Histopathological specimens were acquired using percutaneous needle biopsy and/or aspiration (n=12), bronchoscopic biopsy (n=6), sputum culture (n=3), BAL culture (n=2) and wedge resection (n=2).
The median age of the study patients was 45 years (range 33–65 years), comprising 16 males and 9 females. The underlying diseases necessitating liver transplantation included hepatitis B virus liver cirrhosis (n=17), hepatocellular carcinoma (n=6) and biliary atresia (n=2). All patients received an immunosuppressive regimen consisting of tacrolimus (FK506), which is an effective immunosuppressive agent for the prevention of organ transplant rejection. Approval was obtained from the local institutional review board of our institution, and written informed consent was waived.
Imaging analysis
Chest radiographs were performed routinely as part of a clinical protocol following liver transplantation, even though the patients were asymptomatic in our centre. CT examination was undertaken if any parenchymal abnormality was detected on serial chest radiography or if there was clinical suspicion for pulmonary infection despite normal chest radiography results.
Chest radiographs and CT images of 25 patients with histologically confirmed IPA were reviewed. Chest CT images were obtained from 12 patients after intravenous contrast material injection, and chest CT examinations without contrast material administration were performed on the remaining 13 patients. CT examinations were obtained with a multidetector row CT scanner (Lightspeed Qx/i; GE Medical Systems, Milwaukee, WI). Imaging parameters were as follows: 120 kVp, 180 mAs, 1.25-mm collimation and a pitch of 1.5. The reconstructed slice thickness and interval were 5 mm. The routine chest CT images were obtained from the lung apices through the bases. Images were viewed on standard lung windows (level −600 HU; width 1500 HU) and mediastinal windows (level 40 HU; width 400 HU). Follow-up chest CT examinations were obtained in all patients.
The chest radiographs and CT images were analysed by consensus by two radiologists (JQ and YD, with 10 and 15 years of experience in chest imaging, respectively). The findings and interpretations were based on their consensus opinion. CT images were assessed for the following patterns of pulmonary abnormality: consolidation, nodules, masses and tree-in-bud pattern. Consolidation was considered to be present when the opacities obscured the underlying vessels. Nodules were defined as <3 cm in diameter and a mass was defined as ≥3 cm in diameter. A tree-in-bud pattern was defined as tiny centrilobular nodules in a branching pattern, indicative of fluid or material-filled bronchioles [8]. The number (single or multiple), diameter (smaller than, equal to or larger than 3 cm) and distribution (side distribution: right predominant, left predominant or bilateral; craniocaudality: upper predominant, lower predominant or symmetric) of the lesions were analysed. The level of the tracheal carina was used to divide the lung into upper and lower zones. Central hypointensity in lung consolidations or nodules (hypodense sign) [11], cavity including air crescent sign and air bronchograms were also assessed. Nodules were also assessed as to whether or not they were surrounded by ground-glass opacity (halo sign). Additional findings, such as pleural effusion (unilateral or bilateral) and mediastinal lymph node enlargement, were also recorded.
Serial changes in the chest radiographs and CT findings after treatment for IPA during the follow-up period were assessed and categorised as improved, unchanged or progressed.
Clinical analysis
The patients' clinical records were reviewed by two of the authors (JQ and YF) in order to determine the incidence, time of onset, signs and symptoms, and clinical course. The time of onset of IPA was defined as the time interval from date of transplant to the day of the initial CT examination. Clinical symptoms, including fever, cough, sputum, chest pain or dyspnea, noted at the time of diagnosis, were recorded. The clinical course after each patient's treatment, as well as the mortality, was reviewed. We also recorded the white blood cell (WBC) count on the day that chest CT was performed on each patient. The analysis of clinical data was performed after the evaluation of imaging studies, to avoid possible bias in imaging analysis.
Results
Imaging results
Three main radiological findings were identified: nodules with or without halo sign, masses and consolidations in patchy pattern (Figures 1–3; Table 1). A tree-in-bud pattern was found in 12% (3/25) of patients (Figure 4). The halo sign was seen in 20 liver recipients within 1 week after onset of symptoms. The hypodense sign was observed in larger nodules or masses, which then became cavitary within 4 weeks after onset of symptoms in 17 patients.
Figure 1.
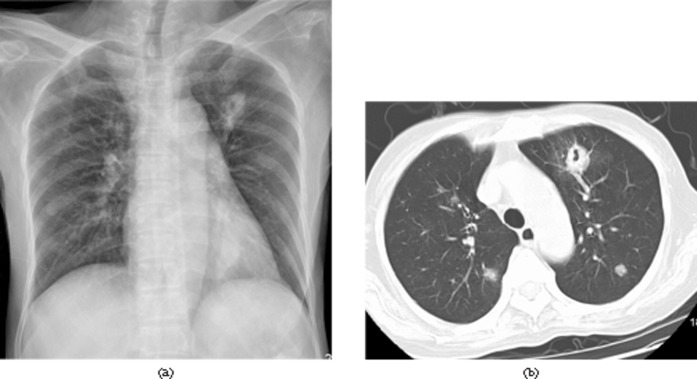
A 34-year-old asymptomatic male 26 days after liver transplantation. Invasive pulmonary aspergillosis was confirmed by percutaneous biopsy. (a) Chest radiograph shows a thick-walled cavity in the left upper lobe. (b) Chest CT image (lung window setting, slice thickness 5-mm) shows a 1.5-cm, thick-walled cavity in the left upper lobe and multiple nodules with random distribution in both lungs.
Figure 3.
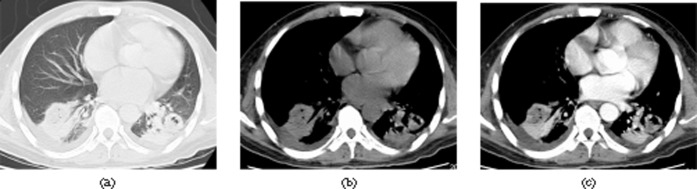
A 43-year-old male with fever, sputum and chest pain 33 days after liver transplantation. Invasive pulmonary aspergillosis was confirmed by bronchoscopic biopsy. Axial CT images of (a) lung window setting (slice thickness 5 mm) (b) mediastinal window setting (slice thickness 5 mm) and (c) contrast-enhanced mediastinal window setting (slice thickness 5 mm) show patchy consolidations and nodules with central low density and containing air bubbles in the both lower lobe. Note also bilateral small pleural effusion.
Table 1. Radiological findings of invasive pulmonary aspergillosis in liver recipients.
| Radiological findings | Number (%) of patients (n=25) |
| Nodulea | 16 (64) |
| Mass | 9 (36) |
| Consolidation in patchy pattern | 5 (20) |
| Tree-in-bud | 3 (12) |
| Halo signb | 20 (80) |
| Hypodense signc | 17 (68) |
| Cavitary lesiond | 17 (68) |
| Air crescent sign | 5 (20) |
| Air bronchogram | 3 (12) |
| Pleural effusion | 9 (36) |
aIncludes nodules with or without halo sign.
bA nodule with perinodular of ground-glass opacity.
cIncludes nodules and masses with hypodense sign.
dIncludes cavitary lesions with or without air crescent sign.
Figure 4.
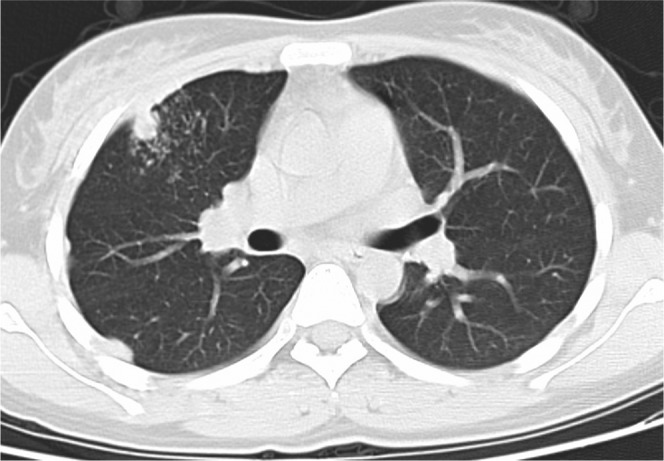
A 45-year-old female with fever and chest pain 15 days after liver transplantation. Invasive pulmonary aspergillosis was confirmed by sputum culture. Axial CT image (lung window setting, slice thickness 5 mm) shows nodule and tree-in-bud nodularity adjacent to the nodule on the right middle lobe and pleural thickening on the right side.
Figure 2.
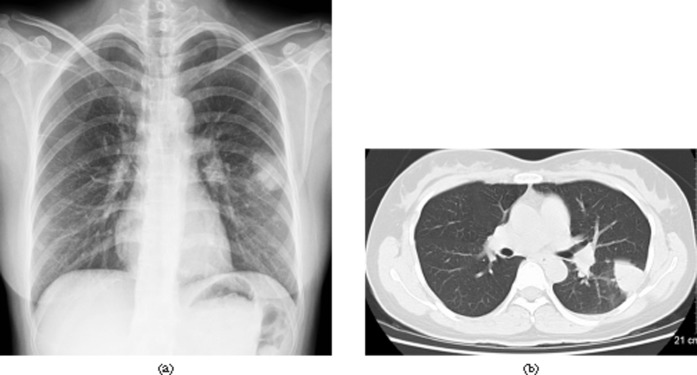
A 35-year-old female with fever and cough 42 days after liver transplantation. Invasive pulmonary aspergillosis was confirmed by wedge resection of left lower lobe. (a) Chest radiograph shows a mass in the left lower lobe. (b) Chest CT image (lung window setting, slice thickness 5-mm) shows a 3.5-cm diameter round mass in the left lower lobe.
In 8 patients (32%), we found a combination of two or more of these signs: 5 (20%) patients presented concurrent nodules accompanied by patchy consolidations and/or tree-in-bud pattern, and 3 (12%) patients showed masses accompanied by large consolidations. The number of nodules or masses was <5 in 15 patients, 5–10 in 6 patients, 10–30 in 2 patients and innumerable in the other 2 patients. When the sizes of the nodules and masses were analysed in 23 patients (excluding the 3 patients with innumerable nodules), 70% (104/149) of the lesions were <3 cm in diameter, whereas 30% (45/149) were >3 cm in diameter. The lesions were distributed in both lungs in 11 patients and in one lung in 14 patients (Figure 5). In a craniocaudal direction, the lesions were distributed evenly throughout the lung in 3 patients, whereas upper lung dominance and lower dominant distribution were noted in 9 and 13 patients, respectively.
Figure 5.
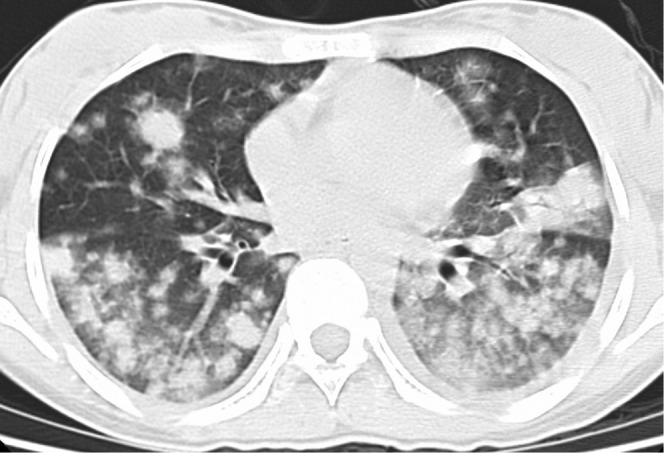
A 39-year-old female with fever, sputum and dyspnea 13 days after liver transplantation. Invasive pulmonary aspergillosis was confirmed by sputum culture. Axial CT scan (lung window setting, slice thickness 5 mm) shows multiple nodules with peripheral halo sign and patchy consolidations in both lungs.
Ancillary findings included pleural effusion (n=9) with bilateral (4/9) or unilateral distribution (5/9). The unilateral effusions were ipsilateral to the parenchymal disease in all five patients. No lymph node enlargement >1 cm in diameter was seen in our cases.
During follow-up, the pulmonary nodules or masses decreased in size and number, and then disappeared with or without scar as seen on the chest radiographs and CT images in 18 patients (Figure 6). The time interval between the diagnosis and complete resolution was a median of 4 months (range 1–15 months). In four patients, the chest radiographs and CT surveys demonstrated the pulmonary opacities were unchanged, despite ongoing antifungal therapy. In the remaining three patients, the parenchymal lesions increased in number and extent despite treatment.
Figure 6.
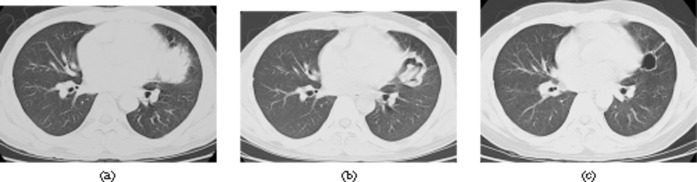
Remission with fibrotic residual lesion in 43-year-old man after liver transplatation. Invasive pulmonary aspergillosis was confirmed by bronchoscopic biopsy. (a) CT scan (lung window setting, slice thickness 5 mm) shows a 4-cm diameter round mass in lingula. (b) 10 days after initial diagnosis, CT scan (lung window setting, slice thickness 5 mm) shows that the mass became an irregular, thick-walled cavity with an intracavitary mass. (c) 30 days after initial diagnosis, the thick-walled cavity has regressed to a thin circular wall of fibrotic tissue without any further dynamics on follow-up CT scans.
Clinical results
The incidence of IPA after liver transplantation was 1.2% (25/2150). The median onset time of IPA was 31 days (range 13–102 days) following transplantation. In 17 of the 25 study patients, the clinical symptoms at presentation were fever (n=15), cough (n=11), sputum (n=8), chest pain (n=6) and dyspnoea (n=5; Table 2). The remaining eight patients were asymptomatic. There was no difference in distribution or extent of disease in the symptomatic and asymptomatic cases.
Table 2. Clinical results of invasive pulmonary aspergillosis in liver transplant recipients.
| Case number | Age (years)/sex (M/F) | Onset (day) | Symptom | Confirmed method | Follow-up |
| 1 | 49/M | 27 | Fever, cough | Percutaneous biopsy | Improved |
| 2 | 54/M | 15 | Fever, cough | Percutaneous biopsy | Improved |
| 3 | 37/F | 27 | None | Percutaneous needle aspiration | Improved |
| 4 | 43/M | 33 | Fever, sputum, chest pain | Bronchoscopic biopsy | Improved |
| 5 | 39/F | 13 | Fever, sputum, dyspnoea | Sputum culture | Progression, death attributed to IPA |
| 6 | 35/F | 42 | Fever, cough | Wedge resection | Improved |
| 7 | 46/M | 102 | Fever, cough, dyspnoea | Wedge resection | Unchanged |
| 8 | 63/F | 64 | Fever, cough | Bronchoscopic biopsy | Unchanged |
| 9 | 38/M | 32 | Fever, cough, sputum, dyspnoea | BAL culture | Progression, death attributed to IPA |
| 10 | 45/F | 15 | Fever, chest pain | Sputum culture | Improved (fibrotic residual lesion) |
| 11 | 58/M | 26 | None | Percutaneous biopsy | Improved (fibrotic residual lesion) |
| 12 | 53/M | 23 | Fever, sputum, chest pain | Percutaneous biopsy | Improved |
| 13 | 33/M | 32 | None | Percutaneous needle aspiration | Deteriorating |
| 14 | 49/M | 27 | Fever, cough | Percutaneous biopsy | Improved |
| 15 | 44/F | 15 | None | Percutaneous biopsy | Improved |
| 16 | 36/M | 17 | None | Percutaneous needle aspiration | Improved |
| 17 | 58/M | 45 | Fever, cough, chest pain | BAL culture | Improved |
| 18 | 39/M | 92 | None | Bronchoscopic biopsy | Deteriorating |
| 19 | 54/F | 42 | None | Percutaneous biopsy | Improved |
| 20 | 46/M | 95 | Fever, cough, dyspnoea | Sputum culture | Unchanged |
| 21 | 63/F | 64 | Fever, sputum, chest pain | Bronchoscopic biopsy | Unchanged |
| 22 | 38/M | 32 | Cough, sputum, dyspnoea | Bronchoscopic biopsy | Progression, death attributed to IPA |
| 23 | 43/M | 27 | Fever, sputum, chest pain | Bronchoscopic biopsy | Improved (fibrotic residual lesion) |
| 24 | 34/M | 26 | None | Percutaneous biopsy | Improved (fibrotic residual lesion) |
| 25 | 36/F | 33 | Cough, sputum | Percutaneous biopsy | Improved |
BAL, bronchoalveolar lavage; F, female; IPA, invasive pulmonary aspergillosis; M, male.
None of the 25 patients was neutropenic at the time of the diagnosis of IPA (median WBC count 6700 mm–3; range 5100–13 700 mm–3). Antifungal therapy prior to diagnosis was routinely administered in all 25 patients. After transplantation, the recipients received tacrolimus (FK506; 0.10–0.20 mg kg–1day–1). Amphotericin B lipid was administered to all IPA cases at a dose of 0.1–5.0 mg kg–1day–1after diagnosis of IPA. The satisfactory global response rate at 12 weeks was 72% (18/25) according to the criteria of resolution of >50% of the pulmonary lesions.
There were three aspergillosis-associated deaths. Aspergillus was cultured from sputum in three cases and from BAL in two. Microscopic examinations of the lesions of lung biopsies, which were performed in 20 patients, demonstrated invasion of the normal lung parenchyma by long, thin, septate fungal hyphae that branched at approximately a 45° angle, a characteristic appearance of Aspergillus. There was vascular invasion and haemorrhage around the nodules in two patients who underwent wedge resection.
Discussion
Our study showed similar findings to that in the literature. Nodules, masses and consolidations were the three most common radiological findings, which usually occurred about 31 days after transplantation. According to other published data, nodules, masses and consolidations were the main radiological findings in patients after solid organ transplant and in other immunocompromised patients [1-5,7-9]. Ground-glass opacity surrounding the nodules, the halo sign, was common in our study (80%). Because the halo sign has also been reported in patients with IPA after HSCT and patients with other immunocompromised conditions, it is not a specific radiological finding of IPA after liver transplantation [9-13]. However, the halo sign is still a useful finding for early diagnosis of IPA and has been pathophysiologically characterised as a discrete nodule of angioinvasive aspergillosis with infarction and coagulative necrosis surrounded by alveolar haemorrhage [9]. Nevertheless, the duration of this halo sign is short. Three-quarters of the initial CT halo signs disappeared within 1 week after IPA diagnosis [9]. To be useful for IPA diagnosis, the CT scan must be performed early in the course of the disease, and probably in the first 5 days after the occurrence of the disease [9-11]. This fact could explain the lower incidence of the halo sign in the same settings in other reports. Horger et al explain that the hypodense sign seen on unenhanced CT images was a relatively specific sign of pulmonary aspergillosis, which could be a precursor to the development of cavitation [11]. Park et al reported that 66.6% (8/12) of patients showed central low attenuation within the nodules or masses [13].
Hypodense signs, air crescent signs and cavitation were common in liver transplantation recipients in our study. We found larger nodules or masses with hypodense sign, which cavitated in 17 patients (68%). A recent retrospective analysis of chest CT data identified that the hypodense sign was thought to be caused by vascular obstruction with secondary lung infarction and sequestration [5-11]. Notably, this imaging finding was generally detected after the finding of a halo sign, thus limiting its utility for early diagnosis of IPA [11]. Thus, our study results agree with the findings of these authors.
Invasive aspergillosis has been reported in 1.5–6.5% of liver transplant patients [5]. In our study, the incidence of IPA after liver transplantation was 1.2%, which was slightly less common than that of IPA in liver transplant recipients previously reported. The difference of risk factors for IPA in our study population probably explains this difference. Improvements in patient selection, management, surgical techniques and immunosuppressive regimens, as well as reduction of environmental exposure may also be responsible for the low incidence. The following variables have been assessed as risk factors for IPA [10-16]:
preoperative variables, including Child–Turcotte–Pugh classification, administration of corticosteroid at a cumulative dose of >0.5 g in the week before living donor liver transplantation
intraoperative variables, including units of blood product transfused, operation time, graft cold ischaemia time
postoperative variables, including duration of intensive care unit stay, duration of mechanical ventilation, requirement for surgical intervention, requirement for administration of corticosteroid and immunosuppressive drugs.
In our study, the pulmonary lesions improved after treatment in more than half of patients (18/25, 72%). There were three aspergillosis-associated deaths. Both of these results demonstrate an improvement on the rates seen in previous series (50–100%) [1-3,17-21]. Early diagnosis coupled with aggressive treatment might be possible reasons for this difference.
Although the presence of nodules, masses and/or patchy or segmental consolidations in patients after liver transplantation should suggest the possibility of IPA, other conditions must be considered in the differential diagnoses. The differential diagnoses include various infectious and non-infectious conditions. Infectious conditions, such as cytomegalovirus and nocardiosis, are prevalent in the early post-transplant period, whereas non-infectious conditions, such as post-transplant lymphoproliferative disorder and recurrent tumour, are common in the late post-transplant period [19-21]. However, it has been reported that pulmonary nodules occurring 1–3 months post-transplant are significantly associated with Aspergillus infection [13,17-20].
Our study has several limitations. Firstly, it was a retrospective study including few patients. However, to our knowledge, it was the largest series analysing the radiological and clinical findings of IPA after liver transplantation. Secondly, the reconstructed slice thickness and interval of chest CT examinations in our study was 5 mm, and this probably influenced our ability to visualise small findings, such as tree-in-bud opacities. Thirdly, we did not compare IPA with other lung pathologies in this study. Further investigation should be performed to differentiate IPA from other pulmonary infections that may overlap many of the imaging findings. Fourthly, comparison of the radiological and histopathological findings was performed retrospectively in a limited number of cases. Further investigation should be undertaken to compare the radiological and histopathological findings.
In summary, the most common radiological findings of IPA after liver transplantation are multiple nodules with or without halo sign, masses and consolidations, which usually appear about 1 month after transplantation. Therefore, knowledge of the radiological findings of IPA in liver transplant recipients can play an important role in early diagnosis, early antifungal therapy and, consequently, improving the chance of survival in these patients.
Footnotes
Jie Qin and Yuan Fang contributed equally to this work as first authors.
References
- 1.Zhu JY, Gao PJ, Li GM, Zhu FX, Huang L, Wang D, et al. Analysis of 565 cases of liver transplantation at a single transplantation center. [Article in Chinese.] Beijing Da Xue Xue Bao 2009;41:368–72 [PubMed] [Google Scholar]
- 2.Blair JE, Kusne S. Bacterial, mycobacterial, and protozoal infections after liver transplantation–part I. Liver Transpl 2005;11:1452–9 [DOI] [PubMed] [Google Scholar]
- 3.Takeda K, Morioka D, Matsuo K, Endo I, Sekido H, Moroboshi T, et al. A case of successful resection after long-term medical treatment of invasive pulmonary aspergillosis following living donor liver transplantation. Transplant Proc 2007;39:3505–8 [DOI] [PubMed] [Google Scholar]
- 4.Mikulska M, Raiola AM, Bruno B, Furfaro E, Van Lint MT, Bregante S, et al. Risk factors for invasive aspergillosis and related mortality in recipients of allogeneic SCT from alternative donors: an analysis of 306 patients. Bone Marrow Transplant 2009;44:361–70 [DOI] [PubMed] [Google Scholar]
- 5.Greene RE, Schlamm HT, Oestmann JW, Stark P, Durand C, Lortholary O, et al. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis 2007;44:373–9 [DOI] [PubMed] [Google Scholar]
- 6.Sherif R, Segal BH. Pulmonary aspergillosis: clinical presentation, diagnostic tests, management and complications. Curr Opin Pulm Med 2010;16:242–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trullas JC, Cervera C, Benito N, de laBellacasa JP, Agustí C, Rovira M, et al. Invasive pulmonary aspergillosis in solid organ and bone marrow transplant recipients. Transplant Proc 2005;37:4091–3 [DOI] [PubMed] [Google Scholar]
- 8.Bruno C, Minniti S, Vassanelli A, Pozzi-Mucelli R. Comparison of CT features of Aspergillus and bacterial pneumonia in severely neutropenic patients. J Thorac Imaging 2007;22:160–5 [DOI] [PubMed] [Google Scholar]
- 9.Brook O, Guralnik L, Hardak E, Oren I, Sprecher H, Zuckerman T, et al. Radiological findings of early invasive pulmonary aspergillosis in immune-compromised patients. Hematol Oncol 2009;27:102–6 [DOI] [PubMed] [Google Scholar]
- 10.Osawa M, Ito Y, Hirai T, Isozumi R, Takakura S, Fujimoto Y, et al. Risk factors for invasive aspergillosis in living donor liver transplant recipients. Liver Transpl 2007;13:566–70 [DOI] [PubMed] [Google Scholar]
- 11.Horger M, Einsele H, Schumacher U, Wehrmann M, Hebart H, Lengerke C, et al. Invasive pulmonary aspergillosis: frequency and meaning of the “hypodense sign” on unenhanced CT. Br J Radiol 2005;78:697–703 [DOI] [PubMed] [Google Scholar]
- 12.Won HJ, Lee KS, Cheon JE, Hwang JH, Kim TS, Lee HG, et al. Invasive pulmonary aspergillosis: prediction at thin-section CT in patients with neutropeniada prospective study. Radiology 1998;208:777–82 [DOI] [PubMed] [Google Scholar]
- 13.Park YS, Seo JB, Lee YK, Do KH, Lee JS, Song JW, et al. Radiological and clinical findings of pulmonary aspergillosis following solid organ transplant. Clin Radiol 2008;63:673–80 [DOI] [PubMed] [Google Scholar]
- 14.Sharifipour F, Rezaeetalab F, Naghibi M. Pulmonary fungal infections in kidney transplant recipients: an 8-year study. Transplant Proc 2009;41:1654–6 [DOI] [PubMed] [Google Scholar]
- 15.Brodoefel H, Vogel M, Hebart H, Einsele H, Vonthein R, Claussen C, et al. Long-term CT follow-up in 40 non-HIV immunocompromised patients with invasive pulmonary aspergillosis: kinetics of CT morphology and correlation with clinical findings and outcome. AJR Am J Roentgenol 2006;187:404–13 [DOI] [PubMed] [Google Scholar]
- 16.Horger M, Hebart H, Einsele H, Lengerke C, Claussen CD, Vonthein R, et al. Initial CT manifestations of invasive pulmonary aspergillosis in 45 non-HIV immunocompromised patients: association with patient outcome? Eur J Radiol 2005;55:437–44 [DOI] [PubMed] [Google Scholar]
- 17.Einollahi B, Lessan-Pezeshki M, Pourfarziani V, Nemati E, Nafar M, Pour-Reza-Gholi F, et al. Invasive fungal infections following renal transplantation: a review of 2410 recipients. Ann Transplant 2008;13:55–8 [PubMed] [Google Scholar]
- 18.Copp DH, Godwin JD, Kirby KA, Limaye AP. Clinical and radiologic factors associated with pulmonary nodule etiology in organ transplant recipients. Am J Transplant 2006;6:2759–64 [DOI] [PubMed] [Google Scholar]
- 19.Althoff Souza C, Müller NL, Marchiori E, Escuissato DL, Franquet T. Pulmonary invasive aspergillosis and candidiasis in immunocompromised patients: a comparative study of the high-resolution CT findings. J Thorac Imaging 2006;21:184–9 [DOI] [PubMed] [Google Scholar]
- 20.Can MF, Yagci G, Gorenek L, Tozkoparan E, Ozerhan I, Cetiner S. Invasive pulmonary aspergillosis after liver transplantation: rapid and complete response to combined and sequential antifungal therapy. Surg Infect (Larchmt) 2008;9:99–104 [DOI] [PubMed] [Google Scholar]
- 21.Moreno R, Berenguer M. Post-liver transplantation medical complications. Ann Hepatol 2006;5:77–85 [PubMed] [Google Scholar]