Abstract
Objective
We retrospectively evaluated the effect of transpulmonary radiofrequency ablation (RFA) of liver tumours on the lung.
Methods
16 patients (10 males and 6 females; mean age, 65.2 years) with 16 liver tumours (mean diameter 1.5 cm) underwent transpulmonary RFA under CT fluoroscopic guidance. The tumours were either hepatocellular carcinoma (n=14) or liver metastasis (n=12). All 16 liver tumours were undetectable with ultrasonography. The pulmonary function values at 3 months after transpulmonary RFA were compared with baseline (i.e. values before RFA).
Results
In 8 of 16 sessions, minor pulmonary complications occurred, including small pneumothorax (n=8) and small pleural effusion (n=1). In two sessions, major pulmonary complications occurred, including pneumothorax requiring a chest tube (n=2). These chest tubes were removed at 4 and 6 days, and these patients were discharged 7 and 10 days after RFA, respectively, without any sequelae. The pulmonary function values we evaluated were forced expiratory volume in 1 s (FEV1.0) and vital capacity (VC). The mean values of FEV1.0 before and 3 months after RFA were 2.55 l and 2.59 l, respectively; the mean values of VC before and 3 months after RFA were 3.20 l and 3.27 l, respectively. These pulmonary values did not show any significant worsening (p=0.393 and 0.255 for FEV1.0 and VC, respectively).
Conclusion
There was no significant lung injury causing a fatal or intractable complication after transpulmonary RFA of liver tumours.
Percutaneous radiofrequency ablation (RFA) has been established as a local therapy for liver cancers, including both hepatocellular carcinoma (HCC) and liver metastasis [1]; meta-analysis that included a total of 5224 treated liver tumours showed a local progression rate of 12% (647/5224) after one or more RFA sessions [2]. In this procedure, a radiofrequency (RF) electrode is generally inserted using the transabdominal and/or transhepatic route under ultrasonographic guidance. However, depending upon the tumour location, it is often difficult to detect these by ultrasonography (e.g. tumours located in the hepatic dome). In such tumours, certain modified approaches, such as artificial pleural effusion [3], artificial ascites [4], artificial pneumothorax [5] or thoracoscopic guidance [6], are often used.
The transpulmonary approach under CT guidance is another method that can be used for needle insertion into such liver tumours. Some investigators have reported the feasibility, effectiveness and safety of transpulmonary RFA of liver tumours [7-12]. Further, certain pulmonary complications—such as pneumothorax and pleural effusion caused by puncture of the pleura, lung and/or diaphragm—have been reported too, along with these being common complications of RFA of liver tumours. To our knowledge, however, although there are no reports of fatal or intractable pulmonary complications of this procedure, there are also no reports that evaluate its effect on the lung.
The purpose of this study is to retrospectively evaluate the effect of transpulmonary RFA of liver tumours on the lung.
Methods and materials
Patient and tumour characteristics
This retrospective study was approved by our institutional review board, and written informed consent was obtained from each patient.
Between August 2007 and November 2010, 116 patients underwent RFA of liver tumours percutaneously at our institution. Of those, 16 patients (10 males and 6 females; mean age 65.2±5.4 years; range 52–71 years) with 16 tumours underwent transpulmonary RFA of liver tumours under CT fluoroscopic guidance. The mean long-axis diameter of the tumours was 1.5±0.4 cm (range 1.0–2.2 cm). The tumours were either HCC (n=14) or liver metastasis from colon cancer (n=2) and located in segment VIII (n=11), in segment IV (n=2), in segment VII (n=2) and in segment I (n=1). All 16 liver tumours were undetectable with ultrasonography. All 14 patients with HCCs had undergone transcatheter arterial chemoembolisation 10.4 days (mean) before RFA. No patients had pulmonary emphysema, active pulmonary infections or severe lung dysfunction. Routine pulmonary function tests were performed with lung spirometry; test parameters included forced expiratory volume in 1 s (FEV1.0) and vital capacity (VC). The mean values of FEV1.0 and VC before RFA were 2.55±0.91 l (range 1.42–4.44 l) and 3.20±1.20 l (range 1.70–5.64 l), respectively.
Radiofrequency ablation
All patients underwent chest and abdominal CT (Aquilion 64; Toshiba, Tokyo, Japan) prior to RFA for assessment and planning of the procedure. Procedural patient positioning was determined depending on the tumour location as follows: supine (n=13) or prone (n=3). In all sessions, intraprocedural pain was treated by using local anaesthesia and intravenous administration of fentanyl. The cardiac and respiratory parameters such as blood pressure, heart rate, electrocardiography and blood oxygen saturation were continuously monitored.
The procedure was always performed percutaneously by CT fluoroscopy (Aquilion 64). In all 16 sessions, a 17-gauge single internally cooled electrode (Cool-tip; Covidien, Mansfield, MA) with a non-insulated tip was used; the non-insulated tip length of the electrode was 2 cm (n=12) or 3 cm (n=4). To reduce radiation exposure, CT fluoroscopy was performed intermittently. Further, the electrode was held with a pair of 19-cm-long plastic forceps during CT fluoroscopy to maintain its position and avoid direct radiation exposure to the operator's hands.
The electrode was introduced into the tumour using a transpulmonary route and connected to an RF generator (CC-1; Covidien). An impedance-control algorithm was selected, and RF energy was applied for 12 min while infusing cold saline into the cooling lumen of the electrode. Immediately after RF application, the temperature of the tumour at the electrode tip was measured. If the temperature had not reached 60 °C, another energy application was added at the same site. Multiple overlapping ablation zones were created when the expected ablation zone per single application was smaller than the tumour plus an ablative margin. While the electrode was being drawn, the needle tract was ablated with low-power RF energy in an attempt to minimise the risk of bleeding and tumour seeding. Immediately after this procedure, contrast-enhanced CT images of the entire lung and liver were obtained to assess the procedure.
A posteroanterior upright chest radiograph was obtained 4 h later and then on the following morning to assess complications, including pneumothorax, intrathoracic haemorrhage and pleural effusion.
Post-radiofrequency ablation evaluation
The therapeutic response and complications of the procedure were assessed on CT images at 1, 3 and 6 months, and subsequently at 6-month intervals using a 64-slice multidetector row CT scanner (Aquilion 64). CT images were obtained before and after the intravenous administration of 100 ml or 150 ml of iohexol (Omnipaque 300; Daiichi-Sankyo, Tokyo, Japan) at a rate of 3–5 ml s−1 in the hepatic arterial phase, portal venous phase and delayed phase. The disappearance of tumour enhancement was considered to indicate complete necrosis. Local tumour progression was defined by the appearance of enhanced tumours adjacent to the zone of ablation. Plain and delayed-phase CT images included the entire chest in addition to the liver.
Pulmonary function tests (FEV1.0 and VC) were performed with lung spirometry at 3 months after RFA.
Statistical analysis
Pulmonary function values (FEV1.0 and VC) at 3 months after RFA were compared with the baseline values (i.e. those before RFA) by using the paired t-test. A p-value of <0.05 was considered significant. Statistical analysis was performed using SPSS® software (v. 14.0; SPSS, Chicago, IL).
Results
In 15 out of 16 sessions the RFA procedure was technically successful (i.e. treatment of the tumour is performed according to protocol and complete tumour coverage is assessed either during or immediately after the procedure [1]; Figure 1). In one patient, although the electrode was successfully inserted into the tumour, RF energy could be applied only for 6 min because of unbearable pain. The mean number of times that the RF electrode punctured the chest wall pleura and the diaphragmatic pleura was 1.4±0.7 (range 1–3) and 1.9±1.0 (range 1–4), respectively. The mean maximum electrode length of transgressed lung parenchyma was 2.4±1.3 cm (range 1.1–6.5 cm). 15 tumours, which were treated completely, showed no local progression with a median follow-up period of 18 months (range 3–41 months).
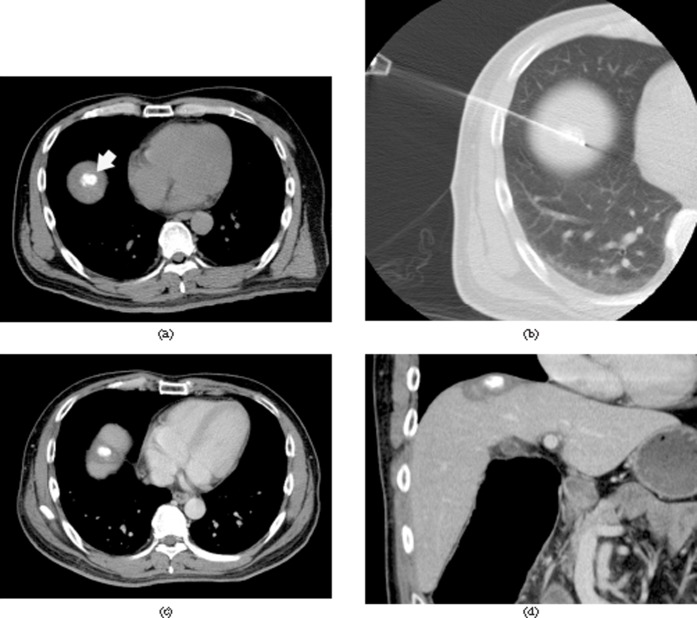
Figure 1.
CT fluoroscopy-guided transpulmonary radiofrequency ablation (RFA) in a 52-year-old man with hepatocellular carcinoma (HCC) in hepatic segment VIII. (a) Plain CT image obtained before RFA shows the targeted HCC of 1.8 cm in diameter with the accumulation of iodised oil (arrow). (b) CT fluoroscopic image obtained during RFA shows radiofrequency needle insertion into an HCC via the transpulmonary route. The electrode length of transgressed lung parenchyma is 1.6 cm. (c, d) Portal venous phase axial (c) and coronal (d) CT images obtained 8 months after RFA show a non-enhancing RFA zone that surrounds the HCC, with no evidence of local progression.
The definition of a major complication was an event that leads to substantial morbidity and disability, increasing the level of care, or results in hospital admission or substantially lengthened hospital stay; minor complications resulted in no sequelae, and they required nominal therapy or a short hospital stay for observation [1].
In 8 of the 16 sessions, minor complications occurred, including small pneumothorax (n=8), small pleural effusion (n=1) and grounding pad burn (n=1). In two sessions, major complications occurred, including pneumothorax requiring a chest tube (n=2). These chest tubes were removed at 4 and 6 days, respectively, and these patients were discharged 7 and 10 days after RFA, respectively, without any sequelae. In 10 of the 16 sessions (62.5%), pulmonary complications (pneumothorax requiring a chest tube, 2; small pneumothorax, 7; small pneumothorax and pleural effusion, 1) occurred. No other pulmonary complications, such as lung bleeding or haemothorax, diaphragmatic injury, tumour seeding in the pleura and/or lung parenchyma, lung abscess or systemic air embolism, occurred during or after RFA. No patients suffered from dyspnoea or required oxygen administration after RFA.
Pulmonary function tests did not show any significant worsening in FEV1.0 and VC at 3 months after RFA as compared with the baseline values. The mean values of FEV1.0 and VC at 3 months after RFA were 2.59±0.93 l (range 1.47–4.46 l; p=0.393) and 3.27±1.18 l (range 1.77–5.84 l; p=0.255), respectively.
Discussion
Some investigators have reported that transpulmonary RFA of liver tumours might be an acceptable treatment based on their results, including the feasibility, effectiveness and safety [7-12]. This procedure was technically feasible and its technique effectiveness was similar to other results of RFA of liver tumours. Kato et al reported that the dynamic CT images showed no local tumour progression in a mean period of 8 months (range 1–29 months) in 22 (92%) of 24 HCCs treated by transpulmonary RFA [7]. Park et al reported that the primary technique effectiveness rates of complete ablation 1, 3, 6 and 12 months after RFA were 97%, 94%, 84% and 74%, respectively, in 97 HCCs in 78 sessions, including 38 transpulmonary sessions [8].
Possible pulmonary complications of CT-guided transpulmonary RF needle insertion for liver tumours include pneumothorax, lung bleeding and haemothorax, pleural effusion, diaphragmatic injury, tumour seeding in the pleura and/or lung parenchyma, lung abscess and systemic air embolism. In previous studies, as well as ours, the most common complication of transpulmonary RFA of liver tumours was observed to be pneumothorax (the incidence of pneumothorax and chest tube placement for pneumothorax was 37.5–71.4% and 0–28.6%, respectively) [7-12]. However, there are no reports of intractable pneumothorax and other pulmonary complications following transpulmonary RFA of liver tumours. We think that it is important to use a single RF needle. It may not be possible to extrapolate the same results when a multitined expandable needle is used.
In this procedure, the reason for the high incidence of pneumothorax may be that the pleura was penetrated twice on both the chest wall and diaphragmatic sides in the transpulmonary approach to liver [7]. Miura et al reported that the main risk factors for pneumothorax after transpulmonary RFA of liver tumours were increased length of needle trajectory through the aerated lung and multiple transpulmonary approaches in one session [9]. Conversely, Park et al reported that the relationship between the occurrence of pneumothorax and the RF electrode length in the lung parenchyma was not statistically significant [8].
Massive pulmonary haemorrhage, including both intraparenchymal and extrapleural haemorrhage, and systemic air embolism are serious complications of transpulmonary RF needle insertion. Although there are no reports of these serious complications in transpulmonary RFA of liver tumours, they have been reported in RFA of lung tumours [13,14]. The reason may be that the number of patients who underwent transpulmonary RFA of liver tumours is much smaller. Tumour seeding may also be a serious complication occurring at a later stage, and it occurred in 0.5% of liver RFA sessions [15] and in 0.3–0.7% in lung RFA sessions [16,17]. Yamakado et al reported that transcatheter arterial chemoembolisation before RFA of HCC may be useful for preventing tumour seeding and may result in an increase in the visibility of HCC on CT images because of the accumulation of iodised oil [10]. We also performed transcatheter arterial chemoembolisation before transpulmonary RFA of all 14 HCCs in order to reduce bleeding and/or tumour seeding, and thus make the target lesions more visible. Additionally, the needle tract was ablated with low-power RF energy while the electrode was being withdrawn in all 16 sessions.
Although the physician may hesitate before selecting the transpulmonary approach to treat the liver tumours because the unaffected lung is injured, there were no fatal pulmonary complications in previous reports [7-12] or in our results. In addition, although the incidence of pulmonary complications was high (10 of 16 RFA sessions; 62.5%), most of our complications were asymptomatic and there was no effect on the pulmonary function at 3 months after transpulmonary RFA. However, if transpulmonary RFA of liver tumours is performed in patients with lung disease such as severe pulmonary emphysema, pulmonary complications may occur more frequently and/or be more intractable. Therefore, such patients are not favourable candidates for this procedure. In patients without lung disease, it appears that CT-guided transpulmonary RFA of liver tumours can be performed as an alternative method when the target is undetectable by ultrasonography.
In patients with subdiaphragmatic liver tumours, if operators want to avoid any form of pulmonary complication, they can use some alternative techniques, such as artificial ascites and CT gantry tilt techniques. Other newer tools include C-arm cone-beam CT [18] and real-time virtual sonography [19]. The former is an advanced three-dimensional imaging technology that incorporates a flat-panel detector angiography system; this technology enables real-time three-dimensional fluoroscopic guidance for needle insertion. In the latter, registration of visualised lesions on CT images is transposed into an ultrasound image for needle guidance.
This study has some limitations. This study included a small number patients in a single institution, and the tumour diameter was small (mean 1.5 cm). If the tumour diameter becomes larger, the number of times that the RF electrode punctures the pleura may increase when treating tumours completely. As a result, the risk of pulmonary complications may increase. We did not evaluate the early effect of the procedure on the lung (i.e. pulmonary function immediately after or 1 month after RFA). Although none of the patients with pulmonary complications clinically suffered from dyspnoea or required oxygen administration after RFA, their pulmonary functions may be slightly decreased in the early period after RFA.
In conclusion, there was no long-term significant lung injury assuming a fatal or intractable complication after transpulmonary RFA of liver tumours.
References
- 1.Crocetti L, de Baere T, Lencioni R. Quality improvement guidelines for radiofrequency ablation of liver tumors. Cardiovasc Intervent Radiol 2010;33:11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg 2005;242:158–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minami Y, Kudo M, Kawasaki T, Chung H, Ogawa C, Shiozaki H. Percutaneous radiofrequency ablation guided by contrast-enhanced harmonic sonography with artificial pleural effusion for hepatocellular carcinoma in the hepatic dome. AJR Am J Roentgenol 2004;182:1224–6 [DOI] [PubMed] [Google Scholar]
- 4.Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation with artificial ascites for hepatocellular carcinoma in the hepatic dome: initial experience. AJR Am J Roentgenol 2008;190:91–8 [DOI] [PubMed] [Google Scholar]
- 5.de Baère T, Dromain C, Lapeyre M, Briggs M, Duret JS, Hakime A, et al. Artificially induced pneumothorax for percutaneous transthoracic radiofrequency ablation of tumors in the hepatic dome: initial experience. Radiology 2005;236:666–70 [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa T, Kohno T, Shibayama T, Fukushima Y, Obi S, Teratani T, et al. Thoracoscopic thermal ablation therapy for hepatocellular carcinoma located beneath the diaphragm. Endoscopy 2001;33:697–702 [DOI] [PubMed] [Google Scholar]
- 7.Kato T, Yamagami T, Hirota T, Matsumoto T, Yoshimatsu R, Nishimura T. Transpulmonary radiofrequency ablation for hepatocellular carcinoma under real-time computed tomography-fluoroscopic guidance. Hepatogastroenterology 2008;55:1450–3 [PubMed] [Google Scholar]
- 8.Park BJ, Byun JH, Jin YH, Won HJ, Shin YM, Kim KW, et al. CT-guided radiofrequency ablation for hepatocellular carcinomas that were undetectable at US: therapeutic effectiveness and safety. J Vasc Interv Radiol 2009;20:490–9 [DOI] [PubMed] [Google Scholar]
- 9.Miura H, Yamagami T, Terayama K, Yoshimatsu R, Matsumoto T, Nishimura T. Pneumothorax induced by radiofrequency ablation for hepatocellular carcinoma beneath the diaphragm under real-time computed tomography-fluoroscopic guidance. Acta Radiol 2010;51:613–18 [DOI] [PubMed] [Google Scholar]
- 10.Yamakado K, Nakatsuka A, Takaki H, Sakurai H, Isaji S, Yamamoto N, et al. Subphrenic versus nonsubphrenic hepatocellular carcinoma: combined therapy with chemoembolization and radiofrequency ablation. AJR Am J Roentogenol 2010;194:530–5 [DOI] [PubMed] [Google Scholar]
- 11.Toyoda M, Kakizaki S, Horiuchi K, Katakai K, Sohara N, Sato K, et al. Computed tomography-guided transpulmonary radiofrequency ablation for hepatocellular carcinoma located in hepatic dome. World J Gastroenterol 2006;12:608–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shibata T, Shibata T, Maetani Y, Kubo T, Itoh K, Togashi K, et al. Transthoracic percutaneous radiofrequency ablation for liver tumors in the hepatic dome. J Vasc Interv Radiol 2004;15:1323–7 [DOI] [PubMed] [Google Scholar]
- 13.Herrera LJ, Fernando HC, Perry Y, Gooding WE, Buenaventura PO, Christie NA, et al. Radiofrequency ablation of pulmonary malignant tumors in nonsurgical candidates. J Thorac Cardiovasc Surg 2003;125:929–37 [DOI] [PubMed] [Google Scholar]
- 14.Okuma T, Matsuoka T, Tutumi S, Nakmura K, Inoue Y. Air embolism during needle placement for CT-guided radiofrequency ablation of an unresectable metastatic lung lesion. J Vasc Interv Radiol 2007;18:1592–4 [DOI] [PubMed] [Google Scholar]
- 15.Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology 2003;226:441–51 [DOI] [PubMed] [Google Scholar]
- 16.Hiraki T, Mimura H, Gobara H, Sano Y, Fujiwara H, Iguchi T, et al. Two cases of needle-tract seeding after percutaneous radiofrequency ablation for lung cancer. J Vasc Interv Radiol 2009;20:415–18 [DOI] [PubMed] [Google Scholar]
- 17.Yamakado K, Akeboshi M, Nakatsuka A, Takaki H, Takao M, Kobayashi H, et al. Tumor seeding following lung radiofrequency ablation: a case report. Cardiovasc Intervent Radiol 2005;28:530–2 [DOI] [PubMed] [Google Scholar]
- 18.Morimoto M, Numata K, Kondo M, Nozaki A, Hamaguchi S, Takebayashi S, et al. C-arm cone beam CT for hepatic tumor ablation under real-time 3D imaging. AJR Am J Roentogenol 2010;194:W452–4 [DOI] [PubMed] [Google Scholar]
- 19.Hirooka M, Iuchi H, Kumagi T, Shigematsu S, Hiraoka A, Uehara T, et al. Virtual sonographic radiofrequency ablation of hepatocellular carcinoma visualized on CT but not on conventional sonography. AJR Am J Roentgenol 2006;186:S255–60 [DOI] [PubMed] [Google Scholar]