Abstract
Objectives
The aim of this study was to compare the pulmonary thin-section CT findings of patients with acute Streptococcus pneumoniae pneumonia with and without concurrent infection.
Methods
The study group comprised 86 patients with acute S. pneumoniae pneumonia, 36 patients with S. pneumoniae pneumonia combined with Haemophilus influenzae infection, 26 patients with S. pneumoniae pneumonia combined with Pseudomonas aeruginosa infection and 22 patients with S. pneumoniae pneumonia combined with methicillin-susceptible Staphylococcus aureus (MSSA) infection. We compared the thin-section CT findings among the groups.
Results
Centrilobular nodules and bronchial wall thickening were significantly more frequent in patients with pneumonia caused by concurrent infection (H. influenzae: p<0.001 and p<0.001, P. aeruginosa: p<0.001 and p<0.001, MSSA: p<0.001 and p<0.001, respectively) than in those infected with S. pneumoniae alone. Cavity and bilateral pleural effusions were significantly more frequent in cases of S. pneumoniae pneumonia with concurrent P. aeruginosa infection than in cases of S. pneumoniae pneumonia alone (p<0.001 and p<0.001, respectively) or with concurrent H. influenzae (p<0.05 and p<0.001, respectively) or MSSA infection (p<0.05 and p<0.05, respectively).
Conclusions
When a patient with S. pneumoniae pneumonia has centrilobular nodules, bronchial wall thickening, cavity or bilateral pleural effusions on CT images, concurrent infection should be considered.
Streptococcus pneumoniae has long been recognised as the most common cause of community-acquired pneumonia (CAP) and is responsible for the increasing frequency of nosocomial pneumonia [1-3]. The mortality associated with pneumonia is linked to inadequate initial antibiotic therapy; therefore, early detection of S. pneumoniae pneumonia is important for reducing morbidity and mortality.
A rapid immunochromatographic membrane test was developed for the detection of S. pneumoniae antigens in urine samples [4]. It is a useful technique for the rapid diagnosis of pneumococcal pneumonia; however, the urinary antigens cannot be detected a few days after S. pneumoniae infection, and this test is unable to detect concurrent pathogen infections.
Most cases of CAP are probably caused by a single pathogen, but dual or multiple infections have been increasingly reported in the literature [5-8]. There is growing concern for the concurrent presence of a second pathogen in a significant proportion of cases of CAP previously thought to be monomicrobial [5,7-10]. De Roux et al [8] reported that in 82 patients with mixed CAP, S. pneumoniae was the most prevalent microorganism (n=44), that the most frequent combination of organisms was S. pneumoniae with Haemophilus influenzae (n=17) and that patients with mixed pyogenic pneumonia more frequently developed shock than patients with single pyogenic pneumonia.
The classic chest radiographic appearances of pneumococcal pneumonia have been described as sublobar, lobar or multilobar opacities, often homogeneous with an air bronchogram [11-13]. As for CT findings, a few studies have been reported in patients with S. pneumoniae pneumonia; Miyashita et al [14] reported CT findings in 68 patients with S. pneumoniae pneumonia who were not infected with any other microorganisms.
However, to the best of our knowledge, no studies have been published that compare CT findings in patients with S. pneumoniae pneumonia alone with those displaying concurrent pneumonia caused by S. pneumoniae and another pathogen. The present study therefore compared the pulmonary thin-section CT findings of patients with acute S. pneumoniae pneumonia alone with those of patients with concurrent S. pneumoniae pneumonia.
Methods and materials
Patients
Our institutional review board approved this retrospective study, and waived the requirement for informed consent. We retrospectively identified 363 patients with acute S. pneumoniae pneumonia (122 patients with S. pneumoniae pneumonia alone and 241 patients with concurrent S. pneumoniae pneumonia) between January 2004 and July 2010 at our institution (Table 1). Among the concurrent pathogens, H. influenzae was the most frequent (n=85), followed by Pseudomonas aeruginosa (n=48) and methicillin-susceptible Staphylococcus aureus (MSSA; n=37). 86 of the 122 patients with S. pneumoniae pneumonia alone and 129 of the 241 patients with concurrent S. pneumoniae pneumonia underwent chest thin-section CT examinations. The study group comprised 86 patients (45 male, 41 female; age range 23–98 years, mean age 61.3 years) with acute S. pneumoniae pneumonia, 36 patients (27 male, 9 female; age range 31–94 years, mean age 67.2 years) with S. pneumoniae pneumonia combined with H. influenzae, 26 patients (13 male, 13 female; age range 56–90 years, mean age 70.2 years) with S. pneumoniae pneumonia combined with P. aeruginosa and 22 patients (13 male, 9 female; age range 47–81 years, mean age 71.7 years) with S. pneumoniae pneumonia combined with MSSA.
Table 1. The underlying diseases and presenting symptoms in 363 patients.
| Characteristics | S. pneumoniae (n=122) | S.pneumoniae with concurrent infections (n=241) | p-value | ||
| Smoking habit | 38 (31.1) | 101 (41.9) | <0.05 | ||
| Pulmonary emphysema | 23 (18.9) | 54 (22.4) | NS | ||
| Malignancy | 23 (18.9) | 80 (33.2) | <0.01 | ||
| Cardiac disease | 20 (16.4) | 50 (20.7) | NS | ||
| Alcoholic | 17 (13.9) | 71 (29.5) | <0.01 | ||
| Diabetes mellitus | 16 (13.1) | 51 (21.2) | NS | ||
| Asthma | 8 (6.6) | 33 (13.7) | <0.05 | ||
| Liver disorder | 8 (6.6) | 28 (11.6) | NS | ||
| Collagen disease | 2 (1.6) | 21 (8.7) | <0.01 | ||
| Presenting symptoms | |||||
| Cough | 104 (85.2) | 187 (77.6) | NS | ||
| Sputum | 79 (64.8) | 222 (92.1) | <0.001 | ||
| Fever | 108 (88.5) | 199 (82.6) | NS | ||
| Dyspnoea | 20 (16.4) | 30 (12.4) | NS | ||
| Delirium | 7 (5.7) | 13 (5.4) | NS | ||
NS, not significant.
Data in parentheses are percentages.
The diagnosis was established by isolation of S. pneumoniae, clinical features and pulmonary infiltrates on chest radiographs. S. pneumoniae was isolated from sputum of 67 patients, tracheal aspirate of 10 patients, bronchoalveolar lavage fluid of 4 patients and blood specimens of 5 patients. Concurrent infections were diagnosed by the isolation of H. influenzae, P. aeruginosa or MSSA (Table 2).
Table 2. Characteristics of 170 patients with each type of pneumonia.
| Characteristics | S. pneumoniae (n=86) | S. pneumoniae with H. influenzae (n=36) | S. pneumoniae with P. aeruginosa (n=26) | S. pneumoniae with MSSA (n=22) | ||||
| M/F | 45/41 | 27/9 | 13/13 | 13/9 | ||||
| Age (year) | ||||||||
| Range | 23–98 | 31–94 | 56–90 | 47–81 | ||||
| Mean | 61.3 | 67.2 | 70.2 | 71.7 | ||||
| Community acquired | 53 (61.6) | 18 (50.0) | 7 (26.9) | 12 (54.5) | ||||
| Nosocomial | 33 (38.4) | 18 (50.0) | 19 (73.1) | 10 (45.5) | ||||
| Culture sample | ||||||||
| Sputum | 67 (77.9) | 30 (83.3) | 24 (92.3) | 20 (90.9) | ||||
| Tracheal aspirate | 10 (11.6) | 5 (13.9) | 1 (3.8) | 1 (4.5) | ||||
| Bronchoalveolar lavage fluid | 4 (4.7) | 1 (2.8) | 1 (3.8) | 1 (4.5) | ||||
| Blood | 5 (5.8) | 0 (0) | 0 (0) | 0 (0) | ||||
F, female; H., Haemophilus; M, male; MSSA, methicillin-susceptible Staphyloccus aureus; P., Pseudomonas; S., Streptococcus.
Data in parentheses are percentages.
A patient was considered to have CAP if, at the time of hospital admission, he/she presented with cough, with or without sputum, fever, leukocytosis or leukopenia, and pulmonary infiltrates on chest radiographs. None of the patients had been admitted to, or treated in, a hospital in the 2 weeks before admission. Of the 86 patients with S. pneumoniae pneumonia alone, 53 had CAP and 33 had nosocomial infections. Of the 36 patients with H. influenzae, 18 had CAP and 18 had nosocomial infections. Of the 26 patients with P. aeruginosa, 7 had CAP and 19 had nosocomial infections, and of the 22 patients with MSSA, 12 had CAP and 10 had nosocomial infections. No patient had HIV infection.
The frequencies of various underlying diseases, alcohol consumption and smoking habits were also evaluated. For the purposes of this study, an alcoholic was defined as an individual with a daily consumption of ≥80 g of alcohol during the past 2 years [15], and a patient was considered to be a heavy smoker if he/she had smoked more than 10 packs of cigarettes per year.
CT examinations
Thin-section CT examinations were performed with 1 mm collimation at 10 mm intervals from the apex of the lung to the diaphragm in 51 patients (26 patients with S. pneumoniae alone, 10 with H. influenzae, 10 with P. aeruginosa and 5 with MSSA), or volumetrically with a multidetector CT system with a 1 mm reconstruction in 119 patients with concurrent pneumonia (n=60, 26, 16 and 17, respectively). CT examinations were performed with the patient in the supine position at full inspiration and were reconstructed using a high-spatial-frequency algorithm. Images were captured at window settings that allowed viewing of the lung parenchyma (window level, –600 to –700 HU; window width, 1200–1500 HU) and the mediastinum (window level, 20–40 HU; window width, 400 HU). The pulmonary CT examination was performed within 1–6 days (mean 3.2 days) after the onset of respiratory symptoms. Intravenously administered contrast material was used for 33 examinations.
Image interpretation
Two chest radiologists (one with 22 and one with 14 years of experience in chest CT image interpretation), who were unaware of the underlying diagnoses, retrospectively and independently interpreted the CT images. Conclusions were reached by consensus.
CT images were assessed for the following radiological patterns: ground-glass attenuation (GGA), consolidation, nodules, centrilobular nodules, bronchial wall thickening, interlobular septal thickening, intralobular reticular opacity, bronchiectasis, enlarged hilar/mediastinal lymph node(s) (>1 cm diameter short axis), cavities and pleural effusion. Areas of GGA were defined as areas showing hazy increases in attenuation without obscuring vascular markings [16,17]. Areas of consolidation were defined as areas of increased attenuation that obscured the normal lung markings [16,17]. Centrilobular nodules were defined as those present around the peripheral pulmonary arterial branches or 3–5 mm from the pleura, interlobular septa or pulmonary veins. Interlobular septal thickening was defined as abnormal widening of the interlobular septa [17]. Intralobular reticular opacity was considered present when interlacing line shadows were separated by a few millimetres [16,17].
The distribution of parenchymal disease was also noted. Whether the abnormal findings were located unilaterally or bilaterally was assessed. If the main lesion was predominantly located in the inner third of the lung, the disease was classified as having a central distribution. Alternatively, if the lesion was predominantly located in the outer third of the lung, the disease was classified as having a peripheral distribution. If the lesions showed no predominant distribution, the disease was classified as having a random distribution. In addition, zonal predominance was classified as upper, lower or random. Upper lung zone predominance meant that most abnormalities were observed at a level above the tracheal carina, while lower zone predominance referred to most abnormalities being below the upper zone. When abnormalities showed no definite zonal predominance, the lung disease was considered to have a random distribution.
Follow-up CT examinations were performed 4 days to 2 months after antibiotic therapy in 44 patients, and follow-up chest radiographs were performed 1 day to 2 months after antibiotic therapy in 110 patients. These follow-up CT images and radiographs were also assessed.
Statistical analysis
Statistical analysis of the frequency of symptoms and CT findings were conducted using Fisher's exact test and the χ2 test. A mean age comparison was conducted using Student's t-test.
Results
Patients' background
The characteristics of all patients are summarised in Table 2. The mean age of the patients with concurrent infection was higher than that of patients with S. pneumoniae infection alone. The proportion of patients with nosocomial infection was significantly higher in cases of concurrent P. aeruginosa than in cases of S. pneumoniae alone (p<0.005).
The underlying conditions and presenting symptoms of all patients are summarised in Tables 1 and 3. Among each type of pneumonia, the frequencies of smoking and asthma were significantly higher in patients with H. influenzae than in those with S. pneumoniae alone (p<0.005 and p<0.005, respectively; Table 3). The frequencies of malignancy, cardiac disease and alcohol consumption were also significantly higher in patients with P. aeruginosa than in those with S. pneumoniae alone (p<0.01, p<0.01 and p<0.001, respectively). With respect to presenting symptoms, the frequency of sputum in patients with H. influenzae or P. aeruginosa was significantly higher than in patients with S. pneumoniae alone (p<0.01 and p<0.05, respectively).
Table 3. Underlying conditions and presenting symptoms.
| Underlying conditions | S. pneumoniae (n=86) | S. pneumoniae with H. influenzae (n=36) | p-value | S. pneumoniae with P. aeruginosa (n=26) | p-value | S. pneumoniae with MSSA (n=22) | p-value | ||||
| Smoking habit | 24 | (27.9) | 20 | (55.6) | <0.005 | 11 | (42.3) | NS | 8 | (36.4) | NS |
| Pulmonary emphysema | 16 | (18.6) | 10 | (27.8) | NS | 3 | (11.5) | NS | 2 | (9.1) | NS |
| Malignancy | 15 | (17.4) | 11 | (30.6) | NS | 11 | (42.3) | <0.01 | 4 | (18.2) | NS |
| Cardiac disease | 14 | (16.3) | 8 | (22.2) | NS | 12 | (46.2) | <0.01 | 4 | (18.2) | NS |
| Alcoholic | 12 | (14.0) | 12 | (33.3) | NS | 13 | (50.0) | <0.001 | 3 | (13.6) | NS |
| Diabetes mellitus | 10 | (11.6) | 6 | (16.7) | NS | 4 | (15.4) | NS | 2 | (9.1) | NS |
| Asthma | 3 | (3.5) | 6 | (16.7) | <0.005 | 2 | (7.7) | NS | 1 | (4.5) | NS |
| Liver disorder | 2 | (2.3) | 2 | (5.6) | NS | 2 | (7.7) | NS | 2 | (9.1) | NS |
| Collagen disease | 1 | (1.2) | 1 | (2.8) | NS | 4 | (15.4) | <0.005 | 2 | (9.1) | <0.05 |
| Presenting symptoms | |||||||||||
| Cough | 77 | (89.5) | 34 | (94.4) | NS | 23 | (88.5) | NS | 17 | (77.3) | <0.05 |
| Sputum | 59 | (68.6) | 34 | (94.4) | <0.01 | 24 | (92.3) | <0.05 | 19 | (86.4) | NS |
| Fever | 84 | (97.7) | 36 | (100.0) | NS | 25 | (96.2) | NS | 19 | (86.4) | NS |
| Dyspnoea | 17 | (19.8) | 11 | (30.6) | NS | 7 | (26.9) | NS | 4 | (18.2) | NS |
| Delirium | 3 | (3.5) | 3 | (8.3) | NS | 2 | (7.7) | NS | 2 | (9.1) | NS |
H., Haemophilus; MMSA, methicillin-susceptible Staphyloccus aureus; P., Pseudomonas; S., Streptococcus.
Data in parentheses are percentages.
CT patterns
The CT findings of the 170 patients are summarised in Table 4. In the 86 patients with S. pneumoniae alone, GGA (n=74, 86.0%) and consolidation (n=65, 75.6%) were most frequently observed, followed by bronchial wall thickening (n=22, 25.6%), centrilobular nodules (n=17, 19.8%) and reticular opacity (n=8, 9.3%; Figure 1). No cavitary lesions were detected in any of the patients.
Table 4. Thin-section CT findings for each type of pneumonia.
| Finding | S. pneumoniae (n=86) | With H. influenzae (n=36) | p-value | With P. aeruginosa (n=26) | p-value | With MSSA (n=22) | p-value | ||||
| Ground-glass attenuation | 74 | (86.0) | 30 | (83.3) | NS | 26 | (100) | NS | 18 | (81.8) | NS |
| Consolidation | 65 | (75.6) | 26 | (72.2) | NS | 20 | (76.9) | NS | 16 | (72.7) | NS |
| Bronchial wall thickening | 22 | (25.6) | 34 | (94.4) | <0.001 | 20 | (76.9) | <0.001 | 18 | (81.8) | <0.001 |
| Centrilobular nodules | 17 | (19.8) | 28 | (77.8) | <0.001 | 20 | (76.9) | <0.001 | 16 | (72.7) | <0.001 |
| Interlobular septal thickening | 8 | (9.3) | 3 | (8.3) | NS | 2 | (7.7) | NS | 2 | (9.1) | NS |
| Reticular opacity | 8 | (9.3) | 6 | (16.7) | NS | 3 | (11.5) | NS | 3 | (13.6) | NS |
| Nodules | 7 | (8.1) | 5 | (13.9) | NS | 2 | (7.7) | NS | 3 | (13.6) | NS |
| Bronchiectasis | 2 | (2.3) | 7 | (19.4) | <0.001 | 4 | (15.4) | <0.01 | 3 | (13.6) | <0.05 |
| Cavity | 0 | (0) | 1 | (2.8) | NS | 5 | (19.2) | <0.001 | 0 | (0) | NS |
| Pleural effusion | 16 | (18.6) | 5 | (13.9) | NS | 16 | (61.5) | <0.001 | 5 | (22.7) | NS |
| Unilateral | 11 | (12.8) | 2 | (5.6) | NS | 3 | (11.5) | NS | 2 | (9.1) | NS |
| Bilateral | 5 | (5.8) | 3 | (8.3) | NS | 13 | (50.0) | <0.001 | 3 | (13.6) | NS |
| Lymph node enlargement | 13 | (15.1) | 4 | (11.1) | NS | 5 | (19.2) | NS | 4 | (18.2) | NS |
| Distribution | |||||||||||
| Unilateral | 51 | (59.3) | 19 | (52.8) | NS | 7 | (26.9) | <0.005 | 7 | (31.8) | <0.05 |
| Bilateral | 35 | (40.7) | 17 | (47.2) | NS | 19 | (73.1) | <0.005 | 15 | (68.2) | <0.05 |
| Central | 10 | (11.6) | 4 | (11.1) | NS | 2 | (7.7) | NS | 2 | (9.1) | NS |
| Peripheral | 43 | (50.0) | 20 | (55.6) | NS | 17 | (65.4) | NS | 8 | (36.4) | NS |
| Upper | 15 | (17.4) | 7 | (19.4) | NS | 4 | (15.4) | NS | 5 | (22.7) | NS |
| Lower | 35 | (40.7) | 17 | (47.2) | NS | 9 | (34.6) | NS | 10 | (45.5) | NS |
H., Haemophilus; MMSA, methicillin-susceptible Staphyloccus aureus; P., Pseudomonas; S., Streptococcus.
Data in parentheses are percentages.
Figure 1.
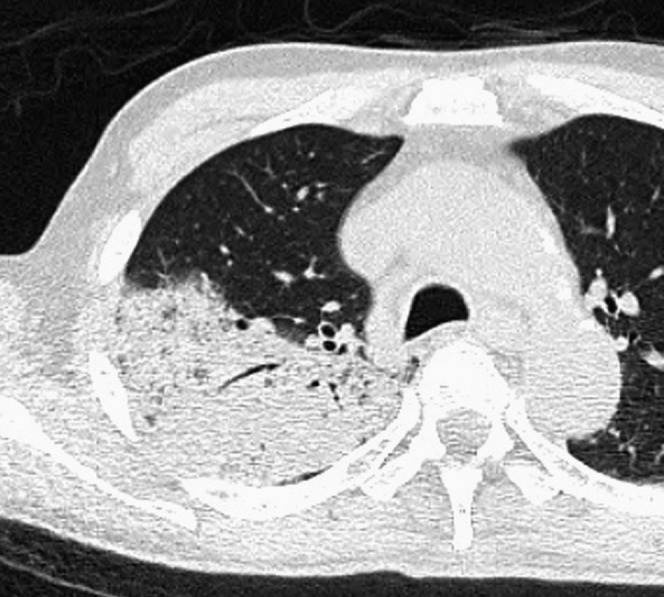
Acute pneumonia caused by Streptococcus pneumoniae alone in a 65-year-old male, 3 days after the onset of fever, cough and dyspnoea. Transverse CT image at the level of the right upper lobe shows consolidation.
In the 36 patients with concurrent H. influenzae, bronchial wall thickening (n=34, 94.4%) and GGA (n=30, 83.3%) were most frequently observed, followed by centrilobular nodules (n=28, 77.8%) and consolidation (n=26, 72.2%; Figure 2). Bronchiectasis (n=17, 19.4%) and cavity (n=3, 8.3%) were also detected.
Figure 2.
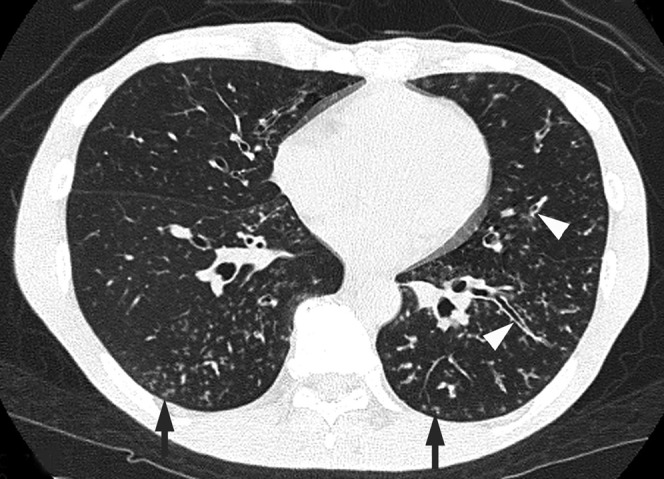
Acute pneumonia caused by Streptococcus pneumoniae along with concurrent Haemophilus influenzae infection in a 51-year-old male alcoholic with a smoking habit and liver disorder, 3 days after the onset of a cough with sputum and fever. Transverse CT image of the lower lobes shows centrilobular nodules (arrows) and bronchial wall thickening (arrowheads).
The frequencies of bronchial wall thickening and centrilobular nodules were significantly higher in patients with concurrent H. influenzae, P. aeruginosa or MSSA compared with patients displaying S. pneumoniae alone (p<0.001 each; Figures 2–4; Table 4). In addition, the frequencies of bronchiectasis were significantly higher in patients with concurrent pathogens compared with those with S. pneumoniae alone (p<0.001, p<0.01 and p<0.05, respectively; Figures 2 and 4). Moreover, cavity was more frequently observed in patients with P. aeruginosa than in patients with S. pneumoniae alone (p<0.001; Figure 3).
Figure 4.
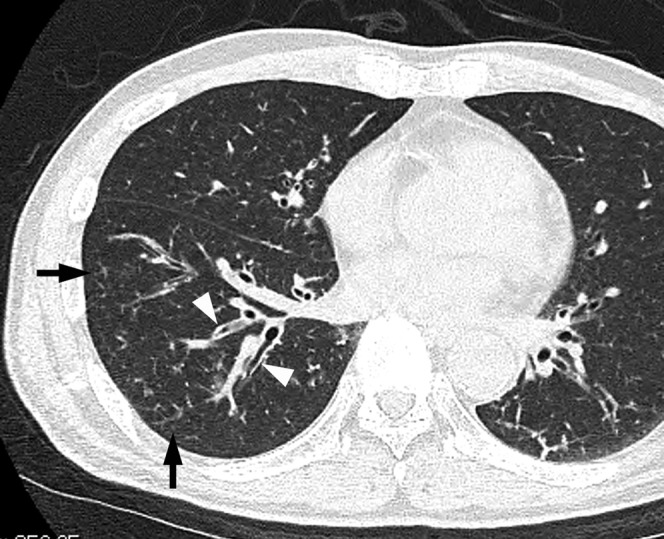
Acute pneumonia caused by Streptococcus pneumoniae along with concurrent methicillin-susceptible Staphylococcus aureus in a 67-year-old male with a smoking habit, liver disorder and lung cancer, 4 days after the onset of a cough with sputum and fever. Transverse CT image of the right lower lobe shows centrilobular nodules (arrows) and bronchial wall thickening (arrowheads).
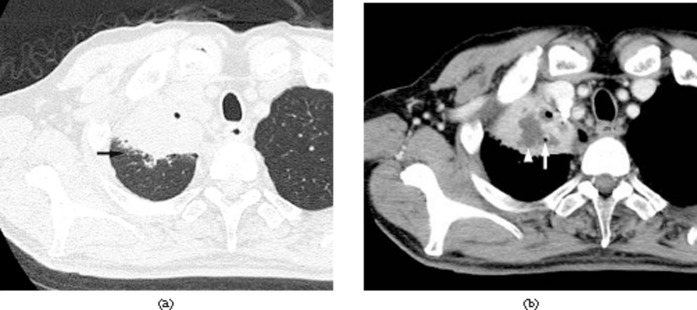
Figure 3.
Acute pneumonia caused by Streptococcus pneumoniae along with concurrent Pseudomonas aeruginosa infection in a 73-year-old male alcoholic with cardiovascular disease and prostatic cancer, 4 days after the onset of a cough with sputum and fever. (a) Transverse CT image of right upper lobe shows consolidation and centrilobular nodules (arrow). (b) Enhanced CT image at the same level shows air collection (arrow) and non-enhanced areas (arrowhead).
Among the three groups of concurrent infections, bronchial wall thickening was more frequently observed in patients with H. influenzae than in those with P. aeruginosa (p<0.05), whereas cavity was more frequently observed in patients with P. aeruginosa than in those with H. influenzae or MSSA (p<0.05 each).
Disease distribution
In the S. pneumoniae alone group, abnormal findings were detected unilaterally in 51 patients (59.3%) and bilaterally in 35 patients (40.7%; Table 4). The predominant zonal distribution was the lower zone in 35 patients (40.7%). In the P. aeruginosa group, abnormal findings were found unilaterally in 7 patients (26.9%) and bilaterally in 19 patients (73.1%). In the MSSA group, abnormal findings were found unilaterally in 7 patients (31.8) and bilaterally in 15 patients (68.2%). The frequency of bilateral abnormal findings was significantly higher in the groups infected with P. aeruginosa or MSSA than in the S. pneumoniae alone group (p<0.005 and p<0.05, respectively). In addition, the frequency of bilateral abnormal findings was higher in the P. aeruginosa group than in the H. influenzae group. There were no significant differences in zonal distributions among the groups.
Effusion and lymph nodes
Pleural effusion was detected in 16 of the 86 patients infected with S. pneumoniae alone (18.6%), and was found to be bilateral in 5 patients (5.8%; Table 4). The frequency of pleural effusion was significantly higher in patients with P. aeruginosa compared with those with S. pneumoniae alone, or with concurrent H. influenzae or MSSA (p<0.001, p<0.001 and p<0.01, respectively). In addition, the frequency of bilateral effusion was also significantly higher in patients with P. aeruginosa than in those with S. pneumoniae alone (p<0.001), or with H. influenzae (p<0.001) or MSSA (p<0.01).
Mediastinal and/or hilar lymph node enlargement was observed in 13 patients (15.1%) with S. pneumoniae alone, 4 patients (11.1%) with H. influenzae, 5 patients (19.2%) with P. aeruginosa and 4 patients (18.2%) with MSSA. There were no significant differences among the groups.
Follow-up study
All 170 patients underwent antibiotic therapy. In 83 of the 86 (96.5%) patients with pneumonia caused by S. pneumoniae alone, abnormal findings showed improvement on a follow-up CT examination or chest radiography. However, in the remaining three patients (3.5%), abnormal findings were found to worsen on follow-up examinations, and all of these patients subsequently died. In comparison, abnormal findings worsened in 4 of the 36 patients with H. influenzae (11.1%), 4 of the 26 patients with P. aeruginosa (15.4%) and 2 of the 22 patients with MSSA (9.1%), and these patients died.
The mortality rate among patients with H. influenzae or P. aeruginosa was significantly higher than among patients with S. pneumoniae alone (p<0.05 each).
Discussion
Recent studies have revealed the presence of more than one causative microorganism in a considerable number of CAP and nosocomial pneumonia cases, and the rates for mixed aetiologies range from 2.5% to 79.6% [6,8,18]. The relevance of these mixed aetiologies in terms of the clinical outcome and the selection of initial empiric antimicrobial treatment remains to be determined.
It has been reported that in cases of mixed CAP, S. pneumoniae was the most prevalent microorganism and severe pneumonia was significantly associated with mixed rather than monomicrobial pneumonia [7,8,19].
In the present study, 241 of 363 patients with acute S. pneumoniae pneumonia (66.4%) were infected by an additional pathogen. The frequencies of underlying disease were significantly higher in patients with concurrent infections than in patients infected with S. pneumoniae alone. It is noteworthy that the frequencies of smoking and asthma were significantly higher in patients with H. influenzae than in those with S. pneumoniae alone, or concurrently with P. aeruginosa or MSSA. This may be explained by the fact that H. influenzae is one of the most common organisms found in patients with acute exacerbation of chronic obstructive pulmonary disease and chronic bronchitis [20].
The radiographic features of pneumococcal pneumonia have been described as lobar consolidation or parenchymal opacities [11-13], sometimes associated with pleural effusion [21,22]. A few studies have reported the CT findings in patients with S. pneumoniae pneumonia. Nambu et al [23] compared thin-section CT findings of 41 patients with S. pneumoniae pneumonia with those of 24 patients with Chlamydia pneumoniae pneumonia. Bronchovascular bundle thickening (p=0.016) and airway dilatation (p=0.034) were found to be significantly less frequent in patients with S. pneumoniae pneumonia than in those with C. pneumoniae pneumonia.
However, to the best of our knowledge, no studies comparing CT findings in patients with S. pneumoniae pneumonia alone with those with S. pneumoniae pneumonia and concurrent infections have been published. We compared the pulmonary thin-section CT findings of 86 patients with acute S. pneumoniae pneumonia alone with those of 84 patients with concurrent S. pneumoniae pneumonia. In the patients with S. pneumoniae alone, GGA and consolidation were most frequently observed, followed by bronchial wall thickening and centrilobular nodules. These results were similar to those of a previous report describing the CT findings in patients with S. pneumoniae pneumonia alone [14]. In the current study, the frequencies of bronchial wall thickening and centrilobular nodules were significantly higher in patients with H. influenzae, P. aeruginosa or MSSA (p<0.001 each) than in patients with S. pneumoniae alone. In addition, the frequencies of bronchiectasis were significantly higher in patients with concurrent pathogen compared with S. pneumoniae infection alone (p<0.001, p<0.01 and p<0.05, respectively).
Recently, we reported thin-section CT findings in 211 patients with acute H. influenzae pulmonary infection who were not infected with any other pathogens [24] and 83 patients with acute MSSA pulmonary infection who were not infected with any other pathogens [25]. The CT findings in the patients infected with H. influenzae consisted mainly of bronchial wall thickening (85.8%) and centrilobular nodules (64.9%). Similarly, the CT findings in the patients with MSSA infection consisted mainly of bronchial wall thickening (75.9%) and centrilobular nodules (63.9%). Moreover, we assessed the differences between the thin-section CT findings in 80 patients with acute pneumonia caused by Klebsiella pneumoniae alone and those in 25 patients with K. pneumoniae pneumonia who were also infected with P. aeruginosa [26]. In cases of K. pneumoniae pneumonia with P. aeruginosa, CT findings of centrilobular nodules, bronchial wall thickening, cavity and pleural effusions were significantly more frequent than in cases of K. pneumoniae pneumonia alone (p<0.001 each).
H. influenzae, P. aeruginosa and MSSA are known to be important pathogens of bronchopneumonia, whereas S. pneumoniae causes air space pneumonia. Classified histologically as bronchopneumonia, nodular features would be expected upon CT evaluation of patients with such infections. Pathologically, bronchopneumonia demonstrates inflammatory changes involving the bronchial and bronchiolar walls, with minimal exudation into adjacent alveoli [27]. The thickened walls of bronchiole and peribronchiolar inflammation can contribute to the centrilobular nodules [28]. Therefore, in cases of S. pneumoniae infection with concurrent H. influenzae, P. aeruginosa or MSSA, CT findings of centrilobular nodules, bronchial wall thickening and bronchiectasis might be significantly more frequent than in cases of S. pneumoniae alone.
Cavity was significantly more frequent in patients with P. aeruginosa than in patients with S. pneumoniae alone, H. influenzae or MSSA (p<0.001, p<0.05 and p<0.05, respectively). In patients with P. aeruginosa, pleural effusions (especially bilateral) were significantly more frequent than in all the other groups. Furthermore, the bilateral distribution of parenchymal abnormalities was also significantly more frequent in patients with P. aeruginosa. Treatment for P. aeruginosa infection does not involve initial empiric antibiotic regimens. Therefore, in patients diagnosed with S. pneumoniae pneumonia, it is important to determine the radiological differences with and without P. aeruginosa. According to the British Thoracic Society guidelines from 2009, CT scanning currently has little role in the routine investigation of CAP [29]. However, in patients diagnosed with S. pneumoniae pneumonia, with underlying conditions such as malignancy, cardiac disease or excessive alcohol consumption, CT examinations may be useful to identify concurrent infection, especially with P. aeruginosa.
It should be noted that there were several limitations to our study. First, this was a retrospective study and CT image interpretation was performed by consensus. Second, thin-section CT images were obtained using different protocols. Third, CT images in patients with concurrent pathogens other than H. influenzae, P. aeruginosa and MSSA were not evaluated.
In summary, underlying diseases were significantly more frequent in patients with pneumonia caused by concurrent infections than in those with pneumonia caused by S. pneumoniae alone. Our CT findings revealed that centrilobular nodules, bronchial wall thickening and bronchiectasis were significantly more frequent in patients with concurrent S. pneumoniae than in those with S. pneumoniae alone.
References
- 1.Craven DE, Steger KA. Epidemiology of nosocomial pneumonia: new perspectives on an old disease. Chest 1995;108(Suppl. 2):1S–16S [DOI] [PubMed] [Google Scholar]
- 2.Chastre J, Fagon JF. Pneumonia in the ventilator-dependent patient. Tobin M, Principles and practice of mechanical ventilation. 1st edn New York, Pa: McGraw-Hill; 1994. pp. 857–90 [Google Scholar]
- 3.American Thoracic Society Hospital-acquired pneumonia in adults: diagnosis, assessment of severity, initial antimicrobial therapy, and preventive strategies: a consensus statement. Am J Respir Crit Care Med 1996;153:1711–25 [DOI] [PubMed] [Google Scholar]
- 4.Dominguez J, Gali N, Blanco S, Pedroso P, Prat C, Matas L, et al. Detection of Streptococcus pneumoniae antigen by a rapid immunochromatographic assay in urine samples. Chest 2001;119:243–9 [DOI] [PubMed] [Google Scholar]
- 5.Heiskanen–Kosma T, Korppi M, Jokinen C, Kurki S, Heiskanen L, Juvonen H, et al. Etiology of childhood pneumonia: serologic results of a prospective, population-based study. Pediatr Infect Dis J 1998;17:986–91 [DOI] [PubMed] [Google Scholar]
- 6.Lieberman D, Schlaeffer F, Boldur I, Lieberman D, Horowitz S, Friedman MG, et al. Multiple pathogens in adult patients admitted with community-acquired pneumonia: a one year prospective study of 346 consecutive patients. Thorax 1996;51:179–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutierrez F, Masia M, Rodriguez JC, Mirete C, Soldan B, Padilla S, et al. Community-acquired pneumonia of mixed etiology: prevalence, clinical characteristics, and outcome. Eur J Clin Microbiol Infect Dis 2005;24:377–83 [DOI] [PubMed] [Google Scholar]
- 8.De Roux A, Ewig S, Garcia E, Marcos MA, Mensa J, Lode H, et al. Mixed community-acquired pneumonia in hospitalized patients. Eur Respir J 2006;27:795–800 [DOI] [PubMed] [Google Scholar]
- 9.Burman LA, Trollfors B, Andersson B, Henrichsen J, Juto P, Kallings I, et al. Diagnosis of pneumonia by cultures, bacterial and viral antigen detection tests, and serology with special reference to antibodies against pneumococcal antigens. J Infect Dis 1991;163:1087–93 [DOI] [PubMed] [Google Scholar]
- 10.Lim WS, Macfarlane JT, Boswell TC, Harrison TG, Rose D, Leinonen M, et al. Study of community acquired pneumonia aetiology (SCAPA) in adults admitted to hospital: implications for management guidelines. Thorax 2001;56:296–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraser RS, Muller NL, Colman NC, Pare PD, Fraser and Pare's diagnosis of disease of the chest. 4th edn Philadelphia, PA: WB Saunders; 1999. pp. 736–43 [Google Scholar]
- 12.Armstrong P, Wilson AG, Dee P, Hansell DM, Armstrong P. Imaging of diseases of the chest. 3rd edn London, UK: Mosby; 2000. pp. 169–71 [Google Scholar]
- 13.Kantor HG. The many radiologic facies of pneumococcal pneumonia. AJR Am J Roentgenol 1981;137:1213–20 [DOI] [PubMed] [Google Scholar]
- 14.Miyashita N, Sugiu T, Kawai Y, Oda K, Tamaguchi T, Ouchi K, et al. Radiographic features of Mycoplasma pneumoniae pneumonia: differential diagnosis and performance timing. BMC Med Imaging 2009;9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torres A, Serra-Batlles J, Ferrer A, Jimenez P, Celis R, Cobo E, et al. Severe community-acquired pneumonia-epidemiology and prognostic factors. Am Rev Respir Dis 1991;144:312–18 [DOI] [PubMed] [Google Scholar]
- 16.Webb WR, Muller NL, Naidich DP. High-resolution computed tomography findings of lung disease. Webb WR, Muller NL, Naidich DP, High-resolution CT of the lung. 3rd edn Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp. 71–192 [Google Scholar]
- 17.Austin JH, Muller NL, Friedman PJ, Hansell DM, Naidich DP, Remy-Jardin M, et al. Glossary of terms for CT of the lungs: recommendations of the Nomenclature Committee of the Fleischner Society. Radiology 1996;200:327–31 [DOI] [PubMed] [Google Scholar]
- 18.Okada F, Ando Y, Honda K, Nakayama T, Kiyonaga M, Ono A, et al. Clinical and pulmonary thin-section CT findings in acute Klebsiella pneumoniae pneumonia. Eur Radiol 2009;19:809–15 [DOI] [PubMed] [Google Scholar]
- 19.Minagawa S, Takayanagi N, Hara K, Takaku Y, Tsutiya Y, Hijikata N, et al. Clinical features of mixed infections in patients with Streptococcus pneumoniae pneumonia. Nihon Kokyuki Gakkai Zasshi 2008;46:278–84 [PubMed] [Google Scholar]
- 20.Moxon ER, Murphy FT. Haemophilus influenzae. Mandell GL, Douglas RG, Beet JE, Principles and practice of infectious diseases. 6th edn New York, NY: Churchill Livingstone; 2005. pp. 2369–78 [Google Scholar]
- 21.Simberkoff MS, El Sadr W, Schiffman G, Rahal JJ., Jr Streptococcus pneumoniae infections and bacteremia in patients with acquired immune deficiency syndrome, with report of a pneumococcal vaccine failure. Am Rev Respir Dis 1984;130:1174–6 [DOI] [PubMed] [Google Scholar]
- 22.Macfarlane JT, Miller AC, Roderick Smith WH, Morris AH, Rose DH. Comparative radiographic features of community acquired Legionnaires' disease, pneumococcal pneumonia, mycoplasma pneumonia, and psittacosis. Thorax 1984;39:28–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nambu A, Saito A, Araki T, Ozawa K, Hiejima Y, Akao M, et al. Chlamydia pneumoniae: comparison with findings of Mycoplasma pneumoniae and Streptococcus pneumoniae at thin-section CT. Radiology 2006;238:330–8 [DOI] [PubMed] [Google Scholar]
- 24.Okada F, Ando Y, Tanoue S, Ishii R, Matsushita S, Ono A, et al. Radiological findings in acute Haemophilus influenzae pulmonary infection. Br J Radiol 2012;85:121–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morikawa K, Okada F, Ando Y, Ishi R, Matsushita S, Ono A, et al. Methicillin-resistant Staphylococcus aureus and methicillin-susceptible Staphylococcus aureus pneumonia: comparison of clinical and thin-section CT findings. Br J Radiol 2012;85:e168–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okada F, Ando Y, Honda K, Nakayama T, Ono A, Tanoue S, et al. Acute Klebsiella pneumoniae pneumonia alone and with concurrent infection: comparison of clinical and thin-section CT findings. Br J Radiol 2010;83:854–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groskin SA. Pneumonia and lung abscess. Heitzman ER, The lung: radiologic–pathologic correlations. 3rd edn St Louis, MO: Mosby; 1993. pp. 194–234 [Google Scholar]
- 28.Okada F, Ando Y, Yoshitake S, Ono A, Tanoue S, Matsumoto S, et al. Clinical/pathologic correlations in 553 patients with primary centrilobular findings on high-resolution CT scan of the thorax. Chest 2007;132:1939–48 [DOI] [PubMed] [Google Scholar]
- 29.Lim WS, Baudouin SV, George RC, Hill AT, Jamieson C, Le Jeune I, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Pneumonia Guidelines Committee of the BTS Standards of Care Committee. Thorax 2009;64(Suppl. 3):iii1–55 [DOI] [PubMed] [Google Scholar]